Abstract
In many B-cell lymphomas, chromosomal translocations are biologic and diagnostic hallmarks of disease. An intriguing subset is formed by the so-called double- hit (DH) lymphomas that are defined by a chromosomal breakpoint affecting the MYC/8q24 locus in combination with another recurrent breakpoint, mainly a t(14;18)(q32;q21) involving BCL2. Recently, these lymphomas have received increased attention, which contributed to the introduction of a novel category of lymphomas in the 2008 WHO classification, “B cell lymphoma unclassifiable with features intermediate between DLBCL and BL.” In this review we explore the existing literature for the most recurrent types of DH B-cell lymphomas and the involved genes with their functions, as well as their pathology and clinical aspects including therapy and prognosis. The incidence of aggressive B-cell lymphomas other than Burkitt lymphoma with a MYC breakpoint and in particular a double hit is difficult to assess, because screening by methods like FISH has not been applied on large, unselected series, and the published cytogenetic data may be biased to specific categories of lymphomas. DH lymphomas have been classified heterogeneously but mostly as DLBCL, the majority having a germinal center phenotype and expression of BCL2. Patients with DH lymphomas often present with poor prognostic parameters, including elevated LDH, bone marrow and CNS involvement, and a high IPI score. All studies on larger series of patients suggest a poor prognosis, also if treated with RCHOP or high-intensity treatment modalities. Importantly, this poor outcome cannot be accounted for by the mere presence of a MYC/8q24 breakpoint. Likely, the combination of MYC and BCL2 expression and/or a related high genomic complexity are more important. Compared to these DH lymphomas, BCL6+/MYC+ DH lymphomas are far less common, and in fact most of these cases represent BCL2+/BCL6+/MYC+ triple-hit lymphomas with involvement of BCL2 as well. CCND1+/MYC+ DH lymphomas with involvement of 11q13 may also be relatively frequent, the great majority being classified as aggressive variants of mantle cell lymphoma. This suggests that activation of MYC might be an important progression pathway in mantle cell lymphoma as well. Based on clinical significance and the fact that no other solid diagnostic tools are available to identify DH lymphomas, it seems advisable to test all diffuse large B-cell and related lymphomas for MYC and other breakpoints.
Introduction
Approximately 40% of all B-cell lymphomas are characterized by the presence of a recurrent reciprocal chromosomal translocations.1-5 In most cases an oncogene is deregulated by juxtaposition to an enhancer of the immunoglobulin (IG) loci, whereas promoter substitution or fusion of genes leading to fusion proteins are less frequent. Certain translocations are characteristic for a specific type of lymphoma and are often considered as cancer-initiating events. For instance, irrespective of being endemic, sporadic, or AIDS associated, the t(8;14)(q24;q32) or variant translocations involving the immunoglobulin light chain loci, are considered as the lymphoma-initiating event in Burkitt lymphoma (BL), constitutively activating the MYC gene. However, similar MYC breakpoints may also occur as secondary events during disease progression in other lymphomas. These secondary events can occur metachronously after a clinically evident phase of indolent lymphoma or synchronously at the moment of clinical presentation.
Lymphomas with recurrent chromosomal breakpoints activating multiple oncogenes, one of which being MYC, are often referred to as “Dual Hit” or “Double Hit” (DH) lymphomas. Rigorously, DH lymphoma is a rather imprecise term because, from the nomenclature point of view, it is neither restricted to B-cell lymphomas (eg, inv(14)-positive T-cell prolymphocytic leukemia may carry a MYC translocation) nor does it exclude 2 translocations activating oncogenes other than MYC (eg, a follicular lymphoma with simultaneous BCL2 and BCL6 translocation). Nevertheless, the term DH lymphoma is mostly used for mature-B-cell lymphomas with a chromosomal breakpoint affecting the MYC locus. For clarity, we will through the text apply a nomenclature, including the affected oncogenes, for example, BCL2+/MYC+, and use the term DH lymphoma for all cases with multiple recurrent breakpoints (triple/quadruple) as well.
Because cases with a MYC/8q24 and BCL2/18q21 breakpoint (BCL2+/MYC+ DH lymphoma) are most common, these casesreceived most attention in the literature. In particular a subset of aggressive lymphomas in elderly patients, previously often diagnosed as Burkitt-like lymphoma, aggressive B-cell lymphoma not otherwise specified (NOS), or diffuse large B-cell lymphomas (DLBCL), appear to represent such DH lymphomas. In the updated classification for malignant lymphomas by the World Health Organization (WHO), it is proposed to classify most if not all cases as “B-cell lymphoma unclassifiable with features intermediate between DLBCL and BL.”6 This novel category is meant to create a (temporary) container for aggressive mature B-cell lymphomas that should not be diagnosed as either BL or DLBCL.
DH lymphomas make up an important part of this novel WHO category, the other part representing heterogeneous cases of aggressive B-cell lymphoma that have features of BL such as a monomorphic proliferation of blasts and a very high proliferation rate, often in combination with a germinal center (GC) phenotype (CD10+, BCL6+, MUM1/IRF4−). The latter lymphomas contain a MYC breakpoint in 30%-50%, an incidence that is higher than seen in regular DLBCL (∼ 10%) but considerably lower than in regular BL (90%-100%). In this review we focus on DH lymphomas as follows. (1) First, we explore the published cytogenetic data for the presence of known and novel recurrent chromosomal abnormalities in DH lymphomas. (2) The biologic function of the involved oncogenes in these lymphomas is briefly discussed. (3) We discuss the timing of the occurrence of the breakpoints in lymphomagenesis as well as the synergistic action of the involved oncogenes. (4) The pathologic and clinical aspects of BCL2+/(BCL6+)/MYC+ DH and triple hit (TH) lymphomas are reviewed. (5) A short section is devoted to other DH B-cell lymphomas. (6) The relationship with “B-cell lymphoma unclassifiable with features intermediate between DLBCL and BL” is discussed. (7) Finally, we draw conclusions and formulate recommendations.
