Abstract
Progress in the last decade has improved the understanding of leukemia biology. Molecular markers in combinations with cytogenetics have improved the risk stratification of acute myeloid leukemia (AML) and informed decision-making. In parallel, several important advances in the transplant field, such as better supportive care, improved transplant technology, increased availability of alternative donors, and reduced-intensity conditioning have improved the safety as well as access of allogeneic hematopoietic cell transplantation (HCT) for a larger number of patients. In this review, the positioning of HCT in the management of patients with AML is evaluated in view of changing risk/benefit ratios associated with both conventional treatments and transplantation, and some of the controversies are addressed in light of emerging data. Increasing data demonstrate outcomes of alternative donor transplantation approaching HLA-identical sibling donors in high-risk AML supporting the inclusion of alternative donors in trials of prospective studies evaluating post remission strategies for high-risk AML. The use of reduced-intensity conditioning has expanded the eligibility of HCT to older patients with AML, and outcome data are encouraging. Continued study of HCT versus alternative therapies is required to optimize patients' outcomes in AML.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment option for patients with acute myeloid leukemia (AML). The curative effect of HCT in patients with AML is contributed both by the chemotherapy and/or radiation in the preparative regimen and more importantly by the immunologic graft-versus-leukemia (GVL) effect.1 Currently, AML is the most common indication for HCT. Data from Center for International Blood and Marrow Transplant Research (CIBMTR) indicate a sustained increase in the number of AML allogeneic transplants in the last decade (Table 1). Although the number of transplants from related donors has remained stable during the last decade, transplants from unrelated donors (URDs) are growing (Table 1). The growth of transplant activity is mainly in adults and is attributable to increased use of URDs, especially in first remission (CR1; Table 1, Figure 1). CIBMTR data demonstrate that 47% of CR1 allografts (all ages) in AML in 2008 were performed with the use of URDs. Among URDs, umbilical cord blood (UCB) is becoming an important graft source in adults and contributed to 11% of adult transplants from URDs in 2008 (Table 1). Despite these trends, there remains wide variation in the application of HCT in AML patients, especially during CR1. The guidelines of various major organizations in Europe and the United States on the use of HCT for AML patients are not consistent.2
Trends in AML allogeneic transplant activity: cases registered with the CIBMTR
| . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of centers reporting to CIBMTR | 162 | 171 | 189 | 208 | 220 | 208 | 222 | 210 | 212 | 197 | 184 |
| AML patients undergoing allo-HCT, all ages | |||||||||||
| All patients | 1778 | 1826 | 2045 | 2119 | 2324 | 2326 | 2621 | 2735 | 2697 | 2581 | 2557 |
| RD | 1286 | 1288 | 1359 | 1319 | 1462 | 1382 | 1444 | 1485 | 1436 | 1284 | 1223 |
| vURD | 443 | 474 | 627 | 697 | 744 | 822 | 1046 | 1091 | 1053 | 1085 | 1114 |
| UCB | 49 | 64 | 59 | 103 | 118 | 122 | 131 | 159 | 208 | 212 | 220 |
| Adult*AML allo-HCT patients using vURD | |||||||||||
| CR1 | 72 | 88 | 153 | 186 | 211 | 273 | 353 | 422 | 441 | 467 | 518 |
| ≥ CR2 | 99 | 104 | 148 | 162 | 173 | 161 | 245 | 258 | 212 | 239 | 221 |
| Not in remission | 126 | 118 | 149 | 155 | 179 | 193 | 242 | 216 | 225 | 173 | 278 |
| Adult*AML allo-HCT patients using UCB | |||||||||||
| CR1 | 3 | 4 | 9 | 9 | 14 | 14 | 16 | 29 | 43 | 39 | 47 |
| ≥ CR2 | 4 | 7 | 4 | 12 | 8 | 8 | 17 | 27 | 30 | 39 | 45 |
| Not in remission | 4 | 4 | 5 | 16 | 5 | 9 | 14 | 11 | 21 | 24 | 38 |
| Graft source on all adult AML patients, % | |||||||||||
| Related donor | |||||||||||
| PB | 43 | 54 | 65 | 74 | 76 | 83 | 83 | 87 | 87 | 88 | 88 |
| BM | 57 | 46 | 34 | 26 | 24 | 17 | 17% | 13 | 13 | 12 | 12 |
| UCB | 0.5 | 0.3 | 0.6 | 0 | 0 | 0 | 0% | 0.1 | 0 | 0.2 | 0 |
| Unrelated donor | |||||||||||
| PB | 4 | 13 | 29 | 39 | 46 | 60 | 68 | 69 | 70 | 71 | 75 |
| BM | 91 | 83 | 68 | 55 | 50 | 34 | 26 | 23 | 19 | 18 | 14 |
| UCB | 5 | 5 | 4 | 6 | 5 | 6 | 6 | 8 | 11 | 11 | 11 |
| . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | 2006 . | 2007 . | 2008 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of centers reporting to CIBMTR | 162 | 171 | 189 | 208 | 220 | 208 | 222 | 210 | 212 | 197 | 184 |
| AML patients undergoing allo-HCT, all ages | |||||||||||
| All patients | 1778 | 1826 | 2045 | 2119 | 2324 | 2326 | 2621 | 2735 | 2697 | 2581 | 2557 |
| RD | 1286 | 1288 | 1359 | 1319 | 1462 | 1382 | 1444 | 1485 | 1436 | 1284 | 1223 |
| vURD | 443 | 474 | 627 | 697 | 744 | 822 | 1046 | 1091 | 1053 | 1085 | 1114 |
| UCB | 49 | 64 | 59 | 103 | 118 | 122 | 131 | 159 | 208 | 212 | 220 |
| Adult*AML allo-HCT patients using vURD | |||||||||||
| CR1 | 72 | 88 | 153 | 186 | 211 | 273 | 353 | 422 | 441 | 467 | 518 |
| ≥ CR2 | 99 | 104 | 148 | 162 | 173 | 161 | 245 | 258 | 212 | 239 | 221 |
| Not in remission | 126 | 118 | 149 | 155 | 179 | 193 | 242 | 216 | 225 | 173 | 278 |
| Adult*AML allo-HCT patients using UCB | |||||||||||
| CR1 | 3 | 4 | 9 | 9 | 14 | 14 | 16 | 29 | 43 | 39 | 47 |
| ≥ CR2 | 4 | 7 | 4 | 12 | 8 | 8 | 17 | 27 | 30 | 39 | 45 |
| Not in remission | 4 | 4 | 5 | 16 | 5 | 9 | 14 | 11 | 21 | 24 | 38 |
| Graft source on all adult AML patients, % | |||||||||||
| Related donor | |||||||||||
| PB | 43 | 54 | 65 | 74 | 76 | 83 | 83 | 87 | 87 | 88 | 88 |
| BM | 57 | 46 | 34 | 26 | 24 | 17 | 17% | 13 | 13 | 12 | 12 |
| UCB | 0.5 | 0.3 | 0.6 | 0 | 0 | 0 | 0% | 0.1 | 0 | 0.2 | 0 |
| Unrelated donor | |||||||||||
| PB | 4 | 13 | 29 | 39 | 46 | 60 | 68 | 69 | 70 | 71 | 75 |
| BM | 91 | 83 | 68 | 55 | 50 | 34 | 26 | 23 | 19 | 18 | 14 |
| UCB | 5 | 5 | 4 | 6 | 5 | 6 | 6 | 8 | 11 | 11 | 11 |
allo-HCT indicates allogeneic hematopoietic cell transplantation; AML, acute myeloid leukemia; BM, bone marrow, CIBMTR, Center for International Blood and Marrow Transplant Research; CR, complete remission; CR1, first complete remission; CR2, second complete remission; PB, peripheral blood; RD, related donor; UCB, umbilical cord blood; and vURD, voluntary unrelated donor.
