In this issue of Blood, Riegel and colleagues demonstrate that inflammatory stimuli induce the expression of the P2Y6 receptor on the vascular endothelium where it serves to enhance systemic inflammatory responses.1
The vascular endothelium plays an important role in orchestrating inflammatory responses in tissues by regulating the production of chemokines that recruit inflammatory cells, and up-regulating adhesion molecules that promote the transendothelial migration of these cells into tissues. Although the importance of endothelial activation is well appreciated and numerous stimuli have been identified, relatively little is known about the endogenous mediators that fine tune these pathways. Understanding the production and mechanism of action of endogenous mediators that regulate endothelial cell activation could provide novel treatment options for diseases where activation of the vascular endothelium is thought to play a major role, including acute lung injury and sepsis.
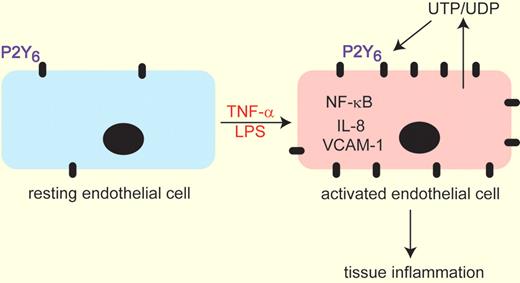
Exposure of resting endothelial cells to TNF-α or LPS leads to increased expression of the P2Y6 purinergic receptor. Simultaneous or subsequent injury to the endothelium promotes the release of UTP/UDP the endogenous ligands of the P2Y6 receptor. These 2 events serve to up-regulate NF-KB signaling leading to increased IL-8 and VCAM-1 expression to collectively enhance tissue inflammation. This model was derived from experiments by Riegel and colleagues1 demonstrating that genetic removal or pharmacologic inhibition of P2Y6 lead to attenuation of these pathways.
Exposure of resting endothelial cells to TNF-α or LPS leads to increased expression of the P2Y6 purinergic receptor. Simultaneous or subsequent injury to the endothelium promotes the release of UTP/UDP the endogenous ligands of the P2Y6 receptor. These 2 events serve to up-regulate NF-KB signaling leading to increased IL-8 and VCAM-1 expression to collectively enhance tissue inflammation. This model was derived from experiments by Riegel and colleagues1 demonstrating that genetic removal or pharmacologic inhibition of P2Y6 lead to attenuation of these pathways.
It is becoming increasingly appreciated that the cellular release of nucleotides after ischemia, hypoxia, or inflammatory stimulation serves to influence cellular and tissue responses to injury.2,3 Once released from cells, nucleotides such as adenosine triphosphate (ATP), uridine triphosphate (UTP), and their metabolites can influence cellular behavior by engaging cell-surface P2 purineric receptors.3 This family of receptors consists of the P2Y receptors that are coupled to G-proteins and the P2X ion channels. These receptors are widely distributed throughout the body and are often elevated in situations of cellular stress or damage.
The P2Y6 receptor has received attention as an important regulator of inflammation in that stimulation of this receptor with its endogenous ligand uridine diphosphate (UDP) can promote the production and release of proinflammatory chemokines.4,5 In the article by Riegel et al1 the laboratories of Holger Eltzschig and Marco Idzko combine their expertise in purinergic signaling and inflammation to demonstrate that the P2Y6 receptor is selectively increased in endothelial cells after inflammatory stimulation in vitro using tumor necrosis factor-α (TNF-α; see figure). There are 8 different P2Y receptors and 7 P2X receptors.3 The authors show that only the P2Y6 receptor is elevated in endothelial cells after TNF-α stimulation; a response that was reproduced in vivo by administration of lipopolysaccharide (LPS). In pursuit of a function for increased P2Y6 expression, the authors demonstrated that exposure of endothelial cells to a selective P2Y6 antagonist could markedly attenuate nuclear factor-κB (NF-κB) activity after TNF-α exposure and could prevent the production of the chemokine interleukin-8 (IL-8) and the cell adhesion molecules VCAM-1 and ICAM-1. These findings suggest that up-regulation of the P2Y6 receptor is part of a pathway for promoting endothelial cell activation after TNF-α exposure.
The authors then examined the functional importance of these in vitro findings in a mouse model of systemic LPS-induced inflammation using P2Y6 knockout mice and a selective P2Y6 antagonist. Their results demonstrated reduction in chemokine and VCAM expression together with diminished tissue injury as indicated by reduced vascular leak. Together, these findings suggest that the P2Y6 receptor plays a role in regulating vascular inflammatory responses in vivo and suggest that P2Y6 antagonist could be useful in the treatment of acute injuries where activation of the endothelium is involved.
The strength of this study clearly lies in the significance of these in vivo findings. It appears that up-regulation of the P2Y6 receptor may be part of an amplification pathway that promotes inflammatory activities in vascular endothelial cells. This could provide attractive new drug targets for systemic inflammatory conditions such as sepsis and inflammatory response syndrome.6 However, relatively little is known about the mechanisms involved. It is not clear how inflammatory stimulation leads to up-regulation of the P2Y6 receptor on endothelial cells, and the downstream signal transduction events associated with the attenuated activation of NF-κB after P2Y6 blockade is not known. Additional studies investigating these mechanisms will be important for understanding how P2Y6 fits into the larger scheme of the cellular injury response, which could in turn provide insight into the involvement of these pathways in other diseases.
It will also be important to keep in mind that extracellular nucleotide signaling is tightly regulated in a temporal and spatial manner. Once released from cells, ATP and UTP can be rapidly dephosphorylated by a series of extracellular enzymes7 and the resulting metabolites can bind other purinergic receptors to elicit either anti- or proinflammatory responses. For example, the breakdown product of ATP, adenosine, elicits anti-inflammatory actions in acute injury conditions by engaging P1 adenosine receptors,8,9 while exhibiting proinflammatory and tissue remodeling properties in chronic disease states.10 An understanding of these discrepancies comes from knowledge of the relative levels of extracellular enzymes of nucleotide metabolism and expression of the various adenosine receptors.10 The current study describes a scenario where P2Y6 signaling is proinflammatory and contributes to tissue injury; however, up-regulation of the P2Y6 receptor is considered tissue protective in the nervous system where it promotes phagocytosis of cellular derbies by microglia.11 Thus, understanding the spatial and temporal expression of the enzymes of extracellular nucleotide catabolism, the relative levels of UDP, and the expression of other P2 receptors on the vascular endothelium will help to further clarify the role of nucleotide signaling in the regulation of vascular inflammation.
In conclusion, this study provides compelling evidence that up-regulation of P2Y6 signaling on endothelial cells plays an important role in the activation of pathways that are important in the regulation of tissue inflammation. These important findings suggest a novel pathway for the amplification of tissue inflammation that could provide novel therapeutic targets.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal