Abstract
Stem cells of highly regenerative organs including blood are susceptible to endogenous DNA damage caused by both intrinsic and extrinsic stress. Response mechanisms to such stress equipped in hematopoietic stem cells (HSCs) are crucial in sustaining hematopoietic homeostasis but remain largely unknown. In this study, we demonstrate that serial transplantation of human HSCs into immunodeficient mice triggers replication stress that induces incremental elevation of intracellular reactive oxygen species (ROS) levels and the accumulation of persistent DNA damage within the human HSCs. This accumulation of DNA damage is also detected in HSCs of clinical HSC transplant patients and elderly individuals. A forced increase of intracellular levels of ROS by treatment with a glutathione synthetase inhibitor aggravates the extent of DNA damage, resulting in the functional impairment of HSCs in vivo. The oxidative DNA damage activates the expression of cell-cycle inhibitors in a HSC specific manner, leading to the premature senescence among HSCs, and ultimately to the loss of stem cell function. Importantly, treatment with an antioxidant can antagonize the oxidative DNA damage and eventual HSC dysfunction. The study reveals that ROS play a causative role for DNA damage and the regulation of ROS have a major influence on human HSC aging.
Introduction
Maintenance of the hematopoietic system requires continual replenishment of mature blood cells from a rare population of bone marrow residing hematopoietic stem cells (HSCs). The alteration of the homeostatic control of hematopoiesis is considered to be a major culprit of drastic increase in pathologic incidences, such as bone marrow failure, anemia, and myeloid leukemia during aging.1 However, the underlying mechanisms of pathogenesis of hematologic malignancy in elderly population remain poorly understood.
Mounting evidence supports the idea that the accumulation of somatic DNA damage is a main cause of aging in multicellular organisms.2-5 Mice with mutations in various DNA repair genes exhibit accelerated aging in the hematopoietic system because of the premature exhaustion of HSCs, indicating that DNA repair is crucial for the maintenance of HSC self-renewal and hematopoietic function.6,7 DNA damage can directly result from genotoxic treatment such as ionizing radiation (IR), or may simply occur as a consequence of genome duplication infidelity or of genotoxic effects of reactive oxygen species (ROS). ROS, such as superoxide anions and hydrogen peroxide, are byproducts of normal oxidative metabolism in eukaryotic cells and are involved in many signaling process. However, they can be harmful to cellular components, including DNA.3,8,9 An uncontrolled elevation of intracellular ROS levels is believed to contribute to cellular aging and the senescence process.3 In fact, an abnormal elevation of intracellular ROS levels has been implicated in the pathogenesis of various diseases, such as ataxia telangiectasia and Fanconi anemia.3 In that sense, the maintenance of ROS levels, through highly regulated mechanisms, is essential for cellular homeostasis.10
Being continuously exposed to oxidants produced during metabolic activity and to external oxidants or oxidant-inducers through normal cellular physiology, DNAs within cells inevitably suffer the oxidative damage. Therefore, an accelerated proliferation of hematopoietic cells, which is expected to occur after clinical HSC transplantation, might lead to DNA damage through overexposure to oxidative stress generated on each cell cycle. Indeed, a hyperproliferation caused an accumulation of oxidative stress and resulted in functional exhaustion of murine HSCs, as shown by the failure to reconstitute hematopoiesis after serial transplantations.11 Taken together, we hypothesize that the continuous production of ROS during long-term repopulation induces an accumulation of genomic damage that leads to exhaustion of human HSCs. We have previously developed a strategy that enables to examine the multipotency of a single human HSC using a reliable surrogate system.12,13 By determining the in vivo repopulating dynamics of individual human HSCs, we demonstrated that the repopulating potential of the majority of human HSCs progressively deteriorated as they underwent extensive repopulation process. Furthermore, the self-renewing long-term repopulating clones stayed mainly in a quiescent state in the recipient bone marrow but expanded extensively on serial transplantation. It has not been clear, however, how the repopulating activity of human HSCs within the stem cell pool is regulated. In addition, functional influences of DNA damage response (DDR) pathway on the self-renewal capacity of human HSCs remain unexplored. In this study, we address the effects of oxidative DNA damage during the lifespan of human HSCs.
Methods
Collection and fractionation of human CD34+ cells
Cord blood (CB) and bone marrow samples were obtained after informed consent in accordance with the Declaration of Helsinki and with approval from the Tokai University Committee on Clinical Investigation. Bone marrow cells were collected from HSC transplant (HSCT) patients who were diagnosed as the complete remission. Information on HSCT patients is listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CD34+ cell fraction was prepared using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec). CD34+-enriched cells were stained with allophycocyanin (APC)–conjugated anti-CD45 mAb (Coulter/Immunotech), and FITC-conjugated anti–lineage-specific antigens; CD2, CD3 (UCHT1), CD41 (P2), glycophorin A (11E4B-7-6), CD14 (MfP9), CD19 (SJ25C1), and CD56 (NCAM16.2; all from BD Biosciences), phycoerythrin (PE)–conjugated anti-CD38 (HB7; BD Biosciences), and PE–Texas Red (ECD)–conjugated anti-CD34 (581; Coulter/Immunotech) mAbs. Cells were gated on lineage marker negative and/or low expression region and Lin−/lowCD34+ cells were fractionated according to their CD38 expression levels using the FACSVantage flow cytometer (BD Biosciences).
Cell cultures
Freshly isolated Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells were incubated with buthionine sulfoximine (BSO; 125μM) for 2 days in αMEM medium supplemented with TPO, SCF, and Flt3L (50 ng/mL each). To exclude the effect of external oxidants and reduce the ground level expression of γ-H2Ax foci, cells were cultured in hypoxic (5% O2) conditions. For some experiments, N-acetyl-L-cysteine (NAC; 100μM, pH 7.0) was added to the culture media.
Human hematopoietic repopulation
NOD/Shi-scid, IL-2Rγcnull (NOG) mice14 were obtained from the Central Institute for Experimental Animals and maintained in the animal facility of the Tokai University School of Medicine in microisolator cages. Mice were fed with autoclaved food and water. Nine- to 20-week-old NOG mice were irradiated with 220 cGy of X-rays. The following day, Lin−CD34+CD38− cells were injected into the retro-orbital plexus of the NOG mice. At the indicated times after transplantation, mice were humanely killed, and bone marrow cells were harvested. Human hematopoietic cells were distinguished from mouse cells by the expression of human CD45. Lin−CD34+CD38− cells isolated from pooled bone marrow cells of 3-5 recipient mice were used for the analyses. All experiments were approved by the animal care committee of Tokai University.
Serial transplantation
Bone marrow cells were obtained from recipient mice at 18 weeks after primary transplantation and injected intravenously into irradiated secondary or tertiary NOG recipients (2 × 107 cells per recipient). To examine the effect of antioxidant, mice were given feed containing 1.3% NAC for the entire repopulation period. CD34+ cells were isolated from these mice, and serially transplanted into recipient mice (1 × 105 cells per recipient).
Detection of intracellular ROS
The intracellular ROS level was measured using a fluorescent probe 2′, 7′-dichlorofluorescene diacetate (DCF-DA). Cells were incubated with 10μM DCF-DA for 30 minutes, and the fluorescence was measured using a flow cytometor. The relative ROS level was calculated on the basis of the mean fluorescence intensity (MFI) of the DCF-DA and was presented as fold induction compared with the control group.
Cell-cycle analysis
Bromodeoxyuridine (BrdU) labeling was performed according to the manufacturer's instructions (BD Biosciences). For in vivo analysis, mice were injected with a single dose (1 mg) of BrdU. After 2 hours, Lin−CD34+CD38− cells were isolated, and BrdU incorporation was quantified by flow cytometry using the BrdU staining kit (BD Biosciences). For in vitro analysis, cells were pulsed with BrdU (10μM) for 30 minutes, and FACS analysis was performed.
Immunofluorescence micoscopy
Cells were seeded onto poly-L-lysine coated slides and fixed with ice-cold 70% ethanol for 15 minutes. After permeabilization with 0.2% Triton X-100 for 20 minutes, slides were treated with PBS containing 5% normal goat serum for 1 hour to block nonspecific binding of antibodies. Primary antibodies were applied overnight at 4°C. The following antibodies were used: γ-H2AX (JBW301; Millipore), ATM (Chemicon), 53BP1 (Cell Signaling Technology), CHK2 (Cell Signaling Technology), FOXO3a (Cell Signaling Technology), caspase-3 (Millipore), or p21CIP1 (12D1; Cell Signaling Technology). Cells were counterstained with DAPI. Images were captured with an LSM510 META confocal microscope (Carl Zeiss) and processed using Adobe Photoshop 7.0 (Adobe Systems). Fifty randomly captured images were used to quantify the foci formation.
Quantitative real-time PCR
RNA was isolated using the RNeasy micro kit (QIAGEN) and reverse transcribed. Each target cDNA was amplified on the same plate using the QuantiTect SYBR Green PCR Master Mix (QIAGEN) and the ABI Prism 7700 Sequence Detection System (Applied Biosystems). The sequence of PCR primers are: p16, 5′-GGGTCGGGTAGAGGAGGTG-3′ and 5′-GCCTCCGACCGTAACTATTCG-3′; p14, 5′-AGTGAGGGTTTTCGTGGTTCA-3′ and 5′-TCCTCAGTAGCATCAGCACGAG-3′; p21, 5′-TGTCTTGTACCCTTGTGCCT-3′ and 5′-AATCTGTCATGCTGGTCTGC-3′; 18S rRNA, 5′-ACTCAACACGGGAAACCTCA-3′ and 5′-AACCAG-ACAAATCGCTCCAC-3′. The relative amounts of target genes were determined in reference to 18S rRNA. A comparative threshold cycle (CT) was used to quantify transcripts. The value was calculated by the expression 2−ΔΔCT. Each reaction was performed at triplicate.
Statistical analysis
Data were statistically analyzed using Prism 5.0 software (GraphPad Software). Results were analyzed by the 2-tailed unpaired Student t test after the evaluation of variants using the pooled datasets. The mean ± SD was presented in each graph. The numbers of γ-H2AX foci per cell were presented using box and whiskers graphs. P values < .05 were considered to be significant.
Results
Repopulating capacity of human HSCs deteriorate during long-term repopulation
A rare fraction of the Lineage−(Lin−)CD34+CD38− cells represents repopulating human HSCs, a candidate human HSCs currently determined as severe immunodeficient mice hematopoietic repopulating cells (SRCs).15 Previously, we demonstrated that quiescent human HSCs were maintained in the stem cell niche under steady-state conditions.13 To examine the cell-cycle status of human HSCs during hematopoietic reconstitution after transplantation, recipient mice were administered with BrdU at several time points, and Lin−CD34+CD38− cells were analyzed for BrdU incorporation rate at 2 hours after the each administration. At 2 weeks after transplantation, Lin−CD34+CD38− cells exited G0/G1 and efficiently entered the S + G2/M phases of the cell cycle, suggesting the activation and expansion of human HSCs (Figure 1A). This cell-cycle transition was confirmed by the up-regulation of cyclin expressions (supplemental Figure 1). Interestingly, the proliferating Lin−CD34+CD38− cells regained quiescence at the later phase of transplantation. Lin−CD34+CD38− cells after secondary transplantation demonstrated the similar cell- cycle dynamics (Figure 1A). These results indicate that the cell-cycle status of human HSCs changes reversibly from quiescence to activation during repopulation.
Functional assessment of long-term repopulating human HSCs. (A) Sublethally irradiated NOG mice were given transplants of CB Lin−CD34+CD38− cells. Recipient mice were administered with BrdU at the indicated time points (3 recipients in each time point), and Lin−CD34+CD38− cells were isolated at 2 hours after each administration. The BrdU incorporation rate for the isolated cells was analyzed by flow cytometry (**P < .01 relative to CB and 18 weeks after transplantation). (B) Relative expressions of p16INK4a, p14ARF, and p21CIP1 in Lin−CD34+CD38− cells were analyzed by quantitative real-time PCR. Each value was normalized to 18S rRNA expression and is presented as a fold induction compared with the levels detected in Lin−CD34+CD38− cells isolated from CB. Data were collected from 4 independent experiments (**P < .01). (C) Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells were obtained from CB and primary and secondary recipients' mouse bone marrow at 18 weeks after transplantation (3 recipients at the each time point). At 3 days after cultivation with cytokines, cells were pulsed with BrdU for 30 minutes, and then the BrdU incorporation rate was analyzed by flow cytometry (*P < .05, **P < .01). (D) Whole bone marrow cells obtained from each primary recipient mouse (n = 6) were serially transplanted into secondary (n = 6) and tertiary (n = 5) recipient mice. Human hematopoietic cell engraftment was assessed by the expression of human CD45 by flow cytometry at 18 weeks after each transplantation (**P < .01 relative to primary recipient).