Adult defined as ≥ 18 years.
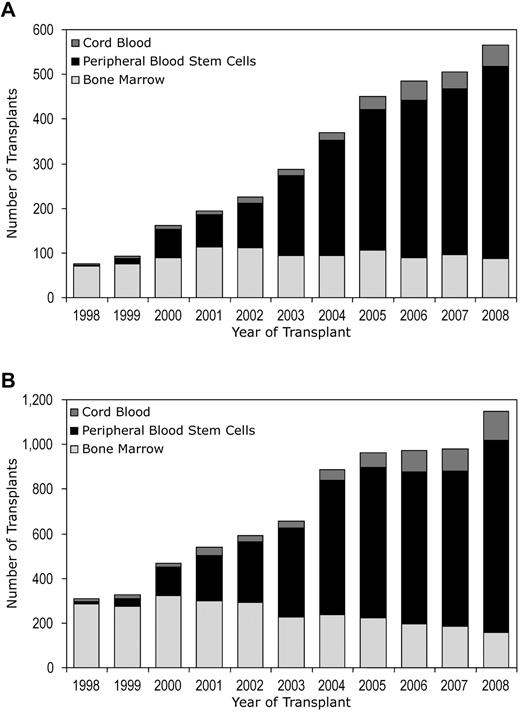
Trends in allogeneic HCT activity in adult AML patients (≥ 18 years) by the use of unrelated donors according to disease status at HCT: cases registered with CIBMTR from 1998 to 2008. (A) Adult AML patients (≥18 years), CR1, CR2, CR3, and not in remission, undergoing unrelated HCT. (B) Adult AML patients (≥ 18 years) undergoing unrelated HCT in CR1.
Trends in allogeneic HCT activity in adult AML patients (≥ 18 years) by the use of unrelated donors according to disease status at HCT: cases registered with CIBMTR from 1998 to 2008. (A) Adult AML patients (≥18 years), CR1, CR2, CR3, and not in remission, undergoing unrelated HCT. (B) Adult AML patients (≥ 18 years) undergoing unrelated HCT in CR1.
Progress in the last 2 decades in understanding the disease biology of AML may guide further practice changes. Cytogenetics is the most robust prognostic marker for risk stratification of AML at the time of diagnosis as well as in selection of postremission treatments.3-5 On the basis of specific structural and numerical cytogenetic abnormalities, patients with AML are divided into favorable, intermediate-, or adverse-risk groups. More recently, new molecular markers such as FMS-related tyrosine kinase 3–internal tandem duplication (FLT3-ITD), CCAAT/enhancer-binding protein-α (CEBPA), and the nucleophosmin-1 (NPM1) mutation have further refined the risk stratification, particularly in patients with cytogenetically normal AML (CN-AML).6 These cytogenetic and molecular markers have helped identify patients at different risk of relapse after achieving CR. In parallel, major advances have developed in the transplant field. Better supportive care, and selection of URD with the use of high-resolution allele-level HLA-typing have improved the safety of transplantation. Increasing use of UCB, and application of reduced-intensity conditioning (RIC) regimens have increased the candidacy for HCT to include a wider group of patients, particularly older or ethnic minority patients who were not well served by earlier HCT and donor search options. In this review, we evaluate the positioning of HCT in the management of patients with AML in the light of changing risk/benefit ratios associated with both conventional chemotherapy and HCT and address controversies highlighted by emerging data.
HCT for adult patients with AML in CR1
Several groups have investigated the role of HLA-matched sibling donor (MSD) transplantation in patients with AML in CR1. The designs of these studies involve genetic assignment substituting for randomization on the basis of the availability of a MSD. These studies were generally not powered to evaluate the outcome of HCT according to cytogenetic risks. Subgroup analyses of these studies on the basis of cytogenetics included small sample sizes. In a recent systematic review and meta-analysis of prospective biologic assignment studies, researchers analyzed 3638 patients with AML in CR1 by cytogenetic risk and showed a significant survival advantage of HCT in comparison with nonallogeneic treatments for AML patients with intermediate and unfavorable cytogenetics, although not for good risk cytogenetics.7 Recent progress in the understanding of leukemia biology has further refined prognostication of intermediate-risk group patients, especially CN-AML, and has contributed to the identification of subgroups among those with favorable risk cytogenetics at greater risk of relapse. Current guidelines and new evidence for AML patients in CR1 on the basis of leukemia biology raise new questions: (1) Should all patients with CN-AML with a suitable donor be referred for HCT in CR1? (2) Is there a role of HCT for a subgroup of patients with good-risk cytogenetics in CR1? (3) If a MSD is not available, is alternative donor HCT indicated in CR1?
Should all patients with CN-AML be referred for HCT in CR1?
CN-AML is a heterogeneous group comprising approximately 40%-50% of AML patients < 60 years, less common in patients > 60, and currently categorized as intermediate risk.3-5 Recently, several molecular alternations have helped researchers to improve the individual risk assessment of CN-AML patients.8 Several authors have shown the prognostic significance for mutations in the NPM1, CEBPA, and FLT3 genes alone or in combination in both younger and older adults with AML. The presence of the FLT3-ITD mutation has been identified as a powerful indicator predicting more frequent and early relapse.9-12 The adverse impact in FLT3-ITD–positive patients may be greater in those with a high level of the mutated allele.10,13 The prognostic significance of FLT3-TKD mutations remains controversial in view of conflicting data.14-16 NPM1 mutation (mNPM1) in the absence of FLT3-ITD mutation in CN-AML has been associated with lower cumulative incidence of relapse, resulting in better leukemia-free survival (LFS) and overall survival (OS).17-20 The outcomes of AML with genotype “mNPM1 without FLT3-ITD” treated with conventional chemotherapy appear favorable and similar to patients with t(8;21) or inv(16).11,19 CN-AML with mutations in CEBPA (mCEBPA) are associated with a favorable prognosis.11,21-24 The role of molecular genetic alterations in AML has been recognized in the 2008 revised World Health Organization classification, and AML with mNPM1 or mCEBPA have been incorporated in the World Health Organization classification as provisional entities.25
In a study from Germany, researchers analyzed the role of mutational status of NPM1, FLT3, CEBPA, along with MLL and NRAS in guiding postremission therapy for CN-AML in CR1.11 They the analyzed data from CN-AML patients treated in 4 prospective clinical trials. Treatment intention was similar in all 4 trials, and patients with a HLA-matched related donor (MRD) were assigned to undergo HCT in CR1; those without a donor were randomly assigned to receive high-dose cytarabine (HiDAC) consolidation or autologous transplant. HCT was performed in 82% of assigned patients. Autologous transplant or consolidation therapy resulted in similar outcomes. An intention-to-treat analysis on the basis of donor availability demonstrated significantly longer relapse-free survival (RFS) in the donor group (P = .009). Data were further analyzed on the basis of mutation status, and patients were further subdivided into 2 groups: patients with “mNPM1 without FLT3-ITD,” and patients with all other genotypes. Because of small numbers, patients with mCEBPA were excluded. No differences in RFS was seen with or without a donor in the favorable genotype patients with mNPM1 without FLT3-ITD (P = .71). Among the patients with CN-AML with genotypes other than “mNPM1 without FLT3-ITD,” superior RFS was observed in those with a donor. At present, there are no comparative data available on outcomes of HCT versus nonallogeneic treatments in CN-AML patients with “mCEBPA.” The outcome of “mCEBPA” patients is similar to “mNPM without FLT3-ITD.”11 Increasing data show that the better outcome of CEBPA mutations is restricted to patients with double mutation only.24,26,27 Therefore among the CN-AML, HCT in CR1 could be deferred in favorable genotype of “mCEBPA or mNPM1 without FLT3-ITD.” HCT should be considered in all other patients with CN-AML in CR1, at least in those up to age 60 years.
The definition of favorable genotype may be further refined with the identification of new mutations, although the favorable subtypes may remain uncommon. Recently identified isocitrate dehydrogenase enzyme isoform 1 mutations (mIDH1) and isoform 2 mutation (mIDH2) have been associated with a poor prognosis in patients with AML.28,29 Of particular note, mIDH1 was seen among 21 of 86 patients with mNPM1. None of the 22 patients with mCEBPA had mIDH1.28 None of the patients with mNPM1 or mCEBPA had mIDH2. The authors suggested that favorable genotype among CN-AML patients should be modified to “mNPM1 or mCEBPA with neither FLT3-ITD nor mIDH1.” Other recently described mutations in the DNA methyltransferase gene DNMT3A are frequent in patients with intermediate-risk cytogenetics and associated with increased relapse risk and poor outcome.30 Of note, a significant benefit of HCT was observed in this small series. Findings from these recent but small studies need further validation before routine application. Evolving technologies such as microarray profiling31-33 and microRNA signatures34 have shown promise in improving the prognostic classification of AML; however, these methodologies are not yet available for routine clinical use.
Is there a role of HCT for patients with favorable risk cytogenetics in CR1?
Patients with core binding factor (CBF) leukemia (ie, t[8;21] or inv[16] or t[16;16]) or acute promyelocytic leukemia (APL) with t(15;17) are considered at relatively lower risk of relapse and thus are considered to have favorable risk cytogenetics.3-5 The meta-analysis of prospective genetic randomization studies showed no benefit of HCT in patients with favorable risk cytogenetics in CR1.7 With all-trans retinoic acid or arsenic trioxide–based treatments, the outcome of APL with t(15;17) has significantly improved. These treatment strategies lead to cure in most patients, and the expected relapse rate is usually 10%-25%.35 However, in CBF-AML, only 50% of patients were alive at 5 years.36 Therefore, some of these patients may still have a high risk of relapse. The role of KIT mutations (mKIT) in CBF-AML has been investigated to identify a high-risk subset in the otherwise-favorable CBF group.37-40 Two commonly identified KIT mutations (exon17 [mKIT 17] and exon 8 [mKIT 8]) in CBF-AML appear to have prognostic relevance; however, the data from these studies are not consistent. Discrepant results may be related to small number of patients and differences in the treatments.
Repetitive cycles of HiDAC as postremission therapy are associated with favorable outcomes in patients with CBF-AML.41,42 In a Cancer and Leukemia Group B (CALGB) study, authors investigated the role of mKIT in 61 adult patients with inv(16) and 49 patients with t(8;21) assigned to postremission therapy with HiDAC.40 In patients with inv(16), the 5-year relapse-risk was significantly greater in patients with mKIT (56% vs 29%, P = .05) and in particular mKIT17 (80% vs 29%, P = .002) in comparison with wtKIT. Similarly, in patients with t(8;21), the 5-year relapse-risk was significantly greater in those mKIT (70% vs 36%, P = .017). This relapse pattern is similar to that seen with patients with adverse cytogenetics. In view of these data, we may suggest that patients with “CBF-AML with mKIT” be considered for HCT in CR1. It is noteworthy that at present, there are no available data to show the benefit of HCT in “CBF-AML with mKIT” although resistance to HiDAC or other consolidation strategies may not imply resistance to the allogeneic GVL effect. The high-risk of relapse in these patients merit the investigation of alternative treatment strategies including HCT or possibly molecular targeted therapies with tyrosine kinase inhibitors in future studies.
Is there a role of alternative donor transplantation in CR1?
Most AML patients with adverse cytogenetics relapse within a year, and likelihood of subsequent CR is very low.43,44 Meta-analysis of prospective biologic assignment studies comparing the role of HCT to non-HCT treatments for patients with adverse cytogenetics in CR1 have shown a strong survival advantage for HCT during CR1.7,45 Most patients in these studies received HCT via the use of MSD, and comparisons of URD HCT to nonallogeneic treatments are limited.46,47 In a recent study from CIBMTR, researchers compared the outcomes of URD and MSD transplants in patients with AML in CR1 with unfavorable cytogenetics.48 This study showed similar LFS and OS of HLA-well matched URD (no known disparity at HLA-A, B, C, or DRB1) in comparison with MSD, whereas outcomes were not as good for human leukocyte antigen (HLA)-partially matched URD (disparity at one locus). Prospective comparisons of MSD and URD for high-risk AML patients in CR1 on a limited number of patients show equivalent outcomes.46,47 Therefore, if a MSD is not available, an HLA-well matched URD is appropriate for patients with AML with adverse cytogenetics in CR1.
There are only scanty data on comparisons of MRD and URD transplantation in intermediate-risk AML in CR1. In a study from Seattle, researchers compared 85 patients with URD versus 135 patients undergoing MRD transplantation in CR1.49 In the intermediate-risk group, 58 and 83 patients underwent HCT via the use of URD and MRD, respectively. Most patients in intermediate-risk had a normal karyotype. Although these sample sizes are modest, the outcomes of URD and MRD appear similar. Among the patients with normal karyotype, those with FLT3-ITD mutations are at a greater risk of relapse. At present, no comparative data are available on outcomes of MSD and URD in AML patients with FLT3-ITD mutations. The high risk of relapse in these patients merits the use of HCT with either related or URD HCT and further investigation of novel molecular targeted therapies. A high risk of early relapse and lengthy time for volunteer URD searches confound the use of URD HCT in CR1 in high-risk AML patients. Therefore, donor searches for such patients should be initiated early, that is, during the initial induction.
An important but as yet incompletely addressed question is the utility of UCB-HCT for patients with high-risk AML in CR1. Previous registry studies have compared the outcomes of unrelated UCB with unrelated bone marrow (URD-BM) grafts in adults with acute leukemia.50,51 A study from the European Group of Blood and Marrow Transplantation (EBMT) showed similar outcomes among the 2 cohorts,51 whereas the study from CIBMTR50 showed lower treatment-related mortality (TRM), treatment failure, and overall mortality among the recipients of HLA-matched BM grafts; similar outcomes were found for patients receiving mismatched UCB transplants and mismatched URD-BM grafts.50 In a recent study from CIBMTR, the authors compared the outcomes of UCB (n = 165) with unrelated peripheral blood (URD-PB; n = 888), URD-BM (n = 472) in patients with acute leukemia.52 The majority of patients receiving UCB graft were 4/6 antigen match (70%). The LFS in patients after 4-6/6 matched UCB-HCT were comparable with that after 8/8 or 7/8 allele-matched URD-PB or URD-BM recipients; however, the TRM was greater after UCB-HCT. A recently published study from Minnesota and Seattle demonstrated similar outcomes in double unit UCB and matched URD compared with MSD after myeloablative HCT in hematologic malignancies.53 Because these studies analyzed impact of graft source in patients with mixture of different disease status in acute leukemia50-52 or hematologic malignancies,53 the results are hard to interpret for AML in CR1.
In a recent study from the Japan, researchers evaluated the disease-specific comparison of unrelated UCB recipients and HLA-allele–matched URD-BM recipients in 484 adult patients with AML (173, CB; and 311, BM).54 In this study, 180 AML patients underwent transplant in CR1 (50, CB; and 130, BM). Multivariate analysis showed inferior survival of patients in CR1 receiving UCB-HCT versus URD-BM (relative risk 2.92 (95% confidence interval 1.38-6.18), P = .005). The inferior survival with UCB-HCT in these patients was associated with greater TRM in the UCB group. Contrary to the commonly held belief that GVL effect after URD or mismatched donor is more potent in comparison with MSD, the studies in which the authors compared different donor sources show that the relapse rate was not decreased after well-matched URD48,55 or mismatched URD/UCB-HCT.48,52,54
Should haplo-identical transplantation (haplo-HCT) be a suitable option for high-risk AML patients in CR1? Very limited data are available on use of haplo-HCT in patients with AML in CR1. In a recent study from the EBMT, authors analyzed the outcomes of haplo-HCT in patients with acute leukemia in remission.56 This study included 86 patients with AML in remission (25 in CR1). The 2-year cumulative incidence of TRM, relapse, and probability of LFS were 36%, 16%, and 48%, respectively in CR1 patients. The effect of donor graft sources in AML from various studies are summarized in Table 2.46,48,49,54,57-59 These data are intriguing but insufficient to guide decisions about URD, UCB, or haplo-HCT, particularly in centers with little experience with either UCB or haplo-HCT where complications of graft failure or slower immune recovery need extra attention, even if graft-versus-host-disease is less common. However, their rapid availability offers an important clinical advantage for patients in whom donor search was delayed or cohorts of greater risk where remission duration may be brief. Current data suggest that these options be used only in the absence of a timely available URD. No data exist on the comparison of UCB and haplo-HCT to nonallogeneic treatments in AML in CR1 at present; emerging data support the inclusion of UCB, haplo-HCT in addition to volunteer URD in prospective studies in which the authors evaluate postremission strategies for high-risk AML in CR1.
Comparative outcomes of various donor sources for HCT in AML
| Study . | Sample size and patient population . | Disease status . | Main comparison . | LFS and OS . | Additional comments . |
|---|---|---|---|---|---|
| Hegenbert et al, 2006,57 Multicenter, Seattle Consortium | 122, age 17-74 y, using NMA conditioning only | CR1 (n = 51), CR2 (n = 39), advanced (n = 32) | MRD (n = 58) vs MUD (n = 64) | No difference between MRD and MUD | Disease status at HCT and cytogenetics most important factors for LFS and OS |
| Moore et al, 2007,58 Multicenter, Australasian Registry | 210, age 16-59 y, using MAC only | CR1 (n = 36), > CR1/others (n = 174) | MSD (n = 105) vs URD (n = 105) | No difference between MSD and URD | Matched case-controlled study |
| Schetelig et al, 2008,59 Multicenter, Germany | 368, age 50-73 y, both MAC and RIC | CR1 (n = 136), advanced, (n = 228), others (n = 4) | MSD (n = 168) vs M/MRD (n = 12) vs MUD (n = 51) vs possibly MUD (n = 68) vs partially MUD (n = 45) vs poorly MUD (n = 24) | No difference between different donor types | Advanced disease at HCT, secondary AML, and high risk cytogenetics associated with poor outcomes |
| Atsuta et al, 2009,54 Multicenter, JMDP/JCBBN | 484, age 16-69 y, using MAC only | CR1 (n = 180), Others (n = 304) | MUD (n = 311) vs UCB-HCT (n = 173) | Inferior outcomes in UCB-HCT attributable to increased TRM | No difference in risk of relapse |
| Walter et al, 2010,49 single center, Seattle | 220, age 18-69 y, using MAC only | CR1, Intermediate cytogenetics (n = 141), high risk cytogenetics (n = 60), others (n = 19) | MRD (n = 135) vs 10/10 MUD (n = 62) vs 9/10 URD (n = 23) | No difference between MRD vs 10/10 MUD vs 9/10 URD | Unfavorable cytogenetics and high HCT-CI score associated with worse outcomes |
| Gupta et al, 2010,48 Multicenter, CIBMTR | 584, age <1-74 y, Both MAC and RIC | AML in CR1 with adverse cytogenetics | MSD (n = 226) vs well-matched URD (n = 254) vs Partially matched URD (n = 104) | Similar between MSD and well-matched URD, inferior for partially matched URD | Lower risk of relapse in patients with chronic GVHD |
| Schlenk et al, 2010,46 Multicenter, Germany and Austria | 162, age 19-61 y, Both MAC and RIC | High-risk AML in CR1, patients refractory to induction therapy | MRD (n = 62) vs MUD (n = 89) vs CB/HaploHCT (n = 11) | Similar between MSD and MUD | Prospective study, also compared with patients who could not get transplant, benefit of HCT seen in comparison with non-HCT patients in landmark analysis |
| Study . | Sample size and patient population . | Disease status . | Main comparison . | LFS and OS . | Additional comments . |
|---|---|---|---|---|---|
| Hegenbert et al, 2006,57 Multicenter, Seattle Consortium | 122, age 17-74 y, using NMA conditioning only | CR1 (n = 51), CR2 (n = 39), advanced (n = 32) | MRD (n = 58) vs MUD (n = 64) | No difference between MRD and MUD | Disease status at HCT and cytogenetics most important factors for LFS and OS |
| Moore et al, 2007,58 Multicenter, Australasian Registry | 210, age 16-59 y, using MAC only | CR1 (n = 36), > CR1/others (n = 174) | MSD (n = 105) vs URD (n = 105) | No difference between MSD and URD | Matched case-controlled study |
| Schetelig et al, 2008,59 Multicenter, Germany | 368, age 50-73 y, both MAC and RIC | CR1 (n = 136), advanced, (n = 228), others (n = 4) | MSD (n = 168) vs M/MRD (n = 12) vs MUD (n = 51) vs possibly MUD (n = 68) vs partially MUD (n = 45) vs poorly MUD (n = 24) | No difference between different donor types | Advanced disease at HCT, secondary AML, and high risk cytogenetics associated with poor outcomes |
| Atsuta et al, 2009,54 Multicenter, JMDP/JCBBN | 484, age 16-69 y, using MAC only | CR1 (n = 180), Others (n = 304) | MUD (n = 311) vs UCB-HCT (n = 173) | Inferior outcomes in UCB-HCT attributable to increased TRM | No difference in risk of relapse |
| Walter et al, 2010,49 single center, Seattle | 220, age 18-69 y, using MAC only | CR1, Intermediate cytogenetics (n = 141), high risk cytogenetics (n = 60), others (n = 19) | MRD (n = 135) vs 10/10 MUD (n = 62) vs 9/10 URD (n = 23) | No difference between MRD vs 10/10 MUD vs 9/10 URD | Unfavorable cytogenetics and high HCT-CI score associated with worse outcomes |
| Gupta et al, 2010,48 Multicenter, CIBMTR | 584, age <1-74 y, Both MAC and RIC | AML in CR1 with adverse cytogenetics | MSD (n = 226) vs well-matched URD (n = 254) vs Partially matched URD (n = 104) | Similar between MSD and well-matched URD, inferior for partially matched URD | Lower risk of relapse in patients with chronic GVHD |
| Schlenk et al, 2010,46 Multicenter, Germany and Austria | 162, age 19-61 y, Both MAC and RIC | High-risk AML in CR1, patients refractory to induction therapy | MRD (n = 62) vs MUD (n = 89) vs CB/HaploHCT (n = 11) | Similar between MSD and MUD | Prospective study, also compared with patients who could not get transplant, benefit of HCT seen in comparison with non-HCT patients in landmark analysis |
CIBMTR indicates Center for International Blood and Marrow Transplant Research; CR1, first complete remission; CR2, second complete remission; GVHD, graft versus host disease; haploHCT, haplo-identical cell transplantation; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic cell transplantation comorbidity index; JCCBN, Japanese cord blood bank network; JMDP, Japanese Marrow Donor Program; LFS, leukemia-free survival; MAC, myeloablative conditioning; NMA, nonmyeloablative; OS, overall survival; M/MRD, mismatched related donor; MRD, matched related donor; MSD, matched sibling donor; MUD, matched unrelated donor; RIC, reduced-intensity conditioning; TRM, treatment-related mortality; UCB, umbilical cord blood; and URD, unrelated donor.
Relapsed AML
The treatment of AML in first relapse is associated with unsatisfactory results and survival is usually poor. There are no prospective studies in which the authors evaluate the outcome of HCT in comparison to conventional chemotherapy in patients with relapsed AML. All reported data are retrospective in nature and have the limitations of treatment heterogeneity and selection bias. However, a prospective study in this area is logistically difficult and unlikely to be performed. HCT is often used in this setting, despite only limited evidence about its outcome.
One study from Europe evaluated the outcomes of 667 AML patients in first relapse among 1540 newly diagnosed non-M3 AML patients (age 15-60 years) entered into 3 consecutive cooperative group trials.60 The authors identified 4 prognostic factors in multivariate analysis: relapse-free interval from CR1, cytogenetics at diagnosis, age at first relapse, and autologous or HCT before first relapse. On the basis of these factors, a weighted prognostic score was proposed to identify 3 risk groups: favorable, intermediate, and poor risk. For patients able to achieve CR2, comparison of chemotherapy versus HCT among these 3 groups showed superior 5-year survival in patients undergoing HCT (favorable, 88% vs 33%; intermediate, 48% vs 31%; poor, 26% vs 6%). Achievement of CR2 and application of salvage HCT are crucial for improving the prognosis of these patients.60,61 Survival of patients in first relapse undergoing salvage HCT was significantly better for those who achieved CR2 compared with those not in remission at HCT (3-year survival 59% vs 21%).61 Few data on comparison of UCB-HCT/ haplo-HCT versus chemotherapy in this setting are reported. Given the poor prognosis associated with chemotherapy alone, UCB or haplo-HCT for patients in CR2 may be valuable, particularly in light of their rapid availability.
HCT in patients with AML not in remission
The utility of HCT for patients with AML with active disease remains controversial. Several authors62-68 have reported the outcomes of HCT in patients with acute leukemia not in remission at the time of transplant with variable outcomes. Small sample sizes; a mixture of patients with AML, ALL, and CML blast crisis; and publication bias confound interpretation of data.
In a recent CIBMTR study, researchers evaluated the outcome of 1673 patients with AML not in remission at the time of HCT.69 Survival at 3 and 5 years was 19% and 17%, respectively. In the multivariate analysis, 5 adverse patient-, disease- and transplant- related factors were identified: first CR duration < 6 months, circulating blasts, donor other than MSD, performance score < 90%, and adverse risk cytogenetics. The 3-year survival of patients with none of these risk factors was 42%, and those with 1, 2, or ≥ 3 risk factors was 28%, 15%, and 6%, respectively. These results provide important guidance in identifying groups where the transplant procedure has reasonable chances of success, as well as identifying patients for whom transplant procedure is likely to be futile. This study included only patients treated with myeloablative conditioning. The role of RIC in patients with active acute leukemia is unclear. Some investigators have suggested that RIC may be an option,67,70,71 whereas others have reported futility of this procedure in such patients.72
For patients with refractory AML (primary induction failure, ie, no CR after 2 cycles of therapy), relapse after a CR1 < 6 months, second or greater relapse, or resistant elapsed disease, success in achieving CR with any further salvage therapy is very low (10%-15% at best). Further chemotherapy extends the risks of opportunistic, often fungal infection and organ toxicities, any of which can increase TRM of future HCT. A sequential approach of use of salvage chemotherapy for reduction of leukemia burden followed by RIC was reported from Germany.71 This approach led to encouraging 3-year LFS and OS of 30% and 32% in patients with refractory AML, yet still reflects substantial patient selection.
Relapse is the major cause of failure in AML patients not in remission at HCT, and novel strategies to improve the efficacy of conditioning such as addition of targeted radiation,73 intensity modulated radiation therapy,74 or post-HCT strategies such as azacytidine, prophylactic donor lymphocyte infusions (DLIs), adoptive transfer of natural killer cells, leukemia-specific T cells or leukemia vaccines aimed at promoting a more potent or durable GvL effect require study.71,75-77
Guidelines for the indications of HCT in AML from various donor sources are summarized in Table 3. With in the framework of these guidelines, each patient should be carefully evaluated for the risk posed by disease itself versus risk from the transplant procedure taking in considerations factors such as age, performance status, comorbidities, and donor-recipient matching. Decisions may further be guided by the likely ability to achieve CR2 in case of relapse.
Guidelines for indications for HCT in adult patients with AML
| . | MSD . | MUD . | UCB . | Haplo-identical . |
|---|---|---|---|---|
| First remission | ||||
| Favorable cytogenetics | ||||
| APL | No | No | No | No |
| CBF-AML* | No | No | No | No |
| With mKIT | Uncertain | Uncertain | Uncertain | Uncertain |
| Without mKIT | No | No | No | No |
| CN-AML* | Yes | Uncertain | Uncertain | Uncertain |
| “mNPM1 without FLT3ITD” | No | No | No | No |
| “mCEBPA”† | No | No | No | No |
| Others than above | Yes | Uncertain | Uncertain | Uncertain |
| Intermediate risk with abnormal cytogenetics | Yes | Uncertain | Uncertain | Uncertain |
| Adverse | Yes | Yes | Yes‡ | Yes‡ |
| Second remission | Yes | Yes | Yes‡ | Yes‡ |
| Not in remission§ | Yes | Yes | Uncertain | Uncertain |
| . | MSD . | MUD . | UCB . | Haplo-identical . |
|---|---|---|---|---|
| First remission | ||||
| Favorable cytogenetics | ||||
| APL | No | No | No | No |
| CBF-AML* | No | No | No | No |
| With mKIT | Uncertain | Uncertain | Uncertain | Uncertain |
| Without mKIT | No | No | No | No |
| CN-AML* | Yes | Uncertain | Uncertain | Uncertain |
| “mNPM1 without FLT3ITD” | No | No | No | No |
| “mCEBPA”† | No | No | No | No |
| Others than above | Yes | Uncertain | Uncertain | Uncertain |
| Intermediate risk with abnormal cytogenetics | Yes | Uncertain | Uncertain | Uncertain |
| Adverse | Yes | Yes | Yes‡ | Yes‡ |
| Second remission | Yes | Yes | Yes‡ | Yes‡ |
| Not in remission§ | Yes | Yes | Uncertain | Uncertain |
Uncertain implies insufficient published data for a recommendation.
APL indicates acute promyelocytic leukemia; CBF, core binding factor [t(8;21) or Inv(16)]; CN-AML, cytogenetically normal AML; FLT3ITD, FMS-related tyrosine kinase 3–internal tandem duplication; HCT, hematopoietic cell transplantation; mCEBPA, mutated CEBPA; mKIT, KIT mutations; MSD, matched sibling donor; mNPM1, mutated NPM1; MUD, matched unrelated donor; and UCB, umbilical cord blood.
If the data on molecular markers are not available.
Increasing data show that the beneficial effect may be restricted only to patients with double mutations.
Only at experienced centers and in the absence of a timely available MUD.
Carefully selected patients with good performance status and low disease burden; CIBMTR risk score may aid the patient selection.
Therapy-related AML
With the expanding pool of cancer survivors, therapy-related AML (t-AML) is increasingly encountered and constitutes approximately 10%-20% of newly diagnosed patients with AML.78 Conventional chemotherapy is not curative, and HCT is a potential treatment for patients with t-AML. However, the best t-AML candidates for HCT are not well defined. There are no prospective data defining the value of HCT in patients with t-AML to guide treatment decisions.
Outcomes of HCT in patients with t-AML are inferior to published data on de novo AML.79-81 This finding is attributable in part to a high number of t-AML patients with poor leukemia biology, including unfavorable cytogenetics, previous therapy-related myelodysplastic syndrome (t-MDS), and active disease at the time of HCT. However in some reports, when adjusted for disease status and cytogenetics, there was no difference in outcomes of t-AML and de novo AML.82,83 The results of t-MDS/AML from 3 major registry studies from the French Society of Bone Marrow Transplantation,81 EBMT,80 and CIBMTR79 are summarized in Table 4. Several common conclusions can be drawn from these studies. HCT yields encouraging outcomes in younger patients in remission with MSD, well-matched URD (8/8), or partially matched URD (7/8). Poor-risk cytogenetics has a significant adverse impact on relapse. On the basis of 4 prognostic factors for survival (age > 35 years, poor risk cytogenetics, AML not in remission or advanced MDS, and donors other than MSD, well-matched or partially matched URD), the CIBMTR study proposed a prognostic scoring system for t-AML undergoing HCT.79 Five-year survival of patients with score 0, 1, 2, 3, and 4 was 50%, 26%, 21%, 10%, and 4%, respectively. This scoring system may be particularly useful to guide selection of subset of patients likely to benefit from HCT and suggest investigational or palliative approaches for those lacking the favorable features.
Cytogenetic classification is an independent prognostic parameter in patients with t-AML.84 However, when comparable cytogenetic groups were evaluated, the survival of t-AML with favorable cytogenetics was inferior to those with de novo AML. Among the CBF-AML, the outcome of t(8;21) in t-AML appears inferior compared with de novo AML,84,85 whereas the outcomes appear similar for the rare patients with inv(16).84 The outcome of t-APL does not appear different from de novo-APL when treated with an all-trans retinoic acid–containing regimen.86 For t-AML, should patients with rare CBF leukemia or CN-AML with favorable molecular profile be referred for HCT in CR1? Currently, there are no data to determine the prognostic impact of “mNPM1 without FLT3-ITD” or “mCEBPA” in the small subset of t-AML patients in comparison to de novo AML patients.
Given the poor prognosis of t-AML with conventional chemotherapy, HCT should be considered during CR1 for all patients in the transplant age group with suitable donors. HCT can be deferred for t-APL, and possibly inv(16) in CR1, but even these data are limited.
Similar to t-AML, AML evolving from preceding myelodysplastic syndrome or myeloproliferative disorder is recognized as high risk and as such, is not curable with conventional chemotherapy. HCT has promise, but no prospective data directly addresses this topic.
HCT in older patients with AML
Conventional chemotherapy options are not curative in a majority of AML patients ≥ 60 years of age.87 High peritransplantation mortality with myeloablative transplant was assumed to be a major barrier resulting in only limited application of HCT in older patients.88 The introduction of RIC has enabled to overcome the barrier of early TRM, and several authors89-91 have shown the feasibility and reasonable outcomes with RIC in older patients with AML ≥ 60 years using related and URDs. It appears that the likelihood of older patients being referred for HCT is very low in comparison to younger patients.92 Hesitancy of treating physicians, uncertainty regarding outcomes of HCT, lack of comparative data on the outcomes of HCT versus nontransplant treatments in older patients, and insurance coverage are some of the barriers contributing to underuse of HCT in older patients.
In a recent study from CIBMTR, authors evaluated the impact of age in 545 patients with AML in CR1 with age ≥ 40 years undergoing RIC (40-54 years, 201; 55-59 years, 149; 60-64 years, 132; ≥ 65 years, 63).93 In this study, 2-year LFS and OS in all age groups were similar, and no impact of age was observed. Two single-center studies showed no impact of age on posttransplant outcomes in patients ≥ 60 years treated with RIC.94,95 In another multicenter study from the Seattle consortium, the outcomes of 274 patients with AML treated with a nonmyeloablative conditioning included 135 patients with AML ≥ 60 years and no impact of age was observed.96 An important observation was that MSD and well-matched URD transplants led to similar survival.93-96 In view of the aforementioned data, HCT can be considered in patients up to the age 70 years. Very few HCT patients older than 70 years of age have been reported. Therefore, suitably fit patients between the age 60-70 years should be informed about the option of HCT, and donor searches should be initiated promptly after diagnosis to allow this option if a CR is achieved.
Most of the comparisons of allogeneic versus non-allogeneic treatments in AML are reported in younger patients up to the age 60 years; at present, there are no prospective comparisons of allogeneic versus conventional treatments in older patients. Preliminary results of a retrospective case-controlled study from the CIBMTR and CALGB comparing the outcomes of RIC transplantation (n = 100) with conventional chemotherapy (n = 96) in patients ≥ 60 years with AML were recently reported.97 To avoid selection bias for the HCT arm, only patients remaining in CR1 for at least 4 months treated on CALGB trials were included in the chemotherapy arm. The 3-year LFS from CR1 for HCT patients was 32% compared with 15% for chemotherapy-treated patients (P = .006). Although relapse-risk in HCT arm was significantly lower (32% vs 81%, P < .001); TRM was significantly greater (22% vs 3%, P < .001) resulting in marginal difference in OS (HCT, 37% vs chemotherapy, 25%;P = .08). These data indicate the importance of ongoing efforts to improve the results of HCT with a focus to decrease TRM as well as novel strategies to decrease the relapse risk in non-allogeneic treatments. Continued efforts should be made to recruit these patients in to prospective studies comparing HCT versus nonallogeneic treatments.
Intensity of conditioning chemotherapy: myeloablative versus reduced?
The preferred intensity of conditioning therapy for AML patients remains a subject of debate. In the last decade, a plethora of RIC regimens have been developed. The spectrum of intensity of RIC regimens vary from minimal to moderately intense. To facilitate comparisons of intensity of conditioning regimens, members of the CIBMTR has developed guidelines to define these regimens, and on the basis of the expected duration of cytopenia and on the requirement for stem cell support, these regimens are defined as(1) myeloablative conditioning (MAC),(2) RIC, and (3) nonmyeloablative (NMA) conditioning.98
Various studies in which authors compared the outcomes of RIC/NMA conditioning with MAC in AML patients are summarized in Table 5.70,72,79-81,99-105 Results of these retrospective studies are limited by significant differences in patient populations and the analysis may be influenced by selection bias. Patients expected to have a high risk of relapse may be selected for MAC; and patients with advanced age and/or comorbidities may more often be selected for RIC/NMA regimens. Missing data on comorbidities and details of the decision-making process for RIC/NMA are other major limitations. Importantly, all studies have reported similar LFS and OS in patients undergoing MAC and RIC, respectively. Some have reported decreased TRM with RIC in comparison with MAC,70,101,104,106 whereas others have reported similar TRM.99,102,103,105 A comparison of NMA versus RIC versus MAC regimens showed similar outcomes of RIC and MAC, whereas outcomes were inferior for NMA as the result of increased relapse.105 A comparison of RIC versus NMA in patients with AML/MDS suggested better outcomes with RIC.107 Relapse rates between MAC and RIC appear similar in patients with leukemia in CR at the time of HCT72,99,105 ; dose intensity appears particularly important for patients with leukemia not in remission at the time of HCT.70,72 Of importance, none of the studies so far has shown superiority of RIC/NMA to MAC in AML. Therefore, RIC/NMA should only be offered to the patients considered ineligible for MAC. At present, the indications of RIC/NMA are not consistent. Tools such as HCT-specific comorbidity index (HCT-CI),108 pretransplant assessment of mortality score,109 and understanding of interaction of comorbidities, performance status, with age may aid in developing objective criteria for candidacy for RIC/NMA. The best regimen for RIC/NMA is not known, but available data support the use of RIC over NMA regimens for AML.
Multicenter studies in which the authors evaluated prognostic risk factors in patients with t-MDS/AML undergoing HCT
| Study . | Sample size . | Adverse prognostic factors for survival . | Other comments . | ||||
|---|---|---|---|---|---|---|---|
| Age . | Cytogenetics . | Disease status . | Donor type . | Other factors . | |||
| Yakoub-Agha et al, JCO, 200081 (The French Society of BMT) | 70 t-MDS = 31 t-AML = 39 | > 37 y | Not evaluated | Not in CR at HCT | Not evaluated, only few URD | Male Recipient CMV Positive Intensive MAC | All received MAC |
| Kroger et al, Haematologica, 200980 (EBMT) | 461 t-AML = 293 t-MDS = 168 | > 40 y | Abnormal ncytogenetics | Not in CR at HCT | MSD vs URD not significant | – | No difference in MAC vs RIC, based on prognostic factors identified 3 groups with different survival at 3 y: 62%, 33%, and 24% |
| Litzow et al, Blood, 201079 (CIBMTR) | 868 t-AML = 545 t-MDS = 323 | > 35 y | Poor risk cytogenetics* | AML not in remission or advanced MDS | Similar outcomes with MSD, well-matched URD (8/8) or partially matched URD (7/8); worse outcomes with mismatched RD or URD | No difference in MAC vs RIC, based on 4 risk factors generated a risk score. Patients with risk score of 0, 1, 2, 3, and 4 had a 5-year survival of 50%, 26%, 21%, 10%, and 4%, respectively | |
| Study . | Sample size . | Adverse prognostic factors for survival . | Other comments . | ||||
|---|---|---|---|---|---|---|---|
| Age . | Cytogenetics . | Disease status . | Donor type . | Other factors . | |||
| Yakoub-Agha et al, JCO, 200081 (The French Society of BMT) | 70 t-MDS = 31 t-AML = 39 | > 37 y | Not evaluated | Not in CR at HCT | Not evaluated, only few URD | Male Recipient CMV Positive Intensive MAC | All received MAC |
| Kroger et al, Haematologica, 200980 (EBMT) | 461 t-AML = 293 t-MDS = 168 | > 40 y | Abnormal ncytogenetics | Not in CR at HCT | MSD vs URD not significant | – | No difference in MAC vs RIC, based on prognostic factors identified 3 groups with different survival at 3 y: 62%, 33%, and 24% |
| Litzow et al, Blood, 201079 (CIBMTR) | 868 t-AML = 545 t-MDS = 323 | > 35 y | Poor risk cytogenetics* | AML not in remission or advanced MDS | Similar outcomes with MSD, well-matched URD (8/8) or partially matched URD (7/8); worse outcomes with mismatched RD or URD | No difference in MAC vs RIC, based on 4 risk factors generated a risk score. Patients with risk score of 0, 1, 2, 3, and 4 had a 5-year survival of 50%, 26%, 21%, 10%, and 4%, respectively | |
CIBMTR, Center for International Blood and Marrow Transplant Research; CMV, CR, complete remission; EBMT, European Group of Blood and Marrow Transplantation; HCT, allogeneic hematopoietic cell transplantation; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MSD, matched sibling donor; RIC, reduced intensity conditioning; t-MDS/AML, therapy-related MDS/AML; and URD, unrelated donor.
Defined according to Southwest Oncology Group/Eastern Cooperative Oncology Group classification for AML patients and International Prognostic Scoring System classification for MDS patients;
Comparisons of myeloablative and reduced intensity or nonmyeloablative conditioning in adult patients with AML
| Study . | Patient population . | Sample size . | LFS . | OS . | Other comments . |
|---|---|---|---|---|---|
| Aoudjhane et al, 2005104 ; Multicenter, EBMT | AML, > 50 y, MSD only | 722, RIC = 315, MAC = 407 | Similar | Similar | Decreased acute GVHD, chronic GVHD, and TRM, but increased relapse with RIC |
| Alyea et al, 200670 ; single center, Boston | AML/MDS, 21-70 y, MRD and URD donors | 136, (AML, 82) RIC = 39, MAC = 97 | Similar | Similar | Decreased TRM, and increased relapse with RIC |
| Scott et al, 2006102 ; single center, Seattle | MDS/sAML with previous MDS, 40-72 y, MRD and URD | 150, (AML, 55) NMA = 38, MAC = 112 | Similar | Similar | No difference in relapse/TRM |
| Shimoni et al, 200672 ; single center, Tel-Hashomer | AML/MDS, 17-70 y, MRD and URD | 112, (AML, 56) RIC = 67, MAC = 45 | Similar | Similar | Similar outcomes for patients in remission at HCT, inferior outcomes of patients with active disease treated with RIC |
| Flynn et al, 2007103 ; single center, Minnesota | AML/MDS, 19-69 y, MRD and URD (included UCB grafts) | 219, (AML, 160) RIC = 32, MAC = 187 | Similar | Similar | Similar TRM, but increase in relapse with RIC |
| Ringden et al, 2009101 ; EBMT multicenter | AML, 16-76 y, URD transplants only | 1555, RIC = 401, MAC = 1154 | Similar | Not reported | Reduced NRM in ≥50 y, and increased relapse in patients <50 y with RIC. |
| Lim et al, 2010106 ; EBMT, multicenter | MDS/sAML with previous MDS, ≥ 50 y, MRD and URD | 1333, (AML, 334), RIC = 833, MAC = 500 | Not reported | Similar | Increased relapse, and decreased TRM with RIC |
| Khabori et al, 201099 ; single center, Toronto | AML/MDS, 40-60 y, MRD and URD transplants | 101, (AML, 77), RIC = 39, MAC = 62 | Similar | Similar | Poor outcome in patients with high-risk disease biology attributable to higher relapse rate |
| Luger et al, CIBMTR105 , multicenter | AML/MDS, 18-70 y, MRD and URD | 5179, RIC/NMA = 1448, MAC = 3731 | Similar between MAC vs RIC, inferior for NMA | Similar between MAC vs RIC, More relapse with NMA | Late TRM negated any advantage offered by RIC or NMA |
| Study . | Patient population . | Sample size . | LFS . | OS . | Other comments . |
|---|---|---|---|---|---|
| Aoudjhane et al, 2005104 ; Multicenter, EBMT | AML, > 50 y, MSD only | 722, RIC = 315, MAC = 407 | Similar | Similar | Decreased acute GVHD, chronic GVHD, and TRM, but increased relapse with RIC |
| Alyea et al, 200670 ; single center, Boston | AML/MDS, 21-70 y, MRD and URD donors | 136, (AML, 82) RIC = 39, MAC = 97 | Similar | Similar | Decreased TRM, and increased relapse with RIC |
| Scott et al, 2006102 ; single center, Seattle | MDS/sAML with previous MDS, 40-72 y, MRD and URD | 150, (AML, 55) NMA = 38, MAC = 112 | Similar | Similar | No difference in relapse/TRM |
| Shimoni et al, 200672 ; single center, Tel-Hashomer | AML/MDS, 17-70 y, MRD and URD | 112, (AML, 56) RIC = 67, MAC = 45 | Similar | Similar | Similar outcomes for patients in remission at HCT, inferior outcomes of patients with active disease treated with RIC |
| Flynn et al, 2007103 ; single center, Minnesota | AML/MDS, 19-69 y, MRD and URD (included UCB grafts) | 219, (AML, 160) RIC = 32, MAC = 187 | Similar | Similar | Similar TRM, but increase in relapse with RIC |
| Ringden et al, 2009101 ; EBMT multicenter | AML, 16-76 y, URD transplants only | 1555, RIC = 401, MAC = 1154 | Similar | Not reported | Reduced NRM in ≥50 y, and increased relapse in patients <50 y with RIC. |
| Lim et al, 2010106 ; EBMT, multicenter | MDS/sAML with previous MDS, ≥ 50 y, MRD and URD | 1333, (AML, 334), RIC = 833, MAC = 500 | Not reported | Similar | Increased relapse, and decreased TRM with RIC |
| Khabori et al, 201099 ; single center, Toronto | AML/MDS, 40-60 y, MRD and URD transplants | 101, (AML, 77), RIC = 39, MAC = 62 | Similar | Similar | Poor outcome in patients with high-risk disease biology attributable to higher relapse rate |
| Luger et al, CIBMTR105 , multicenter | AML/MDS, 18-70 y, MRD and URD | 5179, RIC/NMA = 1448, MAC = 3731 | Similar between MAC vs RIC, inferior for NMA | Similar between MAC vs RIC, More relapse with NMA | Late TRM negated any advantage offered by RIC or NMA |
CIBMTR indicates Center for International Blood and Marrow Transplant Research; EBMT, European Group for Blood and Marrow Transplantation; GVHD, graft versus host disease; HCT, hematopoietic cell transplantation; LFS, leukemia-free survival; MAC, myeloablative conditioning; MRD, matched related donor; MUD, matched unrelated donor; NMA, nonmyeloablative, NRM, nonrelapse mortality; OS, overall survival; RIC, reduced-intensity conditioning; sAML, secondary AML; TRM, treatment-related mortality; UCB, umbilical cord blood; and URD, unrelated donor.
Current data on application of RIC/NMA regimens in AML lack prospective comparison of allogeneic versus nonallogeneic treatments. The authors of a retrospective study evaluated RIC HCT in comparison with chemotherapy in 95 patients with high-risk AML in CR1.110 When an intention-to-treat approach was used, a “donor” versus “no donor” comparison showed better LFS in the donor group (54% vs 30%, P = .01). These encouraging results further need prospective validation including from studies presently in progress.
Relapse after HCT
Relapse is one of most common cause of failure of HCT for AML and is associated with very poor prognosis.111 The preferred management of relapse after HCT is not known. Conventional treatment options include supportive care, chemotherapy, second HCT using the same or alternative donor, DLI, and cytokines. Substantial selection bias confounds interpretation of the reported literature.
Second HCT after failure of first HCT has limited efficacy in patients with AML.112-116 Available literature suggests that duration of remission after first HCT is the most important prognostic factor.114-116 A reasonable outcome can be expected in selected patients, whose duration of remission after first HCT is ≥ 1 year, and who achieve CR before a second HCT.114-116 DLI has limited efficacy in patients with AML. In 2 prospective studies authors have evaluated the strategy of reduction of disease burden with chemotherapy followed by granulocyte colony-stimulating factor-primed DLI in patients with advanced myeloid malignancies.117,118 This strategy was beneficial to those who were able to achieve CR. Patients with remission lasting > 6 months had a greater likelihood of response. The efficacy of DLI was retrospectively studied in a EBMT study, including 399 patients in first relapse after HCT for AML who received DLI (n = 171), or not (n = 228).119 Clinical benefit of DLI was seen only in a minority particularly with those with a lower tumor burden at relapse (< 35% BM blasts), female sex, favorable cytogenetics, and remission before DLI.
Therefore, a second allogeneic treatment can be considered in selected patients, whose duration of remission after first HCT is > 6 months. Decision-making and the selection of second HCT versus DLI should be individualized on the basis of donor availability and achievement of remission before DLI or second HCT. Recently, low-dose azacytidine has been reported to be of benefit in patients relapsing after HCT in small series, and this strategy may be useful tool for disease debulking as an alternative to chemotherapy before DLI/second HCT.75 More research is needed to understand the biology of relapse to develop novel interventions for this difficult problem.
Summary
The trend of growth of numbers of HCT in adult patients with AML can be expected to continue based on acceptance and availability of URD and UCB donor. The highly specialized nature and resource intensity of HCT will lead to major challenges for manpower and resources. Few data are available for the reliable estimates of the number of transplants for AML and the total number of AML patients for whom transplantation is appropriate. With the expansion of donor registries, and availability of UCB grafts a suitable donor can potentially be found for nearly all patients with AML.46 However, there appears a great discrepancy in the number of newly diagnosed AML patients in the transplant age group and the number of cases reported to the CIBMTR. Volunteer URD searches limit timely availability of graft as patients with acute leukemia may relapse while waiting for transplant.120 In addition, although race and ethnicity may limit URD availability, significant barriers such as age, sex, socioeconomic status, donor registry and center funding plus insurance restrictions, and other unknown barriers for access to HCT still exist.121 Addressing these challenges will be required to harness the full potential of HCT for patients with AML.
Acknowledgment
We acknowledge assistance from the CIBMTR for the data on trends in AML HCT.
Authorship
Contribution: V.G. prepared the initial draft of manuscript; M.ST. and D.J.W. provided further knowledge, insights, and helped in critical review; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Vikas Gupta, MD, FRCP, FRCPath, Blood and Marrow Transplant Program, Princess Margaret Hospital, Suite 5-217, 610 University Ave, Toronto, M5G 2M9, ON, Canada; e-mail: vikas.gupta@uhn.on.ca.