Published DH lymphomas
To get an impression on the incidence of MYC breakpoint positive and DH lymphomas diagnosed as DLBCL, we analyzed the available fluorescence in situ hybridization (FISH) studies on series of unselected DLBCL for the presence of the most common chromosomal breakpoints. Table 1 shows the results from 8 larger studies, 3 being incomplete with respect to BCL2 and BCL6. These studies show a wide range of MYC breakpoints in 3%-16% of the cases and DH lymphomas in 0%-12%. The wide range of BCL2 breakpoints and therefore also DH cases may be partially because of the inclusion of one series from Asia in which the incidence of t(14;18)–carrying lymphomas may be lower than in Western countries.7
Incidence of chromosomal breakpoints in unselected series of diffuse large B cell lymphoma
| Study . | N* . | MYC+ total, n (%) . | MYC+ SH, n (%) . | BCL2+/MYC+ DH, n (%) . | BCL6+/MYC+ DH, n (%) . | BCL2+/BCL6+/MYC+ TH, n (%) . | All DH and TH, n (%) . | All DH & TH / MYC cases, n/N (%) . | BCL2+ SH, n (%) . | BCL6+ SH, n (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrans et al114 2010† | 245-264 | 35 (14) | 6 (2) | 19 (8) | 3 (1) | 7 (3) | 29 (12) | 29/35 (83) | 55 (21) | 64 (24) |
| Obermann et al130 2009‡ | 220 | 9 (4) | 7 (3) | 1 (0) | 1 (0) | 0 | 2 (1)§ | 2/9 (22) | NA | NA |
| Yoon et al129 2008‡ | 137-156 | 14 (7) | 11 (7) | 1 (1) | 1 (1) | 1 (1) | 3 (3) | 3/14 (21) | 3 (2) | 22 (16) |
| Tibiletti et al131 2009†‖ | 74 | 12 (16) | 3 (4) | 4 (7) | 4 (7) | 1 (1) | 9 (12) | 9/12 (75) | 12 (15) | 30 (39) |
| Copie-Bergman et al132 2009‡ | 68-71 | 2 (3) | 2 (3) | 0 | 0 | 0 | 0 | 0 | 14 (20) | 21 (30) |
| van Imhoff et al133 2006†¶ | 58-59 | 9 (15) | 5 (8) | 3 (5) | 1 (2) | 0 | 4 (7) | 4/9 (44) | 7 (12) | 14 (24) |
| Savage et al128 2009‡ | 135 | 12 (9) | 9 (7) | 3 (2) | NA | NA | NA | 3/12 (25) | NA | NA |
| Klapper et al108 2008‡# | 117 | 14 (8) | NA | NA | NA | NA | NA | NA | NA | NA |
| Study . | N* . | MYC+ total, n (%) . | MYC+ SH, n (%) . | BCL2+/MYC+ DH, n (%) . | BCL6+/MYC+ DH, n (%) . | BCL2+/BCL6+/MYC+ TH, n (%) . | All DH and TH, n (%) . | All DH & TH / MYC cases, n/N (%) . | BCL2+ SH, n (%) . | BCL6+ SH, n (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrans et al114 2010† | 245-264 | 35 (14) | 6 (2) | 19 (8) | 3 (1) | 7 (3) | 29 (12) | 29/35 (83) | 55 (21) | 64 (24) |
| Obermann et al130 2009‡ | 220 | 9 (4) | 7 (3) | 1 (0) | 1 (0) | 0 | 2 (1)§ | 2/9 (22) | NA | NA |
| Yoon et al129 2008‡ | 137-156 | 14 (7) | 11 (7) | 1 (1) | 1 (1) | 1 (1) | 3 (3) | 3/14 (21) | 3 (2) | 22 (16) |
| Tibiletti et al131 2009†‖ | 74 | 12 (16) | 3 (4) | 4 (7) | 4 (7) | 1 (1) | 9 (12) | 9/12 (75) | 12 (15) | 30 (39) |
| Copie-Bergman et al132 2009‡ | 68-71 | 2 (3) | 2 (3) | 0 | 0 | 0 | 0 | 0 | 14 (20) | 21 (30) |
| van Imhoff et al133 2006†¶ | 58-59 | 9 (15) | 5 (8) | 3 (5) | 1 (2) | 0 | 4 (7) | 4/9 (44) | 7 (12) | 14 (24) |
| Savage et al128 2009‡ | 135 | 12 (9) | 9 (7) | 3 (2) | NA | NA | NA | 3/12 (25) | NA | NA |
| Klapper et al108 2008‡# | 117 | 14 (8) | NA | NA | NA | NA | NA | NA | NA | NA |
All cases had DLBCL as morphology.
SH indicates single hit; DH, double hit; TH, triple hit; and NA, not available.
Number of cases on which FISH was performed; variable numbers because of some failures in individual tests.
FISH on conventional tissue sections.
FISH on tissue microarray.
Original paper included 1 CCND1+/MYC+ DH that is not shown in table.
DLBCL was selected for primary nodal localization.
Cases from clinical trials were selected for patients with poor-risk DLBCL.
No FISH for BCL2 and BCL6 was performed in the study.
FISH analysis only informs on the targets for which probes are used. To get a better idea about the nature of all DH lymphomas and to search for other types than BCL2+/MYC+ and BCL6+/MYC+ lymphomas, we explored the Mitelman Database of Chromosome Aberrations in Cancer, edition February 2010 (see supplemental Tables 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).8 This large publicly available database contains virtually all published cytogenetic data on a wide variety of malignancies, including B-cell lymphomas. We selected reports with a MYC breakpoint published after the Revised European-American Lymphoma classification to be confident about the classification of the cases.9 We realize that this has introduced a certain bias toward DH lymphomas, because several of these publications specifically addressed this phenomenon.10-15 We included only mature B-cell malignancies (see supplemental data for strategies and sources). Plasma cell neoplasms were excluded because they represent a different disease and are characterized by genetic aberrations different from those seen in aggressive B-cell lymphomas. Translocations in myelomas involve both primary translocations (CCND1, CCND3, FGFR3 and MMSET, c-MAF, and MAFB) and secondary translocations involving MYC.16,17 DHs involving these genes and also combinations thereof were frequently seen in the Mitelman database (5%-25%; data not shown).8 Of note, a selection bias may have occurred because myelomas with MYC translocations probably have a higher success rate for culturing and karyotyping. Plasmablastic lymphomas were also excluded. A recent report identified MYC rearrangements in 49% of these lymphomas but no concomitant rearrangement of BCL2, BCL6 or PAX5.18
From the 689 MYC+ breakpoint–positive lymphomas 326 were DH lymphomas (47%). From the 804 cases diagnosed as DLBCL, 139 cases had a MYC breakpoint (17%). This incidence was similar to that reported for the period 1980-1995 (16%; data not shown). However, although between 1995 and 2009, 109 of 804 DLBCLs were BCL2+/MYC+ or BCL6+/MYC+ DH cases (14%), only 12 of 445 similar DH DLBCLs were reported in the period before 1995 (3%; data not shown). As stated earlier, this increase in the fraction of DH lymphomas after 1994 may be due to a growing interest in these lymphomas, as well as by changes in classification.
Taking these limitations into consideration, BCL2+/MYC+ DH lymphomas formed the great majority of DH lymphomas (62%; Table 2). BCL6+/MYC+ DH lymphomas were relatively rare (8% of all cases), and in fact TH lymphomas that involved MYC, BCL2 and BCL6 (16%) were more frequent than BCL6+/MYC+ DH cases. In DLBCL, 25 of 139 cases (18%) had a BCL6 breakpoint, whereas 84 of 139 (60%) had a BCL2 breakpoint (see supplemental Table 1). This preference for BCL2+/MYC+ is at least partially because of an underrepresentation of BCL6 breakpoints in the database, because these breakpoints at the very tip of chromosome 3 may have been missed by conventional cytogenetics. However, a very strong preference for BCL2 involvement in DH lymphomas was also found by FISH (Table 1) and suggests a selective complementary role for MYC and BCL2.
DH and TH lymphomas in the Mitelman database
| DH lymphomas . | N . | Percentage of all 326 DH cases . | MYC-IG fusion, % . | MYC-IGK or IGL fusion (% of cases with MYC-IG fusion)* . |
|---|---|---|---|---|
| BCL2+/MYC+ | 203 | 62 | 66 | 49 |
| BCL6+/MYC+ | 26 | 8 | 31 | 13 |
| BCL2+/BCL6+/MYC+ | 53 | 16 | 53 | 50 |
| CCND1+/MYC+ | 34 | 10 | 20 | 43 |
| BCL3+/MYC+ | 5 | 2 | ||
| 9p13+/MYC+ | 4 | 1 | ||
| BCL3+/9p13+/MYC+ TH | 1 | 0 | ||
| Total DH and TH cases | 326 | 100 | ||
| MYC only | ||||
| Burkitt lymphoma | 205 | 98 | 18 | |
| Other lymphomas | 158 | 61 | 34 | |
| Total | 689 |
| DH lymphomas . | N . | Percentage of all 326 DH cases . | MYC-IG fusion, % . | MYC-IGK or IGL fusion (% of cases with MYC-IG fusion)* . |
|---|---|---|---|---|
| BCL2+/MYC+ | 203 | 62 | 66 | 49 |
| BCL6+/MYC+ | 26 | 8 | 31 | 13 |
| BCL2+/BCL6+/MYC+ | 53 | 16 | 53 | 50 |
| CCND1+/MYC+ | 34 | 10 | 20 | 43 |
| BCL3+/MYC+ | 5 | 2 | ||
| 9p13+/MYC+ | 4 | 1 | ||
| BCL3+/9p13+/MYC+ TH | 1 | 0 | ||
| Total DH and TH cases | 326 | 100 | ||
| MYC only | ||||
| Burkitt lymphoma | 205 | 98 | 18 | |
| Other lymphomas | 158 | 61 | 34 | |
| Total | 689 |
MYC+ indicates 8q24 breakpoint; BCL2+, 18q21; BCL6+, 3q27; CCND1+, 11q13; BCL3+, 19q13; and 9p13+, yet unidentified locus.
One case had a complex t(8;14;22)(q24;q32;q11), another case both a t(8;14)(q24;q32) and t(8;22)(q24;q11). Arbitrarily, both cases were considered as having two MYC-IG events.
CCND1+/MYC+ DH lymphomas (N = 34) formed 10% of all cases. In fact, 5% of all mantle cell lymphomas (MCLs) in the database were CCND1+/MYC+ DH cases. Other recurrent DH lymphomas were 5 BCL3+/MYC+ cases with t(14;19)(q32;q13), 4 cases with t(9;14)(p13;q32) involving a yet unidentified locus on 9p13,19,20 and 1 case with all 3 loci involved. A MYC breakpoint without any other recurrent breakpoint (MYC+ SH) was seen in 363 of 689 cases (53%), from which 205 cases (56%) were diagnosed as BL (supplemental Table 1).
Although in typical BL MYC is almost always juxtaposed to an IG locus (in our dataset 98%), this was only found in 66% of BCL2+/MYC+ DH lymphomas (Table 2). Moreover, in 49% of the cases with juxtaposition to an IG locus one of the IG light chain genes was involved, which is far more than the 18% in BL. We explored the direct partners of MYC in the other DH lymphomas. Interestingly, in the group of BCL2+/BCL6+/MYC+ DH lymphomas the commonest non-immunoglobulin partner was 9p13 (N = 19; 7%). This translocation was never seen in the BCL6+/MYC+ DH and CCND1+/MYC+ DH groups. Other recurrent translocations in the BCL2+/MYC+ group were t(1;8)(p36;q24) in 5 cases and t(2;8)(p11;q24) in 5 cases, the latter probably representing MYC-IGK fusion. Both in the BCL6+/MYC+ DH and BCL2+/BCL6+/MYC+ TH groups BCL6 itself was a MYC partner in 4 and 7 cases, respectively.
Oncogenes involved in DH lymphomas
In this section we briefly discuss the function of the known oncogenes involved in DH lymphomas.
MYC
MYC is a transcription factor controlling the expression of a large set of target genes involved in cell cycle regulation, metabolism, DNA repair, stress response, and protein synthesis.21 MYC exerts its function by dimerization with MAX and subsequent binding to specific consensus DNA sequences (CACGTG) called an “E-Box.”22-24 Many genes are directly (de)regulated by MYC, including LDH-A and TERT.25 In addition MYC is involved in the regulation of micro-RNA (miRNA) expression,26-28 (de)regulating many target genes in an indirect way as well. Interestingly, MYC represses many miRNAs, which corroborates the idea that MYC generally is an activator of other genes. MYC expression in the GCs is, surprisingly, lower compared with naive and memory B cells.29 This low expression could protect against MYC-induced genomic instability in the GC. For a detailed review about the role of MYC in lymphomagenesis, see Klapproth and Wirth.30 Genomic alterations of the MYC gene include chromosomal translocations, mutations affecting regulatory sequences and promoter regions, as well as copy number increase. In contrast to early reports, most chromosomal breakpoints that involve MYC and the IGH locus are mediated by activation-induced cytidine deaminase (AICDA) and not by recombinase activating gene 1/2 (RAG1/2).31-34 Thus, these breakpoints should have their origin from erroneous somatic hypermutation or class switch recombination.35 On the basis of transgenic mouse models, additional factors are needed for malignant transformation.36,37 This is supported by the finding that MYC-IG translocation can be detected in nonneoplastic conditions, both in humans and mice.38,39 The mechanism responsible for translocations affecting MYC and non-IG loci, for instance 9p13 in the t(8;9)(q24;p13) translocation, is unknown.15,40
BCL2
BCL2 was first described in the early 1980s by its involvement in the t(14;18) in follicular lymphoma.41 As a member of a large BCL2 family of proteins, it has potent antiapoptotic functions. BCL2 is widely expressed in immature B cells and memory B cells but is temporarily down-regulated in GC B cells, partially because of repression by BCL6.29,42-44 With the occurrence of t(14;18), transcription of BCL2 is “constitutively” deregulated with high transcription activity from the translocated BCL2 allele.42,45 This leads to a survival advantage of the involved B cells. Recent research has shown that BCL2 overexpression in B cells also leads to impaired DNA repair by blocking nonhomologous end-joining activities of Ku proteins essential for repair of both RAG1/2- and AICDA-mediated breakpoints.46
With the exception of rare cases,47 t(14;18) translocations are thought to occur early in B-cell development.48,49 Chromosomal breaks are mediated by RAG1/2, probably in combination with low levels of AICDA.50 Extensive mapping studies of the breakpoints as well as sequence analysis have shown that the BCL2 breakpoints are strongly clustered at cytosine-phosphate-guanosine (CpG) islands. One hypothesis is that these CpG island are first deaminated by low levels of AICDA and that the resulting T:G mismatches are subsequently targeted by RAG1/2.50 An in-depth discussion about the occurrence of the t(14;18) at later stages of B-cell development (including even the GC) and (secondary) involvement of RAG1/2 and/or other mechanisms herein51-54 goes beyond the scope of this review.
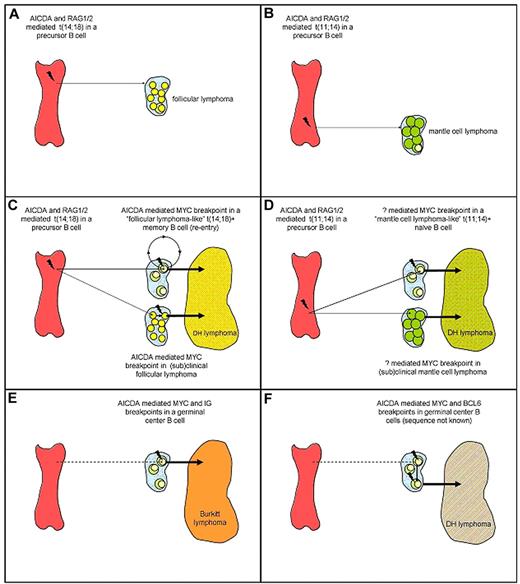
Apparently, the t(14;18) is insufficient to cause follicular lymphoma because IGH-BCL2 transgenic mice do not develop lymphomas, and t(14;18)-carrying mature B cells can also be found in healthy persons and probably arise by the same mechanisms.51,55-59 Hence, the translocation may rather facilitate than directly cause malignant transformation. The secondary genetic changes responsible for development into follicular lymphoma are not known. Importantly, these t(14;18)-carrying B cells in healthy persons, called “follicular lymphoma-like B cells,” usually enter the GCs, undergo somatic hypermutations and a limited degree of immunoglobulin gene class switching, and circulate as memory B cells.60,61 This passage across the GC cell reaction with exposure to high levels of AICDA may make these cells susceptible for additional genomic alterations such as a MYC or BCL6 mutations and breakpoints. Thus, BCL2+/MYC+ DH lymphomas may arise in 2 ways: either they arise from a clinically overt or subclinical follicular lymphoma or they directly arise from the much more prevalent B cells with a t(14;18) that otherwise had not attained any malignant potential (Figure 1). Under both circumstances MYC translocation may function as a progression event,48,62,63 although some follicular lymphomas with MYC breakpoints but without evidence of morphologic transformation have been described (see “Classification”).
Schematic scenarios for the origin of follicular lymphoma, MCL, BL (MYC-IG single hit lymphomas), and DH lymphomas. (A) Follicular lymphoma; (B) MCL; (C) BCL2+/MYC+ DH lymphoma with 2 scenarios, one with an origin from relatively common benign “follicular lymphoma–like B cells” that can be detected in most healthy persons, and the other from a preexistent follicular lymphoma. (D) CCND1+/MYC+ DH lymphoma with 2 scenarios, one with an origin from rare benign “MCL-like B cells” that can be detected in few healthy persons, and the other from a preexistent MCL. (E) BL; (F) BCL6+/MYC+ DH lymphoma with unknown order of events. For readability possible occurrence of the t(14;18) at later stages of B-cell development (including the GC) and (secondary) involvement of RAG1/2 and/or other mechanisms herein are not displayed in the figure (see “Oncogenes involved in DH lymphomas”). AICDA indicates activation-induced cytidine deaminase; RAG1/2, recombinase activating gene 1/2. Not drawn to scale.
Schematic scenarios for the origin of follicular lymphoma, MCL, BL (MYC-IG single hit lymphomas), and DH lymphomas. (A) Follicular lymphoma; (B) MCL; (C) BCL2+/MYC+ DH lymphoma with 2 scenarios, one with an origin from relatively common benign “follicular lymphoma–like B cells” that can be detected in most healthy persons, and the other from a preexistent follicular lymphoma. (D) CCND1+/MYC+ DH lymphoma with 2 scenarios, one with an origin from rare benign “MCL-like B cells” that can be detected in few healthy persons, and the other from a preexistent MCL. (E) BL; (F) BCL6+/MYC+ DH lymphoma with unknown order of events. For readability possible occurrence of the t(14;18) at later stages of B-cell development (including the GC) and (secondary) involvement of RAG1/2 and/or other mechanisms herein are not displayed in the figure (see “Oncogenes involved in DH lymphomas”). AICDA indicates activation-induced cytidine deaminase; RAG1/2, recombinase activating gene 1/2. Not drawn to scale.
BCL6
BCL6 is a zinc finger transcription factor with an N-terminal POZ domain. The gene is localized at 3q27, a position at the very end of the chromosome. BCL6 is widely expressed in many tissues, but in B cells it is mostly restricted to GC B cells.64,65 BCL6 is required for the formation of GCs because mice deficient for BCL6 lack these structures.66,67 Within the GC BCL6 acts as an transcriptional repressor of many target genes involved in apoptosis, DNA-damage response, cell cycle control, proliferation, and differentiation.68-70 Important direct targets are BCL2, TP53, IRF4, and BLIMP1, the latter being essential for maturation into plasma cells.71,72 Interestingly, BLIMP1 is a repressor of both BCL6 and MYC in plasma cells. As a result of the BCL6-mediated repression of TP53,73 somatic hypermutation and class switch recombination are facilitated. Interestingly, the AICDA-mediated somatic hypermutation machinery can target many non-IG genes, including BCL6 itself. By facilitating activating mutations or chromosomal translocations, BCL6 activation may therefore indirectly lead to its own mutation and constitutive activation.74,75 Deregulated expression of BCL6 in a mouse model that mimicks BCL6 translocations resulted in lymphoproliferative disease and ultimately in a disorder resembling DLBCL.76
Only half of the translocations involving BCL6 affect an IG locus; in other cases the translocation partner is very diverse. Translocations involving BCL6 can be found in ∼ 30%-40% of all DLBCLs, some follicular lymphomas, and even some marginal zone B-cell lymphomas.
CCND1
The gene encoding cyclin D1 (CCND1) is located on 11q13 and is involved in cell cycle progression from the G1 to the S phase, by forming a complex with CDK4 and activating the RB1-E2F1 complex, allowing E2F1 to be released. Although CCND2 and CCND3 are expressed in normal B cells, CCND1 is not. In consequence, CCND1 is almost exclusively expressed in neoplastic B cells with genetic alterations of 11q13, that is, translocation or copy number increase. In most MCLs and in a substantial fraction of multiple myelomas 11q13 translocations are observed.77-80 In addition to these malignancies, also hairy cell leukemia and some cells in the proliferation centers of chronic lymphocytic leukemia as well as extremely rare DLBCL cases may express CCND1, the mechanism being unknown.81 Like the t(14;18), the t(11;14) in MCL is mediated by RAG1/2, probably in combination with low levels of AICDA as well as other mechanisms.50,82 Similar to the t(14;18), also CCND1 breakpoints are strongly centered at CpG islands, indicating a concerted action of AICDA and RAG1/2 in precursor B cells.50 As seen for the t(14;18), also occasional t(11;14)+ cells can be found in the blood of healthy persons, however at much lower frequencies than t(14;18)+ cells.83 This low frequency may be because these cells are not expanded in the GC cell reaction. This fits with the finding that MCL represents a pre-GC B-cell lymphoma. Of note, the t(11;14) in myeloma has a different configuration of the breakpoint with strong indications of an AICDA-mediated breakpoint initiated in GC B cells.84
BCL3
BCL3 is a distinct member of the Iκβ protein family and resides on 19q13. Its expression is dependent on the stage of B-cell differentiation with higher expression in mature than immature B cells.85 Experiments in BCL3-deficient mice have shown that the gene is involved in GC formation.86 The t(14,19)(q32;q13) leads to increased BCL3 transcription and has been described in a large variety of lymphomas and leukemias,87 including atypical chronic lymphocytic leukemia.88,89 Analysis of t(14;19) breakpoints indicates that this translocation is mediated by illegitimate class switch recombination.90-92 Eμ-BCL3 transgenic mice overexpressing BCL3 show lymphoid hyperplasia but do not develop lymphomas.93
Timing and synergy of translocations in DH lymphoma
DH mature B-cell lymphomas are by definition characterized by a MYC breakpoint in combination with another recurrent chromosomal breakpoint. Most MYC breakpoints are probably mediated by AICDA in mature B cells. In contrast, and with the exception of CCND1 in myeloma, BCL2 and CCND1 breakpoints are most probably mediated by RAG1/2 in precursor B cells. This strongly suggests that the MYC/8q24 breakpoint is a secondary event in the cases with a BCL2/18q21 or CCND1/11q13 breakpoint (Figure 1). This sequence of events is also supported by the fact that ∼ 5% of all follicular lymphoma with a BCL2/18q21 breakpoint will acquire a MYC/8q24 breakpoint during the course of the disease and that at the cytogenetic level incidental cases show ≥ 2 subclones, one with only a t(14;18) and the other with both translocations.48 Another argument for the secondary nature may be that, in comparison to the “primary” MYC breakpoints in BL whereby 82% affect the IGH locus at 14q32 (Table 2), many more of these “secondary” breakpoints affect the light chain loci. Probably, tumor cells still require a functional heavy chain protein for signaling and cell survival, because cells with disruption of both IGH alleles are deleted.94
For BCL6+/MYC+ and BCL3+/MYC+ DH lymphomas the timing of events is less clear because most breakpoints affecting MYC, BCL6, and BCL3 are probably mediated by the same mechanism.
As shown in Table 2 there are interesting differences between BCL2+/MYC+ DH and CCND1+/MYC+ DH lymphomas with respect to the partner of the MYC gene. These differences might shed light on the mechanisms causing the MYC breakpoint. In the majority of the BCL2+/MYC+ DH lymphomas (66%) the MYC partner is an IG locus, which might reflect a high activity of AICDA that can induce mutations and breakpoints in both the IG and MYC loci. This may be because the t(14;18) translocation forces tumor cells to accumulate as GC B cells in which high AICDA levels are present. Exposure to high levels of AICDA may then lead to a MYC-IG breakpoint. In contrast, in CCND1+/MYC+ DH lymphomas MYC is in only 20% of the cases joined to an IG locus (see also Table 2 and supplemental Table 2). Indeed, the t(11;14) translocation does not result in accumulation of GC B cells because the tumor cells are already blocked in an earlier stage of development. Likely, the occasional MYC breakpoints without an IG partner in these cases are not caused by AICDA or by AICDA expression in extrafollicular B cells.
What could be the biologic synergy of acquiring 2 or 3 breakpoints, and thus activating both oncogenes? This is again most evident for BCL2 and MYC whereby BCL2 is antiapoptotic without mediating proliferative signals. Instead, MYC drives the cells in an active proliferative and metabolic state, for instance by allowing anaerobic glycolysis in an anaerobic state by up-regulating lactate dehydrogenase A.25 Moreover, in normal cells MYC induces DNA stress and activates the TP53 checkpoint leading to apoptosis; in consequence tumor cells with constitutive MYC activation frequently have acquired inactivating TP53 mutations or other mechanisms to protect them from apoptosis. In that view a preexistent BCL2 activation may also protect the cells from apoptosis. This synergy may be further enhanced by the fact that BCL2 can also repress important proteins involved in repair of non–homologous end joining–mediated DNA double-strand breaks.46 When cells carrying a t(14;18)(q32;q21) enter the GC, this might facilitate an increased accumulation of chromosomal abnormalities, including MYC translocations, as a result of the processes of somatic hypermutation and class switch recombination. In that respect it would be interesting to study whether a combination of MYC and BCL2 translocation favors a molecular signature of genomic instability and therefore could explain the high genomic complexity that is so frequent in this type of lymphoma.95
As discussed, BCL6 is a strong repressor of many genes, including BCL2 and MYC.44,68,70 Therefore, both constitutive activation of MYC and BCL2 by chromosomal translocation might be of advantage for BCL6+ tumor cells. In reverse, because DNA damage induced by MYC can repress BCL6 expression, constitutive activation of BCL6 by a translocation might be of selective advantage for MYC-overexpressing tumor cells.
For CCND1 and MYC, the synergy may be based on the fact that cyclin D mediates G1-S phase transition. Activation of MYC may bring the cells in an advantageous metabolic state, allowing cells to progress further. This synergy has also been shown in CCND1-MYC transgenic mice.96,97 Indeed acquisition of a MYC translocation is associated with a dramatic morphologic change in MCL (blastic98 or even mimicking BL99 ).
Clinicopathologic aspects of mature DH B-cell lymphomas involving MYC+, BCL2+, and BCL6+
Classification
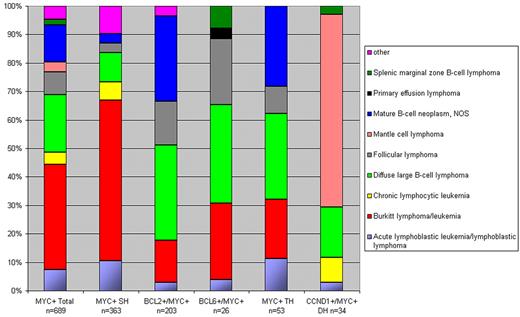
As can be concluded from the analysis of the Mitelman database8 and the original publications therein, DH lymphomas show heterogeneous morphologies, most of the BCL2+/MYC+ and BCL6+/ MYC+ DH cases being classified as DLBCL or mature B-cell lymphoma NOS (Figure 2). Except for the BCL6+/MYC+ DH cases, < 20% were classified as BL. Of note, the category of “mature B-cell neoplasm NOS,” was frequently called “Burkitt-like lymphoma” in the past and therefore possibly was lumped with BL, also in the Mitelman database.8 On the basis of these literature data and our own experience, there are no unique unifying morphologic features of DH lymphomas. Rare cases were classified as lymphoblastic lymphoma/leukemia. These cases express CD10 and terminal deoxynucleotidyl transferase (TdT) and lack expression of immunoglobulins.48,100,101 Intriguingly, at least some of these cases do not really represent a neoplasm of precursor B cells but B cells that may have reexpressed TdT, because they have accumulated somatic hypermutations, a hallmark of GC B cells.102,103 Whatever the nature of these exceptional cases may be, many DH lymphoblastic cases in the Mitelman database8 are incompletely documented without data on expression of TdT or CD34.
Distribution of morphologies according to breakpoints. For readability of the figure BCL3+/MYC+ and 9p13+/MYC+ DHs (n = 10) are omitted. SH indicates single hit.
Distribution of morphologies according to breakpoints. For readability of the figure BCL3+/MYC+ and 9p13+/MYC+ DHs (n = 10) are omitted. SH indicates single hit.
On the basis of the difficulties to classify many DH cases and because they represent a subset of highly aggressive lymphomas (see this and following sections), it was considered that these lymphomas, in particular the BCL2+/MYC+ DH cases, should be separated from other lymphomas. In the 2008 WHO classification,6 these cases are therefore called “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL.” Discussion is ongoing whether otherwise morphologically regular DLBCL with a BCL2+/MYC+ DH should be placed in this category as well. As discussed below, both molecular and clinical data indeed suggest this should be done.
Certainly not all DH lymphomas represent morphologic aggressive lymphomas. In the BCL2+/MYC+ group rare cases represented morphologic untransformed follicular lymphoma, whereas other cases had blastoid features or were classified as follicular lymphoma grade 3a or 3b.14 In 3 studies that systematically addressed MYC rearrangements in follicular lymphoma, the frequency was 2%-8%.104-106 However, all studies showed deficits in histology (grading, Ki67 proliferation index) or clinical follow-up. One interesting observation was that MYC translocation may be associated with a blastic/blastoid morphology of the tumor cells (4 of 7 cases being DH follicular lymphoma),106 which is usually associated with progressive disease. The implications of the presence of MYC rearrangement at initial diagnosis in follicular lymphoma thus deserves further study.
Immunophenotype
We collected immunophenotypical data from larger studies on DH lymphoma.10-15 Most lymphomas had a GC phenotype with expression of CD10 (107 of 122 cases; 88%) and BCL6 (45 of 60; 75%) and lack of MUM1/IRF4 (12 of 69; 17%). This corroborates the observation that BCL2 translocations are mainly found in GC type of DLBCL, and that also MYC translocations are associated with a GC molecular profile in DLBCL.107,108 Most importantly, the BCL2 protein was detected in 101 of 106 cases (95%). The Ki67/MIB1 proliferation rate varied between 50% and 100% with a median of 90% in the 58 cases for which accurate data were given. Thus, although not very specific, coexpression of CD10, BCL6, BCL2, and a high Ki67 proliferation index might be used to select potential DH lymphomas in tumors morphologically diagnosed as DLBCL.
Gene expression profile
So far only 2 studies on BL and gray zone lymphomas between BL and DLBCL addressed gene expression specifically for BCL2+/MYC+ DH lymphomas.109,110 In a collaborative study of the German network Molecular Mechanisms in Malignant Lymphoma (MMML) project on 220 aggressive B-cell lymphomas,109 16 cases represented DH lymphomas with a BCL2+/MYC+, BCL6+/MYC+ DH or TH configuration. All cases except one with a borderline profile had a gene expression profile that was “intermediate between Burkitt lymphoma and DLBCL” or “non-BL.” With the use of a molecular algorithm in which the molecular profiles were constructed in a different way, the Lymphoma/Leukemia Molecular Profiling Project consortium investigated the gene expression profile in 3 BCL2+/MYC+ DH lymphomas.110 Morphologically, these lymphomas had not been diagnosed as BLs but had a molecular BL score of 98% or 99% and thus were classified as discrepant lymphomas. These discrepant cases had much higher genomic imbalances than true BL (6.9 ± 4.4 versus 1.5 ± 1.8 in BL), suggesting that they are nevertheless different from real BL.111 Importantly, 6 “regular” DLBCL cases with a MYC breakpoint (no DH) did not have a BL type of gene expression in the Lymphoma/Leukemia Molecular Profiling Project study, indicating that some MYC translocations might be insufficient to enforce a full-blown MYC-driven gene expression program, probably because the partner is a non-IG gene locus with different regulatory properties than IG loci or because the genetic or cellular background interacts with the possibility to fully express a MYC program.40
An additional interesting finding by the MMML consortium was that in 14 of the 35 MYC breakpoint-positive cases that lacked the molecular BL signature, a non-IG partner was involved in the MYC breakpoint. Thus, in particular the MMML study suggests that DH lymphomas are biologically and clinically different from both classical BL and DLBCL. In fact, they cannot be classified easily, probably because the profile has shifted toward molecular BL after acquiring a MYC translocation.
Clinical aspects
In this section we focus on DH lymphomas involving MYC, BCL2, and BCL6. We collected clinical features of DH cases from 8 studies with ≥ 10 DH cases and with sufficient clinical data (Table 3).10-15,40,112 Median age for the DH lymphomas ranged from 51 to 65 years. DH lymphomas are extremely rare in children younger than 18 years of age.113
Clinical features of DH lymphomas
| Study . | No. of DH/total study size (%) . | DHs with prior history of indolent lymphoma, n/N (%)* . | Age, median (range) . | Stage III/IV, n/N (%) . | LDH > ULN, n/N (%) . | BM+, n/N (%) . | CNS+, n/N (%) . | >1E site, n/N (%) . | IPI HI-H, n/N (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Bertrand et al40 2007 | 10/17 (59) | 1/10 (10) | 58 (45-81) | 7/10 (70) | NA† | NA | NA | NA | 5/9 (56)‡ |
| Johnson et al15 2009 | 54/54 (100) | 20/54 (46) | 62 (24-93) | 41/54 (76) | 27/54 (50) | 32/45 (71)§ | NA | 19/54 (35) | 38/54 (70)‖ |
| Kanungo et al13 2006 | 14/14 (100) | None | 55 (29-72) | NA | 13/14 (93) | 11/14 (79) | 3/14 (21) | 8/14 (57)¶ | NA |
| Le Gouill et al10 2007 | 16/16 (100) | 4/16 (25) | 61 (36-73) | 16/16 (100) | 16/16 (100) | 15/16 (94) | 8/16 (50) | 14/16 (88) | 13/16 (81) |
| Macpherson et al112 1999# | 15/39 (38) | 6/13 (46) | 65** | 12/13 (92) | 8/10†† (80) | 9/13 (69) | NA | 8/13 (62) | 9/10 (90)†† |
| Niitsu et al12 2009 | 19/19 (100) | None | 61 (29-79) | 19/19 (100) | 19/19 (100) | 16/19 (84) | 4/19 (21) | 12/19 (63) | 17/19 (89) |
| Snuderl et al14 2010‡‡ | 20/20 (100) | 3/20§§ (15) | 64 (32-91) | 18/19 (95) | 18/18 (100) | 10/17 (59) | 5/11 (45) | 6/20 (30)¶ | 17/20 (85) |
| Tomita et al11 2009‖‖ | 27/27 (100) | 4/23 (17) | 51 (36-79) | 22/23 (96) | 25/27 (93) | 15/23 (65) | 2/23 (9) | 15/23 (65) | 20/23 (87) |
| Study . | No. of DH/total study size (%) . | DHs with prior history of indolent lymphoma, n/N (%)* . | Age, median (range) . | Stage III/IV, n/N (%) . | LDH > ULN, n/N (%) . | BM+, n/N (%) . | CNS+, n/N (%) . | >1E site, n/N (%) . | IPI HI-H, n/N (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Bertrand et al40 2007 | 10/17 (59) | 1/10 (10) | 58 (45-81) | 7/10 (70) | NA† | NA | NA | NA | 5/9 (56)‡ |
| Johnson et al15 2009 | 54/54 (100) | 20/54 (46) | 62 (24-93) | 41/54 (76) | 27/54 (50) | 32/45 (71)§ | NA | 19/54 (35) | 38/54 (70)‖ |
| Kanungo et al13 2006 | 14/14 (100) | None | 55 (29-72) | NA | 13/14 (93) | 11/14 (79) | 3/14 (21) | 8/14 (57)¶ | NA |
| Le Gouill et al10 2007 | 16/16 (100) | 4/16 (25) | 61 (36-73) | 16/16 (100) | 16/16 (100) | 15/16 (94) | 8/16 (50) | 14/16 (88) | 13/16 (81) |
| Macpherson et al112 1999# | 15/39 (38) | 6/13 (46) | 65** | 12/13 (92) | 8/10†† (80) | 9/13 (69) | NA | 8/13 (62) | 9/10 (90)†† |
| Niitsu et al12 2009 | 19/19 (100) | None | 61 (29-79) | 19/19 (100) | 19/19 (100) | 16/19 (84) | 4/19 (21) | 12/19 (63) | 17/19 (89) |
| Snuderl et al14 2010‡‡ | 20/20 (100) | 3/20§§ (15) | 64 (32-91) | 18/19 (95) | 18/18 (100) | 10/17 (59) | 5/11 (45) | 6/20 (30)¶ | 17/20 (85) |
| Tomita et al11 2009‖‖ | 27/27 (100) | 4/23 (17) | 51 (36-79) | 22/23 (96) | 25/27 (93) | 15/23 (65) | 2/23 (9) | 15/23 (65) | 20/23 (87) |
LDH indicates lactate dehydrogenase; ULN, upper limit of normal; BM+, bone marrow involvement; CNS+, central nervous system involvement; >1E, involvement of >1 extranodal site; IPI, International Prognostic Index; HI, high-intermediate (IPI score 3); H, high (IPI score 4 or 5); and NA, no specific information available.
Follicular lymphoma, n = 29; chronic lymphocytic leukemia, n = 1; and low-grade lymphoma (not otherwise specified), n = 8.
LDH values available but no ULN provided.
IPI not available in 1 case.
Not available in 9 cases.
IPI score at least low-intermediate (IPI score 2 or higher). No specific information about distribution between patients in low-intermediate and HI groups available.
Involvement of > 1 extranodal site calculation was based on data presented in the original paper.
For the clinical parameters only information for BCL2+/MYC+DH cases (n = 13) was available; for BCL6+/MYC+ DH (n = 2) cases no specific information was available.
No age-range for DHs available.
LDH and IPI not available in all cases.
For some cases not all clinical parameters were available.
Three cases with confirmed preexisting follicular lymphoma.
Lymphoma type DH (n = 23), leukemia type DH (n = 4).
A prior history of indolent lymphoma was documented only in a minority of cases. In the majority of cases elevated lactate dehydrogenase and an advanced stage of disease was reported. In addition patients often had extranodal involvement. The bone marrow and central nervous system (CNS) were most frequently involved, although the frequency of CNS involvement varied widely between studies (9%-50%). In addition, pleural effusions were commonly reported.10-12 Most patients had a high-intermediate or high International Prognostic Index (IPI) risk profile (IPI score 3 or 4/5).
Therapy and outcome
In Table 4, a summary is given for the most relevant published clinical studies in which patients with DH lymphoma could be identified. Patients were treated with a variety of regimens (including doxorubicin-based chemotherapy regimens as well as high-dose chemotherapy regimens with stem cell transplantation). In some instances only palliative therapy was given. With these limitations taken into account, DH lymphomas generally tended to have a poor survival, with a median overall survival of only 0.2-1.5 years.10-15,40,112
Treatment and survival of DH lymphomas
| Study . | No. of DH/total study size (%) . | Treatment regimen* . | Overall response rate, n/N (%)† . | Median survival, y . |
|---|---|---|---|---|
| Bertrand et al40 2007 | 10/17 (59) | NA | 5/10‡ (50) | < 1§ |
| Johnson et al15 2009 | 54/54 (100) | RCHOP (11/54);HDC+/− SCT‖ (6/54); CHOP (23/54); P (14/54) | NA | HD, 0.26; RCHOP, 1.40; CHOP-like, 0.42; P 0.07 |
| Kanungo et al13 2006 | 14/14 (100) | CT-NOS (11); R¶ (1); CT and BMT (1); CT, BMT, and RT (1) | NA | < 1§ |
| Le Gouill et al10 2007 | 16/16 (100) | CEEP/COPADM + Auto-SCT/BEAM (1); CHOP/IVAM (1); COPADM/CYVE (3); COPADM (1); COPADM + Auto-SCT/BEAM (1); COPADM + Allo-SCT/Bu/Cy (1) CEEP/DHAP + Auto-SCT/BEAM (1); RCHOP (4); CHOP (1); Steroids# (1); R-CEEP Allo-SCT/TBI/Cy (1) | 12/16 (75) | 0.42 |
| Macpherson et al112 1999 | 15/39 (38) | CHOP-variant or cyclophosphamide + MTX (6); HDC +/− SCT (3); P (4) | NA | 0.21 |
| Niitsu et al12 2009 | 19/19 (100) | CyclOBEAP (6); CHOP + HD MTX (3); CHOP (4); RCHOP (3), CyclOBEAP + R (3) | 17/19 (89) | 1.50 |
| Snuderl et al14 2010 | 20/20 (100) | R-ICE + MTX/ASCT (1); CHOP (1); RCHOP (3); RCHOP + MTX (6); RCHOP + MTX + ASCT (1); R-EPOCH + MTX (3); CODOX- + MTX/R-IVAC (3); P (1); NK(1) | 10/20** (50) | 0.38 |
| Tomita et al11 2009 | 27/27 (100) | CHOP or CODOX-M/IVAC or HyperCVAD (+ R, n = 14; -R, n = 8)†† | 6/23 (26)†† | 0.50‡‡ |
| Study . | No. of DH/total study size (%) . | Treatment regimen* . | Overall response rate, n/N (%)† . | Median survival, y . |
|---|---|---|---|---|
| Bertrand et al40 2007 | 10/17 (59) | NA | 5/10‡ (50) | < 1§ |
| Johnson et al15 2009 | 54/54 (100) | RCHOP (11/54);HDC+/− SCT‖ (6/54); CHOP (23/54); P (14/54) | NA | HD, 0.26; RCHOP, 1.40; CHOP-like, 0.42; P 0.07 |
| Kanungo et al13 2006 | 14/14 (100) | CT-NOS (11); R¶ (1); CT and BMT (1); CT, BMT, and RT (1) | NA | < 1§ |
| Le Gouill et al10 2007 | 16/16 (100) | CEEP/COPADM + Auto-SCT/BEAM (1); CHOP/IVAM (1); COPADM/CYVE (3); COPADM (1); COPADM + Auto-SCT/BEAM (1); COPADM + Allo-SCT/Bu/Cy (1) CEEP/DHAP + Auto-SCT/BEAM (1); RCHOP (4); CHOP (1); Steroids# (1); R-CEEP Allo-SCT/TBI/Cy (1) | 12/16 (75) | 0.42 |
| Macpherson et al112 1999 | 15/39 (38) | CHOP-variant or cyclophosphamide + MTX (6); HDC +/− SCT (3); P (4) | NA | 0.21 |
| Niitsu et al12 2009 | 19/19 (100) | CyclOBEAP (6); CHOP + HD MTX (3); CHOP (4); RCHOP (3), CyclOBEAP + R (3) | 17/19 (89) | 1.50 |
| Snuderl et al14 2010 | 20/20 (100) | R-ICE + MTX/ASCT (1); CHOP (1); RCHOP (3); RCHOP + MTX (6); RCHOP + MTX + ASCT (1); R-EPOCH + MTX (3); CODOX- + MTX/R-IVAC (3); P (1); NK(1) | 10/20** (50) | 0.38 |
| Tomita et al11 2009 | 27/27 (100) | CHOP or CODOX-M/IVAC or HyperCVAD (+ R, n = 14; -R, n = 8)†† | 6/23 (26)†† | 0.50‡‡ |
NA indicates not available; RCHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; HDC, high-dose chemotherapy; SCT, stem cell transplantation; P, palliative; R, rituximab; CT-NOS, intensive combination chemotherapy, not otherwise specified; BMT, bone marrow transplantation; RT, radiotherapy; CEEP, cyclophosphamide, etoposide, epidoxorubicin, and cisplatin; COPADM, cyclophosphamide, vincristine, prednisone, doxorubicin, and high-dose methotrexate; BEAM, carmustine, etoposide, cytarabine, and melphalan; IVAM, ifosfamide, etoposide, cytarabine, and methotrexate; CYVE, cytarabine and etoposide; Bu, busulfan; Cy, cyclophosphamide; DHAP, dexamethason, high-dose cytarabine, and cisplatin, TBI, total body irradiation; MTX, methotrexate; CyclOBEAP, cyclophosphamide, vincristine, bleomycin, etoposide, doxorubicin, prednisone; HD, high dosage; R-ICE, rutuximab plus ifosfamide, carboplatin, and etoposide plus rituximab; ASCT, autologous stem cell transplantation; EPOCH, rituximab plus etoposide, doxorubicin, vincristine, prednisone, and cyclophosphamide; CODOX, cyclophosphamide, vincristine, and doxorubicin; IVAC, ifosfamide, etoposide, and high-dose cytarabine; NK, not known; and CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone.
For details about the treatment regimens we refer to the original papers.
Overall response rate (ORR), complete remission (unconfirmed) + partial response.
Dead before treatment, n = 1.
Calculation of median survival is based on data presented in the original paper.
Auto-SCT, n = 3; Allo-SCT, n = 1.
Given for low-grade B-cell lymphoma, NOS.
Steroids as palliative therapy.
Therapy ongoing, n = 1.
Lymphoma-type DH only, n = 23.
Twenty-three DH lymphomas and 4 DH leukemias.
In a recent study on 303 patients with DLBCL treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone, 35 had a MYC rearrangement of which 27 (77%) were DH cases.114 All MYC-rearranged cases had an inferior outcome, also compared with the individual IPI categories. Additional breakpoints of BCL2 or BCL6 had no significant additional effect on survival, but this may have been because only 8 of the 27 patients did not have a DH lymphoma. Few data are available for other treatment modalities. All 4 patients with DLBCL with a DH who received high-intensity chemotherapy with cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine died within 5 months after the start of treatment.115
How can the dismal outcome in DH lymphomas be explained?
Several biologic mechanisms may arise to explain the dismal outcome in patients with DH lymphoma:
First, it could be that activation of MYC is directly responsible. However, the observation that adult patients with BL have a much more favorable outcome is against this hypothesis and suggests that additional factors are essential.
Second, the synergistic action of MYC and BCL2 might be responsible for this behavior. Observations in transgenic mice (see “Oncogenes involved in DH lymphomas” and “Timing and synergy of translocations in DH lymphomas”) as well as some clinical observations support this hypothesis. For instance in one report with relatively large numbers of cases and relatively homogeneous therapy the 19 BCL2+/MYC+ cases had a worse survival than the 24 patients with a single MYC+ or the 18 patients with a single BCL2+ translocation, as well as all 333 patients with other DLBCL.12
Third, it could be that other molecular features play an important role as well. DH lymphomas often have a complex karyotype with many additional genetic alterations, and the poor outcome may reflect many of these alterations. The role of genomic complexity as such is suggested by the studies of Hummel et al109 and Seegmiller et al,116 both indicating that the genomic complexity correlates with survival in lymphomas with a MYC rearrangement. Interestingly, on the basis of their biologic functions, it might be speculated that MYC and BCL2 themselves play a role in the generation of this genomic complexity.
Clinicopathologic aspects of other DH lymphomas
In the Mitelman database8 as analyzed by us, 34 DH lymphoma cases had a CCND1+/MYC+ combination (Table 2; Figure 2). CCND1+/MYC+ DH cases accounted for 5% of all MCLs. Many of these cases were leukemic and had a blastoid, pleomorphic, or even a BL-like morphology. Perhaps because of the retained and easily detectable cyclin D1 protein expression in tissue sections, most of these cases were readily diagnosed as MCL. An overrepresentation of such cases in the database may have also occurred because leukemic MCLs are possibly more frequently karyotyped than nonleukemic MCLs, and most of these cases presented with overtly leukemic disease. Intriguingly, some CCND1+/MYC+ DH cases had aberrant expression of CD10 and BCL6, which parallels the observation that most MYC-rearranged DLBCL also express CD10. Because most cases were studied on leukemic cells, only few cases have been documented for the Ki67 proliferation index.99,118,119 Six of 8 documented cases had a Ki67 index of > 75%, suggesting that MYC might confer an important additional proliferative boost to the tumor cells in which proliferation is a strong driving force.120 Interestingly, a recent gene expression study of 65 MCL cases showed that high MYC expression is the most important factor for outcome but only marginally was associated with the Ki67 proliferation index, suggesting a role of MYC in the (TP53) DNA damage pathway rather than in the proliferation pathway.121 As far as can be concluded from the reports, MCLs with involvement of 8q24 tend to have an aggressive clinical course, the average survival of CCND1+/MYC+ MCL being only 8 months.119
The reported numbers of the rare other DH lymphomas involving BCL3 or other loci are too few to draw any conclusions. One interesting feature that needs more attention is a subset of lymphomas in which 9p13 is involved, (see “Published DH lymphomas” and supplemental Table 2).
DH lymphomas and “B-cell lymphomas, unclassifiable with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma”
According to the 2008 WHO classification, the category “B-cell lymphomas, unclassifiable with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma”6 is a heterogeneous category of lymphomas that for biologic and clinical reasons should not be classified as BL lymphoma or DLBCL. It is meant as a temporary category, necessary until better discriminating criteria and more distinct categories of lymphomas are available. Apart from the DH lymphomas there are 3 other problematic issues with respect to the diagnosis of BL versus DLBCL that justified this category:
First, the most problematic area is formed by non-DH lymphomas that are diagnosed as DLBCL but nonetheless share several morphologic and immunophenotypical features with BL, in particular a cohesive growth pattern, a very high Ki-67 proliferation index, and expression of GC associated proteins such as CD10. The dimension of this diagnostic problem is difficult to assess but certainly is different between pediatric and adult patients. Many of such cases in children contain an MYC-IG breakpoint, do not have any other breakpoint, and have a gene expression profile similar to BL and, therefore, probably should be better diagnosed as BL.122 In contrast, for adult patients no data are available for large series of DLBCLs collected in population-based studies in which all ancillary tests to exclude BL or DH lymphomas have been applied. According to the Nordic Lymphoma Group,123 which more or less reflects a population-based registry, 10% of all 185 DLBCL cases had a Ki-67 proliferation index of ≥ 90%. This should mean that after exclusion of DH lymphomas and DLBCL with a phenotype not compatible with BL, far < 10% of all DLBCL are problematic in this respect.109,124
Second, BL has a characteristic expression of CD20, CD10, BCL6 and the absence of BCL2 protein, whereas MUM1/IRF4 may be expressed at low levels. However, in ≤ 20% of all otherwise classical BLs some immunophenotypic abnormalities have been reported, for instance weak expression of BCL2 protein in 0%-20%109,125 or aberrant expression of T-cell markers such as CD4 or CD5.126 As already discussed in this review, such cases should only be accepted as BL after vigorous exclusion of a DH lymphoma.
Finally, in ∼ 10% of all lymphomas that are otherwise indistinguishable from BL, including endemic and pediatric BLs, a MYC breakpoint is not detectable by current FISH methods. It might be considered to restrict the diagnosis of BL to cases with a proven MYC-IG breakpoint and to consign all other cases to the “intermediate” group. Likely, it is too early to do so because certain MYC breakpoints are missed with the current (FISH) methods and because in rare cases of BL a similar high MYC expression can be induced by down-regulation of miRNA-34B.127
These dilemmas and the fact that many recent publications suggest that the presence of a MYC breakpoint in DLBCL, either or not involved in a DH, has an important prognostic value,108,109,114,117,128,129 suggest that all aggressive mature B lymphomas should be systematically studied with ancillary methods, in particular FISH analysis, to provide the best possible diagnosis and therapeutic prospects.
Conclusions
DH and TH lymphomas are B-cell lymphomas characterized by a recurrent chromosomal translocation in combination with a MYC/8q24 breakpoint, the latter mostly as a secondary event involved in transformation. The compiled cytogenetic and FISH data strongly suggest that many lymphomas other than BL with a MYC breakpoint represent DH lymphomas. This implies that the studies that only focused on the effect of MYC breakpoints in DLBCL have to be reconsidered. Most DHs have a BCL2+/MYC+ combination, and most BCL6+/MYC+ DH lymphomas represent BCL2+/BCL6+/MYC+ TH lymphomas. CCND1+/MYC+ DH lymphomas may be more frequent than anticipated and should receive more attention.
In view of the frequency of these aberrations and their clinical effect, it seems timely to test all aggressive B-cell lymphomas, including the MCLs with a high-proliferation index or blastic morphology, for MYC (and MYC-IG) breakpoints by FISH. In selected cases BCL2 and BCL6 FISH tests should be performed as well, for instance in those cases with a MYC breakpoint and concomitant BCL2 protein expression. A BCL2+/MYC+ DH lymphoma should be considered in all aggressive and highly proliferating B-cell lymphomas with a distinct GC B phenotype in combination with BCL2 expression, in particular when the patient presents with extensive disease, including bone marrow or CNS involvement or both. However. these parameters are insufficient to identify all cases. Moreover, individual morphologic and immunophenotypical parameters may have a low reproducibility. Although we realize that detection of MYC, BCL2, and BCL6 breakpoints does not reflect all biologic aspects of these complex tumors, we suggest to perform these assays until more biologic data and better tests become available.
Patients with DH lymphoma generally have rapidly progressive disease and a dismal outcome, even with high-intensity chemotherapy. The course of disease might reflect not only the synergistic actions of the ≥ 2 oncogenes involved but also the high genomic complexity in most of these tumors.
The online version of this article contains a data supplement.
Acknowledgments
We thank Dr Itziar Salaverria for critically reading the manuscript.
This work was supported by the Deutsche Krebshilfe (Network Project “Molecular Mechanisms in Malignant Lymhpoma” [R.S.]) and BMBF (Network Project “HämatoSys” [R.S.]). S.M.A. is a fellow of the Junior-Scientific-Masterclass-UMCG MD-PhD program.
Authorship
Contribution: S.M.A. performed literature and database searches and wrote the manuscript; R.S. contributed to the design and supervised the cytogenetic/molecular investigations; E.S. contributed to the scientific cytogenetic/molecular part of the manuscript; G.W.v.I. contributed to the design of the manuscript and wrote parts of the manuscript; H.C.K.-N. and E.-J.B. contributed to the design of the manuscript; P.M.K. contributed to the design of the manuscript and wrote parts of it.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.-J.B. is Department of Surgery, Maasstad Hospital, Rotterdam, The Netherlands.
Correspondence: Sieste M. Aukema, Department of Pathology and Medical Biology, University Medical Center Groningen, Hanzeplein 1, 9700 RB Groningen, The Netherlands; e-mail: s.m.aukema@student.rug.nl.