Functional assessment of long-term repopulating human HSCs. (A) Sublethally irradiated NOG mice were given transplants of CB Lin−CD34+CD38− cells. Recipient mice were administered with BrdU at the indicated time points (3 recipients in each time point), and Lin−CD34+CD38− cells were isolated at 2 hours after each administration. The BrdU incorporation rate for the isolated cells was analyzed by flow cytometry (**P < .01 relative to CB and 18 weeks after transplantation). (B) Relative expressions of p16INK4a, p14ARF, and p21CIP1 in Lin−CD34+CD38− cells were analyzed by quantitative real-time PCR. Each value was normalized to 18S rRNA expression and is presented as a fold induction compared with the levels detected in Lin−CD34+CD38− cells isolated from CB. Data were collected from 4 independent experiments (**P < .01). (C) Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells were obtained from CB and primary and secondary recipients' mouse bone marrow at 18 weeks after transplantation (3 recipients at the each time point). At 3 days after cultivation with cytokines, cells were pulsed with BrdU for 30 minutes, and then the BrdU incorporation rate was analyzed by flow cytometry (*P < .05, **P < .01). (D) Whole bone marrow cells obtained from each primary recipient mouse (n = 6) were serially transplanted into secondary (n = 6) and tertiary (n = 5) recipient mice. Human hematopoietic cell engraftment was assessed by the expression of human CD45 by flow cytometry at 18 weeks after each transplantation (**P < .01 relative to primary recipient).
To further investigate the quiescent status of HSCs at the different time points of the serial transplantation, we examined the expressions of cell-cycle inhibitors p16INK4a, p14ARF, and p21CIP1 in Lin−CD34+CD38− cells isolated from fresh CB and recipients' bone marrow at 18 weeks after primary and secondary transplantation. Serial transplantation resulted in marked up-regulation of cell- cycle inhibitors among the human HSC population (Figure 1B). In contrast, the expression levels of cell-cycle inhibitors in Lin−CD34+CD38+ cells were unchanged throughout the same repopulation period (data not shown). Proliferation activity of human HSCs, determined as S-phase entry in response to cytokine stimulation, was assessed by BrdU incorporation in vitro. Although there were no significant changes in the percentage of BrdU incorporation among committed progenitors (Lin−CD34+CD38+ cells), the proliferative capacity of human HSCs was reduced gradually during serial transplantation (Figure 1C). Consistent with these observations, the repopulating capacity of human HSCs in recipient mice was reduced drastically after the secondary transplantation (Figure 1D). These data suggest that self-renewal capacity of quiescent HSCs before and after transplantation is distinctly different even though they appear similar based on more than one criterion, that is, the cell-surface phenotype and cell-cycle status.
Decline of self-renewal potential of human HSCs is associated with an accumulation of DNA damage
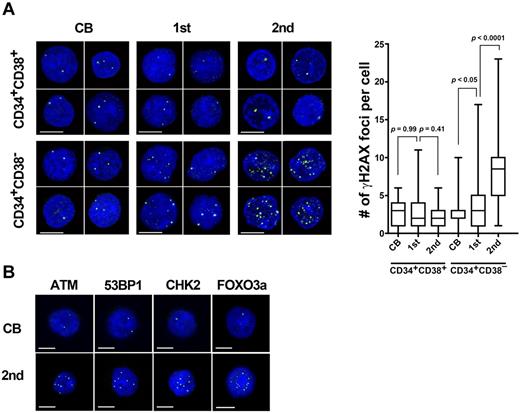
Mice deficient in several genomic-maintenance pathways demonstrated a functional exhaustion of HSCs, because of an accumulation of genomic damage within HSC population.6,7 To examine whether serial transplantation induces an accumulation of DNA damage in human HSCs, the number of γ-H2AX (γ-H2AX; pS139) foci, an indicator of the extent of DNA damage, was quantified in individual human cells recovered from recipient mice. The analysis revealed that whereas only an occasional γ-H2AX focus was observed in freshly isolated CB HSCs, the majority of Lin−CD34+CD38− cells isolated from serially transplanted mice exhibited multiple foci (Figure 2A). Furthermore, these cells exhibited cytologic evidence of activated DDR pathways, that is, up-regulation of DDR markers including ataxia telangiectasia mutated (ATM; pS1981), p53 binding protein (53BP1; pS1778), checkpoint kinase 2 (CHK2; pT68), and forkhead box O3a (FOXO3a; Figure 2B and quantitative data provided in supplemental Figure 2). Interestingly, no obvious accumulation of γ-H2AX foci was observed in committed progenitor cells (Lin−CD34+CD38+) at any time of reconstitution (Figure 2A).
An accumulation of DNA damage in long-lived human HSCs. (A) Lin−CD34+CD38− cells or Lin−CD34+CD38+ cells isolated from CB and recipient mice bone marrow at 18 weeks after primary (n = 10) and secondary (n = 15) transplantation were immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue; left). All bars represent 5 μm. The box and whisker plot shows the number of γ-H2AX foci per cell. More than 50 cells in random fields on a slide were counted for 5 independent experiments. (B) Lin−CD34+CD38− cells were immunostained with antibodies for ATM phosphorylated on Ser 1981 (green), 53BP1 phosphorylated on Ser 1778 (green), CHK2 phosphorylated on Thr 68 (green), or FOXO3a (green). DAPI (blue) was used to stain nucleus. All bars represent 5 μm.
An accumulation of DNA damage in long-lived human HSCs. (A) Lin−CD34+CD38− cells or Lin−CD34+CD38+ cells isolated from CB and recipient mice bone marrow at 18 weeks after primary (n = 10) and secondary (n = 15) transplantation were immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue; left). All bars represent 5 μm. The box and whisker plot shows the number of γ-H2AX foci per cell. More than 50 cells in random fields on a slide were counted for 5 independent experiments. (B) Lin−CD34+CD38− cells were immunostained with antibodies for ATM phosphorylated on Ser 1981 (green), 53BP1 phosphorylated on Ser 1778 (green), CHK2 phosphorylated on Thr 68 (green), or FOXO3a (green). DAPI (blue) was used to stain nucleus. All bars represent 5 μm.
Hematopoietic repopulation stimulates the excessive production of ROS in HSCs
Being cell-intrinsic byproducts of normal metabolism, ROS can cause persistent DNA damage in any living cells and may contribute to cellular aging.3,8 Therefore, we evaluated the ROS levels of reconstituting human HSCs at 18 weeks after transplantation. Lin−CD34+CD38− cells were isolated from serial transplant recipients, and the intracellular concentration of ROS was determined by the intensity of DCF-DA staining using a flow cytometer. A gradual but marked increase in ROS levels was observed during serial transplantation (Figure 3A), reminiscent of the kinetics of DNA damage accumulation (Figure 2A-B). We also found that higher ROS levels were associated with increased DNA damage in human HSCs (Figure 3B). To investigate whether intracellular ROS levels influence the stem cell function, serial transplantations using ROSlow and ROShigh populations were performed. While ROSlow human HSCs retained the repopulation potential, the repopulating capacity of ROShigh human HSCs was almost diminished by the tertiary recipients (Figure 3B). These results suggest that a series of bone marrow reconstitution causes the elevation of intracellular ROS level and accumulation of DNA damage in human HSC, which lead to the reduction in stem cell function.
Increased ROS levels in long-lived human HSCs. (A) Lin−CD34+CD38− cells were isolated from CB (n = 4) and recipient mouse bone marrow at 18 weeks after primary (n = 4) and secondary transplantation (n = 4). Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometor. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from CB (*P < .05). (B) Representative sorting profiles of DCF-DA stained Lin−CD34+CD38− cells isolated from secondary recipients' bone marrow cells. Gray shade indicates a DCF-DA–staining of Lin−CD34+CD38− cells isolated from CB. Purified DCF-DAhigh cells and DCF-DAlow cells were immunostained for γ-H2AX. The box and whisker plot shows the number of γ-H2AX foci per cell. More than 50 cells in random fields on a slide were counted for 3 independent experiments (**P < .01). Bars represent 5 μm. DCF-DAlow and DCF-DAhigh Lin−CD34+CD38− cells isolated from secondary recipient were transplanted into sublethally irradiated NOG mice. Twelve weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (3 recipients in each group; **P < .01). (C) Apoptosis in Lin−CD34+CD38− cells was assessed by annexin V/PI staining (n = 3 in each group). Inserted images demonstrate the immunostaining results of Lin−CD34+CD38− cells from secondary recipient. Positive staining for γ-H2AX (green) but not for active form caspase-3 (red) was detected.
Increased ROS levels in long-lived human HSCs. (A) Lin−CD34+CD38− cells were isolated from CB (n = 4) and recipient mouse bone marrow at 18 weeks after primary (n = 4) and secondary transplantation (n = 4). Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometor. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from CB (*P < .05). (B) Representative sorting profiles of DCF-DA stained Lin−CD34+CD38− cells isolated from secondary recipients' bone marrow cells. Gray shade indicates a DCF-DA–staining of Lin−CD34+CD38− cells isolated from CB. Purified DCF-DAhigh cells and DCF-DAlow cells were immunostained for γ-H2AX. The box and whisker plot shows the number of γ-H2AX foci per cell. More than 50 cells in random fields on a slide were counted for 3 independent experiments (**P < .01). Bars represent 5 μm. DCF-DAlow and DCF-DAhigh Lin−CD34+CD38− cells isolated from secondary recipient were transplanted into sublethally irradiated NOG mice. Twelve weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (3 recipients in each group; **P < .01). (C) Apoptosis in Lin−CD34+CD38− cells was assessed by annexin V/PI staining (n = 3 in each group). Inserted images demonstrate the immunostaining results of Lin−CD34+CD38− cells from secondary recipient. Positive staining for γ-H2AX (green) but not for active form caspase-3 (red) was detected.
As a coping mechanism for internal and external cellular damage, the DDR machinery induces cell-cycle arrest in damaged cells. These cells may resume to cell-cycle progression once damage has been repaired, or cells that suffered irreparable DNA damage undergo apoptosis or permanent cell-cycle arrest.3 We examined whether the DDR in human HSCs led to apoptotic cell death and found no evidence of apparent apoptotic cells among the long-lasting HSC population of primary and secondary transplants (Figure 3C). The results indicate that as a consequence of DDR, the long-lived HSCs may undergo senescence-like changes rather than apoptosis, or senescence-like cells may survive after damaged cells are cleared by apoptosis, either way leading to the persistence of DNA damaged foci in the stem cell compartment.
Oxidative DNA damage causes human HSC dysfunction
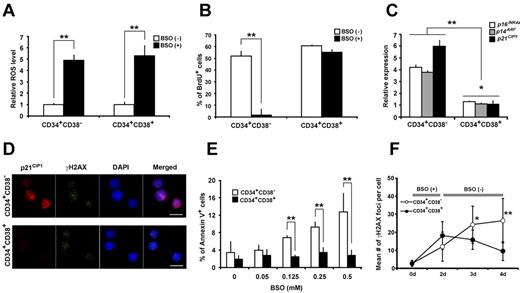
We then asked whether an increased ROS caused functional defects in the human HSC compartment. We added BSO, an inhibitor of glutathione synthetase, to the culture media to experimentally increase the intracellular ROS level of human HSCs (Figure 4A). To exclude the effect of external oxidants and to reduce the ground level expression of γ-H2AX foci, cells were cultured in a hypoxic (5% O2) condition. BSO-treated cells and control cells were immunostained for γ-H2AX. The majority of the BSO-treated human HSCs presented multiple foci, contrasting to the control human HSCs in which only sporadic γ-H2AX foci were observed (Figure 4B). Further analyses revealed the cytologic evidence of activated DDR in BSO-treated human HSC cultures. The activated form of ATM, 53BP1, CHK2, and FOXO3a was detected (Figure 4C and quantitative data provided in supplemental Figure 3), thus demonstrating an activation of a series of DDR pathways initiated by the increased ROS levels in human HSCs. We therefore asked whether the oxidative DNA damage played a causative role in the functional defect in the human HSC compartment. The reconstitution activity of human HSCs was evaluated. As expected, the repopulating capacity of BSO-treated human HSCs was significantly reduced (Figure 4D). These data indicate that an accumulation of DNA damage in the HSC compartment initiated by the increased ROS level results in human HSC dysfunction.
Oxidative DDR in human HSCs. (A) CB Lin−CD34+CD38− cells were cultured for 2 days with or without BSO. Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells cultured without BSO. Data were collected from 3 independent experiments (**P < .01). (B) CB Lin−CD34+CD38− cells cultured with or without BSO were immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue). The number of γ-H2AX foci per cell is shown (right; **P < .01). Bars represent 5 μm. (C) CB Lin−CD34+CD38− cells treated with BSO were immunostained for ATM (p-S1981: green), 53BP1 (p-S1778: green), CHK2 (p-T68: green), and FOXO3a (green). All bars represent 5 μm. (D) BSO treated or nontreated CB Lin−CD34+CD38− cells were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry. Data were obtained from a total of 6 recipients in each group (3 independent experiments, **P < .01).
Oxidative DDR in human HSCs. (A) CB Lin−CD34+CD38− cells were cultured for 2 days with or without BSO. Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells cultured without BSO. Data were collected from 3 independent experiments (**P < .01). (B) CB Lin−CD34+CD38− cells cultured with or without BSO were immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue). The number of γ-H2AX foci per cell is shown (right; **P < .01). Bars represent 5 μm. (C) CB Lin−CD34+CD38− cells treated with BSO were immunostained for ATM (p-S1981: green), 53BP1 (p-S1778: green), CHK2 (p-T68: green), and FOXO3a (green). All bars represent 5 μm. (D) BSO treated or nontreated CB Lin−CD34+CD38− cells were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry. Data were obtained from a total of 6 recipients in each group (3 independent experiments, **P < .01).
It has been reported that the over-production of proinflammatory cytokines, such as IFN and TNF, is one element of the activation of the oxidative DNA damage checkpoint mechanisms in HSCs.16,17 To address this issue, we examined whether the pro-inflammatory cytokines were involved in DNA damage observed in our human-to-mouse transplantation settings. First, we examined the production of murine and human TNF after transplantation in the hosts and found that the level of the cytokine was under detection limit throughout the reconstitution period (supplemental Figure 4A). We then investigated the expressions of TNF and IFNγ in recipients' bone marrow cells and the effect of murine and human inflammation cytokines on the accumulation of DNA damage in human CB HSCs in vitro. Although the local production of murine TNF was slightly up-regulated (supplemental Figure 4B), the effect of this cytokine on DNA damage on human HSCs was negligible, given that no cross-reactivity of murine cytokines on human cells was confirmed (supplemental Figure 4C). Therefore, the effect of human proinflammatory cytokines appeared limited in our experimental model. We conclude that the DNA damages in human HSCs observed in our xenotransplantation settings are induced by a proinflammatory cytokine-independent manner. However, the proinflammatory cytokines may have a substantial influence on the stability of HSCs in the case of severe transplant-related diseases, such as GVHD or infection.
HSCs are predisposed for persistent DNA damage
Earlier in this study, we demonstrated that the extent of DNA damage in the Lin−CD34+CD38+ progenitor cells was less prominent than in Lin−CD34+CD38− HSCs (Figure 2A). This may suggest the difference(s) in the response mechanism to oxidative DNA damage between HSCs and progenitors. To investigate this possibility, the biologic responses to oxidative DNA damage were compared between Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells. At day 2 of BSO treatment, no detectable difference was found in the induction of intracellular up-regulation of ROS levels (Figure 5A) or the number of γ-H2AX foci in the individual cells (Figure 5F) between HSCs and progenitor fractions. However, their biologic response to oxidative stress was quite different. The upsurge of intracellular ROS levels halted the cell cycle in HSCs almost completely (Figure 5B). This cell-cycle arrest of human HSCs in response to oxidative stress was further documented by the up-regulation of cell-cycle inhibitors p16INK4a, p14ARF, and p21CIP1 (Figure 5C). No such growth arrest (Figure 5B) or cell- cycle inhibitor up-regulation was observed in Lin−CD34+CD38+ progenitor cells (Figure 5C). The oxidative DDR-induced cell- cycle inhibitor up-regulation in HSCs was confirmed by immunofluorescent microscopic analysis. Progenitor cells exhibited no apparent p21CIP1 expression (Figure 5D). We assessed the apoptotic response in these cells by annexin V/PI staining and found that a significantly higher percentage of cells underwent apoptosis in human HSC compartment than progenitor cells in vitro (Figure 5E). We then examined the kinetics of DNA lesions after BSO treatment. At the end of 2-day culture with BSO, cells were washed and continued to be cultured in BSO-free conditions for the indicated time period, and DDR was analyzed. The number of γ-H2AX foci per cell in Lin−CD34+CD38+ cells declined comparatively faster than in Lin−CD34+CD38− cells (Figure 5F). We further examined the kinetics of DNA lesions after BSO treatment using Lin−CD34+CD38−CD90+CD45RA− cells, a more HSC-enriched population, and CD90−CD45RA+ multilymphoid progenitors (MLPs). The experiment resulted in the same outcome, confirming that the HSCs are predisposed for persistent DNA damage (supplemental Figure 5). These data indicate that HSCs are intrinsically more sensitive to the elevation of intracellular ROS levels than progenitors and explain why DNA lesions are less likely to be accumulated in progenitors in vivo (Figure 2A). The results support and clarify the interpretation for our earlier in vivo observation (Figure 3C) that the long-lasting HSCs are survivors of DNA damage that undergo senescence-like changes as a result of DDR.
Distinctive response of human HSCs and progenitors to oxidative DNA damage. (A) CB Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells were cultured with or without BSO for 2 days. Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometor. The relative ROS level is presented as a fold induction compared with the MFI value detected in cells cultured without BSO. Data from 2 independent experiments are shown (**P < .01). (B) Two days after BSO treatment, cells were pulsed with BrdU and analyzed by flow cytometry. Data from 2 independent experiments are shown (**P < .01). (C) Relative expressions of p16INK4a, p14ARF, and p21CIP1 in Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells cultured with or without BSO were analyzed at the end of 2-day culture by quantitative real-time PCR. Each value was normalized to 18S rRNA expression and is presented as a fold increase compared with the levels detected in cells cultured without BSO (**P < .01). (D) CB Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells cultured with BSO for 2 days were stained with γ-H2AX and p21CIP1 mAbs (γ-H2AX: green; p21CIP1: red; DAPI: blue). Bars represent 5 μm. (E) At 2 days after BSO-treatment, the frequency of apoptotic cells was determined by annexin V/PI staining. Data from 3 independent experiments are shown (**P < .01). (F) The number of γ-H2AX foci per cell in Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells at the indicated time points. Cells cultured with BSO for 2 days were washed, replaced with fresh BSO-free media, and cultured for additional 1, 2, or 3 days (n = 4 in each time point; *P < .05, **P < .01, Lin−CD34+CD38− cells vs Lin−CD34+CD38+ cells).
Distinctive response of human HSCs and progenitors to oxidative DNA damage. (A) CB Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells were cultured with or without BSO for 2 days. Intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometor. The relative ROS level is presented as a fold induction compared with the MFI value detected in cells cultured without BSO. Data from 2 independent experiments are shown (**P < .01). (B) Two days after BSO treatment, cells were pulsed with BrdU and analyzed by flow cytometry. Data from 2 independent experiments are shown (**P < .01). (C) Relative expressions of p16INK4a, p14ARF, and p21CIP1 in Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells cultured with or without BSO were analyzed at the end of 2-day culture by quantitative real-time PCR. Each value was normalized to 18S rRNA expression and is presented as a fold increase compared with the levels detected in cells cultured without BSO (**P < .01). (D) CB Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells cultured with BSO for 2 days were stained with γ-H2AX and p21CIP1 mAbs (γ-H2AX: green; p21CIP1: red; DAPI: blue). Bars represent 5 μm. (E) At 2 days after BSO-treatment, the frequency of apoptotic cells was determined by annexin V/PI staining. Data from 3 independent experiments are shown (**P < .01). (F) The number of γ-H2AX foci per cell in Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells at the indicated time points. Cells cultured with BSO for 2 days were washed, replaced with fresh BSO-free media, and cultured for additional 1, 2, or 3 days (n = 4 in each time point; *P < .05, **P < .01, Lin−CD34+CD38− cells vs Lin−CD34+CD38+ cells).
Inhibition of ROS elevation rescues human HSCs from functional deterioration
We then asked whether the pharmacologic inhibition of intracellular ROS elevation could protect human HSCs from functional degradation. Lin−CD34+CD38− cells were cultured with BSO and an antioxidant NAC. Treatment of human HSCs with the antioxidant prevented cells from the BSO-induced accumulation of DNA damage (Figure 6A) and apoptosis (Figure 6B). The pharmacologic inhibition of ROS elevation in the human HSC compartment also maintained the cytokine responding proliferation (Figure 6C) and repopulating activity (Figure 6D) of human HSCs.
Antioxidant treatment prevents quantitative and qualitative loss of stem cell function in vitro and in vivo. (A) CB Lin−CD34+CD38− cells were cultured with BSO or BSO plus NAC and immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue). The number of γ-H2AX foci per cell was obtained. The pooled data from 3 independent experiments are presented as the box and whisker plot (**P < .01 relative). (B) CB Lin−CD34+CD38− cells were cultured with BSO or BSO plus NAC for 2 days. The frequency of apoptotic cells was determined by annexin V/PI staining (n = 3 in each BSO concentration; **P < .01). (C) Cultured cells were pulsed with BrdU, and the BrdU incorporation was analyzed by flow cytometry (n = 4 in each culture condition; **P < .01). (D) Cells cultured under the indicated conditions were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (6 recipients in each group; **P < .01). (E) Lin−CD34+CD38− cells were isolated from secondary recipients that had been fed with control or NAC-containing food, and intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from control mice. Data were collected from 3 independent experiments (a total of 5 recipients in each group; *P < .05). (F) Lin−CD34+CD38− cells were isolated from secondary recipients that had been fed with control or NAC-containing food, and immunostained for γ-H2AX. The number of γ-H2AX foci per cell was counted. The pooled data from 3 independent experiments are presented as the box and whisker plot (9 recipients in each group; **P < .01). (G) Lin−CD34+CD38− cells isolated from NAC-treated or nontreated secondary recipients were cultured with cytokines for 3 days and then pulsed with BrdU. The percentage of BrdU positive cells was determined by flow cytometry. Data were collected from 3 independent experiments (a total of 9 recipients in each group; **P < .01). (H) The absolute number of Lin−CD34+CD38− cells in the recipient mice treated with or without NAC (5 recipients in each group; **P < .01). (I) Primary recipients were injected with 1 × 104 Lin−CD34+CD38− cells isolated from CB. Secondary and tertiary transplantation was performed at the 18th week of engraftment. A total of 1 × 105 CD34+ cells pooled from 2-5 reconstituted recipients was transplanted into new groups of irradiated recipients. Engraftment levels of human hematopoietic cells were assessed by flow cytometry. Black circles indicate recipients that had been treated with NAC. White circles indicate nontreated recipients. Data were collected from 4 independent experiments (a total of 8 recipients in each group; **P < .01).
Antioxidant treatment prevents quantitative and qualitative loss of stem cell function in vitro and in vivo. (A) CB Lin−CD34+CD38− cells were cultured with BSO or BSO plus NAC and immunostained for γ-H2AX (γ-H2AX: green; DAPI: blue). The number of γ-H2AX foci per cell was obtained. The pooled data from 3 independent experiments are presented as the box and whisker plot (**P < .01 relative). (B) CB Lin−CD34+CD38− cells were cultured with BSO or BSO plus NAC for 2 days. The frequency of apoptotic cells was determined by annexin V/PI staining (n = 3 in each BSO concentration; **P < .01). (C) Cultured cells were pulsed with BrdU, and the BrdU incorporation was analyzed by flow cytometry (n = 4 in each culture condition; **P < .01). (D) Cells cultured under the indicated conditions were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (6 recipients in each group; **P < .01). (E) Lin−CD34+CD38− cells were isolated from secondary recipients that had been fed with control or NAC-containing food, and intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from control mice. Data were collected from 3 independent experiments (a total of 5 recipients in each group; *P < .05). (F) Lin−CD34+CD38− cells were isolated from secondary recipients that had been fed with control or NAC-containing food, and immunostained for γ-H2AX. The number of γ-H2AX foci per cell was counted. The pooled data from 3 independent experiments are presented as the box and whisker plot (9 recipients in each group; **P < .01). (G) Lin−CD34+CD38− cells isolated from NAC-treated or nontreated secondary recipients were cultured with cytokines for 3 days and then pulsed with BrdU. The percentage of BrdU positive cells was determined by flow cytometry. Data were collected from 3 independent experiments (a total of 9 recipients in each group; **P < .01). (H) The absolute number of Lin−CD34+CD38− cells in the recipient mice treated with or without NAC (5 recipients in each group; **P < .01). (I) Primary recipients were injected with 1 × 104 Lin−CD34+CD38− cells isolated from CB. Secondary and tertiary transplantation was performed at the 18th week of engraftment. A total of 1 × 105 CD34+ cells pooled from 2-5 reconstituted recipients was transplanted into new groups of irradiated recipients. Engraftment levels of human hematopoietic cells were assessed by flow cytometry. Black circles indicate recipients that had been treated with NAC. White circles indicate nontreated recipients. Data were collected from 4 independent experiments (a total of 8 recipients in each group; **P < .01).
We then attempted to rescue human HSCs from functional exhaustion during serial transplantation by treating recipients with the antioxidant. To minimize unavoidable superficial differences between treated recipients, recipients were transplanted with a limited number of cells. Recipients were fed with control or NAC- containing food during the entire reconstitution period starting immediately after the transplantation. The qualitative and quantitative changes in human HSC compartment were analyzed. Both the intracellular ROS level and the number of γ-H2AX foci were reduced in the human HSCs isolated from NAC-treated secondary recipients (Figure 6E-F). Consequently, the cytokine responsive proliferation (Figure 6G), the HSC compartment size (Figure 6H), and the self-renewal capacity of human HSCs (Figure 6I) were all maintained. Taken together, the results show that the elevated ROS level is the primary cause of DNA damage in human HSCs, and the pharmacologic inhibition of ROS elevation effectively prevents quantitative and qualitative deterioration of stem cell function in vitro and in vivo.
Accumulation of oxidative DNA damage is reproduced in the HSCs of elderly individuals and transplant recipients
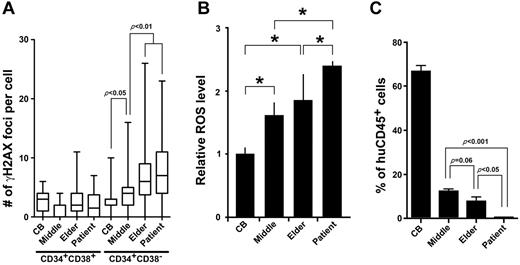
We have shown so far that a repeated transplantation leads to functional decline among stem cell population. Thus, accumulated genomic damage in HSCs appears to be a physiologic indicator of stem cell aging. To test whether the DNA damage accumulation and subsequent stem cell dysfunction observed in the human-to-mouse xenotransplantation experiments is also applicable to human-to-human stem cell transplantation and normal human aging, we examined DNA lesions in Lin−CD34+CD38− cells and Lin−CD34+CD38+ cells isolated from bone marrow of elderly healthy individuals and HSCT patients. Similar to the HSCs of the xenotransplantation experiments, the majority of the Lin−CD34+CD38− cells from elderly individuals (72-84 years; median: 79 years, n = 10) and HSCT recipients (34-56 years; median: 48 years, n = 8) exhibited multiple foci. Also consistent was no obvious accumulation of γ-H2AX foci in the Lin−CD34+CD38+ progenitor cells (Figure 7A). Interestingly, the number of γ-H2AX foci of Lin−CD34+CD38− cells from middle- aged individuals (44-56 years; median: 50 years, n = 6) placed approximately in the middle of CB and aged samples. We compared the intracellular ROS level in the Lin−CD34+CD38− cells from each cell source. While elder HSCs exhibited higher ROS levels, although not statistically significant, than middle-aged HSCs (P = .068, Figure 7B), HSCs from transplant patients had a significantly higher level of intracellular ROS concentration than any other sources of HSCs (Figure 7B). This indicates that the HSC transplantation induces excessive production of ROS in the donor HSCs. We then investigated the repopulation activity of CD34+ cells isolated from each group. As expected, human HSCs from aged individuals and HSCT recipients demonstrated deterioration in the repopulating capacity compared with CB samples (Figure 7C). Of note, HSCs from HSCT recipients exhibited a significantly higher number of γ-H2AX foci per cell and diminished repopulating activity compared with those of age-matched middle-aged individuals. These results indicate that DNA damage and functional decline of human HSCs of transplant recipients and elderly individuals are indeed recapitulated in oxidative DDR-induced HSC dysfunction in our xenotransplantation experiments.
DDR in physiologic HSC aging. (A) Bone marrow Lin−CD34+CD38− cells isolated from middle-aged (n = 6) and elderly individuals (n = 10) as well as HSCT patients (n = 8) were immunostained for γ-H2AX. The number of γ-H2AX foci per cell for each subject is shown as the box and whisker plot. More than 50 cells in random fields on a slide were counted. (B) Lin−CD34+CD38− cells were isolated from HSCT patients and elderly individuals, and intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from CB. Data were collected from 3 independent experiments (*P < .05). (C) Bone marrow CD34+ cells (1 × 104 cells) isolated from middle-aged and elderly individuals and HSCT patients were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (4 recipients in each group).
DDR in physiologic HSC aging. (A) Bone marrow Lin−CD34+CD38− cells isolated from middle-aged (n = 6) and elderly individuals (n = 10) as well as HSCT patients (n = 8) were immunostained for γ-H2AX. The number of γ-H2AX foci per cell for each subject is shown as the box and whisker plot. More than 50 cells in random fields on a slide were counted. (B) Lin−CD34+CD38− cells were isolated from HSCT patients and elderly individuals, and intracellular ROS concentrations were determined by the intensity of DCF-DA staining using a flow cytometer. The relative ROS level is presented as a fold induction compared with the MFI value detected in Lin−CD34+CD38− cells isolated from CB. Data were collected from 3 independent experiments (*P < .05). (C) Bone marrow CD34+ cells (1 × 104 cells) isolated from middle-aged and elderly individuals and HSCT patients were transplanted into NOG mice. Eight weeks after transplantation, engraftment levels of human hematopoietic cells were assessed by flow cytometry (4 recipients in each group).
Discussion
DNA damage has been well accepted as a cause for cellular dysfunction and carcinogenesis.2-5 In recent years, many theories have been proposed to explain the age-related functional decline of cells and tissues. One is the free-radical theory: the excess amounts of ROS, such as hydrogen peroxides, superoxides, and hydroxyl radicals, cause detrimental cellular damages that lead to premature aging.3,10 ROS have previously been identified as a cause for the HSC defects in mice.16,18-21 In mice lacking an oxidative stress responder, Atm or FoxO,19-21 the HSC defect can be rescued by the addition of an antioxidant agent, thus indicating that the proper regulation of ROS level is indispensible for maintaining quiescent HSCs and sustaining hematopoiesis throughout the lifetime of an individual. The emerging question is whether ROS are responsible for the DNA damage leading to the HSC defect. Our study demonstrated that a regeneration-induced intracellular up-regulation of ROS resulted in the accumulated and persistent DNA lesions in human HSCs. In these HSCs, both ATM and FOXO3a, the oxidative stress responders also known to contribute to DDR pathways,22,23 were activated. Importantly, the accumulation of DNA lesions was demonstrated in HSCs of HSCT patients and aged healthy individuals as well. The results of the inhibition of DNA damage accumulation with antioxidant treatment indicate that HSCs are highly sensitive to ROS elevation. The results also indicate that ROS play a causative role in DNA damage accumulation in human HSCs and have a major impact on stem cell aging.
A possible mechanism for the DDR observed in the human HSCs involves replication stress. Under steady-state conditions, the majority of human HSCs are maintained in a quiescent state, where they divide only infrequently, and at the same time, they produce proliferating progenitors that eventually give rise to mature hematopoietic cells and sustain blood homeostasis.13,24,25 In this study, the transplanted HSCs had to undergo intensive proliferation to produce massive numbers of primitive progenitor cells to replace hematopoiesis in the myeloablated host environment. Such a forced stimulation of stem-cell proliferation is likely to create replication stress that involves ROS production and initiates DDR activation. The activation of DDR induces premature senescence in human HSCs, which results in the progressive loss of stem cell functions, in particular the capacity to self-renew. Such replication- induced DNA damage could occur in the normal aging process as well, and perhaps it plays a significant role in a variety of premature bone marrow failure syndromes or engraftment failure.
The accumulation of DNA damage during aging is a possible cause for the increased incidence of cancer in elderly populations.6 In this study, long-lasting HSCs that underwent regenerative stress or normal aging process demonstrated persistent DNA damage without a significant increase in apoptotic cells. Long-lived HSCs that had escaped apoptosis demonstrated an accumulation of DNA lesions and the activation of cell-cycle inhibitors (p16INK4a, p14ARF, and p21CIP1), an indication of senescence. Senescence could be characterized as a net accumulation of senescent cells in tissues or limitation of the regenerative potential within the stem cell pools, both of which may simultaneously contribute to aging of stem cells. The enormous functional demands and longevity of stem cells suggest that stem cells, particularly those from highly regenerative compartments, such as the skin and blood, are equipped with effective DDR mechanisms to ensure the genomic integrity over a lifetime. Induction of premature senescence in response to DNA damage under stress conditions could be one mechanism that protects stem cells from acquiring mutations that lead them to malignant transformation to putative leukemic stem cells. Progenitor cells play versatile functional roles to support the daily life of organisms, as opposed to stem cells, whose primary role is to provide and maintain the hematopoietic homeostasis. Our study showed that Lin−CD34+CD38+ progenitor cells exhibited far fewer damaged lesions than HSCs, indicating efficient DNA repair mechanisms functioning in progenitor cells. Our findings are consistent with a notion that as a population the committed progenitors are more resistant to DNA damage.6,26,27 An alternative explanation is that during long-term repopulation, the frequency of damaged HSCs with higher ROS is increased gradually and markedly, and then only HSCs without oxidative DNA damage may successfully divide and contribute to the maintenance of hematopoietic homeostasis. As a result, those progenitor cells derived from lesser-damaged HSCs continue to have fewer damaged lesions.
In addition to our xenotransplantation model, ROS-induced accumulation of DNA damage was confirmed in human HSCs of HSCT patients and elderly individuals. Although the extent of DNA damage was similar in the 2 groups, HSCs from HSCT patients demonstrated far inferior ability to engraft in the murine host, suggesting that regenerating HSCs must have suffered much more stress than normal aging. This is consistent with an anecdotal clinical observation that hematopoietic recovery after chemotherapy takes longer in HSCT recipients. Successful outcome of clinical HSCT depends on several factors including the age of HSC donors. In a previous murine study, when a limited number of aged HSCs was transplanted into young recipients under competitive conditions, they showed an overall reduction in long-term repopulating potential, suggesting that aged HSCs are no longer able to meet the demand for blood production.28 We showed that the ability to reconstitute hematopoiesis of murine host was significantly reduced in human HSCs from elderly individuals compared with HSCs from middle-aged individuals, indicating an age-related decline of stem cell function. Therefore, it seems relevant to set a limit at a certain age when selecting donors for clinical HSCT, not only because the physiologic stress for bone marrow collection may be unbearable for elderly donors but also because patients may not receive stem cells with optimal quality.
Regulatory mechanisms that ensure the maintenance of homeostasis and protection from tumorigenesis must be evolutionarily conserved in stem cells in highly regenerative tissues such as blood. To understand how stem cells cope with DNA damage caused by the endogenous and exogenous stress, much work has been invested on cell-intrinsic mechanisms in response to IR and revealed unique survival mechanisms furnished in stem cells.26,27,29,30 In the present study, we focused on transplantation-induced DDR in clinical HSCT and aging-related DDR of human cells, which reflect a replication of stress under clinical procedures and physiologic cellular aging, respectively. Our study showed that a replication-induced intracellular elevation of ROS resulted in DNA damage in transplanted human HSCs and treatment with an antioxidant could antagonize the ROS-induced DNA damage and subsequent HSC dysfunction in a xenotransplantation model. Thus, the replication-induced DNA damage may offer a physiologic way of understanding the evolution of tumor from normal stem cells. Our findings would lead a new direction of optimization of HSCT condition and prevention of carcinogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Research Center for Regenerative Medicine of Tokai University for helpful discussion and assistance. They also thank members of the animal facility of Tokai University for meticulous care of the experimental animals; and members of the Tokai Cord Blood Bank for their assistance.
This work was supported by a Grant-in-Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology of Japan (T.Y. and K.A.), and a Tokai University School of Medicine Project Research (T.Y.).
Authorship
Contribution: T.Y. conceived and designed the study, collected and assembled data, performed data analysis and interpretation, and wrote the manuscript; Y.M. wrote the manuscript; T.T., A.A.I., T.U., and Y.S. collected data; M.O., M.I., and S.K. provided study material; and K.A. conceived and designed the study and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Kiyoashi Ando, Division of Hematopoiesis, Research Center for Regenerative Medicine, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan; e-mail: andok@keyaki.cc.u-tokai.ac.jp.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal