Abstract
Investigation of the effects of rituximab (anti-CD20) on B-cell-activating factor of the tumor necrosis factor family (BAFF) and B cells would better define the significance of B-cell homeostasis in chronic graft-versus-host disease (cGVHD) pathophysiology. We studied 20 cGVHD patients at a median of 25 months after rituximab treatment when most patients had recovered total B-cell numbers. A total of 55% of patients had stable/improved cGVHD, and total B-cell numbers in these patients were significantly higher compared with rituximab-unresponsive patients. Although total B-cell number did not differ significantly between cGVHD groups before rituximab, there was a proportional increase in B-cell precursors in patients who later had stable/improved cGVHD. After rituximab, BAFF levels increased in all patients. Coincident with B-cell recovery in the stable/improved group, BAFF/B-cell ratios and CD27+ B-cell frequencies decreased significantly. The peripheral B-cell pool in stable/improved cGVHD patients was largely composed of naive IgD+ B cells. By contrast, rituximab-unresponsive cGVHD patients had persistent elevation of BAFF and a predominance of circulating B cells possessing an activated BAFF-RLoCD20Lo cell surface phenotype. Thus, naive B-cell reconstitution and decreased BAFF/B-cell ratios were associated with clinical response after rituximab in cGVHD. Our findings begin to delineate B-cell homeostatic mechanisms important for human immune tolerance.
Introduction
Evidence that donor B cells play a role in the development of chronic graft-versus-host disease (cGVHD) in humans has led to several phase 1/2 trials of B cell-directed therapy with rituximab, a monoclonal antibody specific for CD20, in steroid-refractory cGVHD.1,2 Clinical efficacy of rituximab has provided compelling evidence that B cells play an important role in human cGVHD, but the mechanisms that promote and sustain B-cell involvement remain poorly studied. The durability of clinical responses to rituximab in patients with cGVHD also remains unclear.1,2 In patients with autoimmune diseases, initial clinical responses to rituximab are inevitably followed by clinical relapse in the majority of patients. Because increased plasma B-cell-activating factor of the tumor necrosis factor family (BAFF) levels are found in patients with autoimmune disease after rituximab treatment, concern has been raised that high BAFF in this setting contributes to clinical relapse in these patients.3-6 Achievement or degree of B lymphopenia after rituximab does not appear to correlate with efficacy of this agent.3 Variable B-cell recovery was previously found in patients treated with rituximab for autoimmune diseases.7-10 In addition, increased frequencies of memory and post-germinal center (GC) plasmablast-like cells after rituximab may be associated with relapse in patients with autoimmune diseases.7,8,11 Thus, although clinical responses to rituximab are compelling, inefficient elimination of potentially autoreactive B cells in a postrituximab, BAFF-enriched environment has been hypothesized.3,6,10,12
Altered B-cell homeostasis leads to the disruption of the BAFF tolerance checkpoint and an autoimmune phenotype in murine models, but this mechanism of B-cell tolerance has not yet been fully elucidated in humans.13,14 Study of patients who undergo allogeneic hematopoietic stem cell transplantation (HSCT) and then develop the autoimmune manifestations found in cGVHD represent a unique opportunity to examine human B-cell reconstitution during constant exposure to alloantigens and neoautoantigens. Patients who develop cGVHD after allogeneic HSCT do not regain B-cell homeostasis.15,16 In a previous study, we found that, despite normal B-cell numbers, cGVHD patients had high BAFF/B-cell ratios and circulating activated CD27+ B-cell populations.16 The patients who did not develop cGVHD after HSCT had supranormal numbers of naive B cells and a proportional increase in the most recent bone marrow emigrant (transitional) B-cell populations before cGVHD development. To evaluate the potential importance of the peripheral B-cell pool composition in human B-cell tolerance, we characterized 20 patients with cGVHD who had been B-cell depleted with rituximab. We found that patients with stable/improved cGVHD had recovery of a naive B-cell pool associated with significantly decreased BAFF/B-cell ratios. Measurable autoantibody responses in these patients were also decreased relative to the rituximab-unresponsive cGVHD group. Taken together, our data suggest that recovery of the B-cell compartment is required for cGVHD improvement after rituximab therapy.
Methods
Patients
BAFF and B-cell subset analyses were performed on all samples available from cGVHD patients who had received rituximab treatment approximately 2 years before analysis on clinical protocol at Dana-Farber Cancer Institute (Table 1). All patient samples were collected after written informed consent was obtained according to the Declaration of Helsinki with approval by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center. Of the 20 patients reported in the current study, clinical outcome at one year after rituximab in 15 patients has been previously reported by Cutler et al.2 Of the 21 patients reported in that phase 1/2 trial, 6 had hematologic malignancy relapse or died or had no fresh whole blood sample available for flow cytometry 2 years after receipt of the first rituximab dose and were not included in the current report. In addition to the 15 patients previously reported and available patients, 5 previously unstudied cGVHD patients who had received rituximab on protocol 2 years before sample collection were included in the present analysis. The patients were grouped by a clinician blinded to the laboratory information, according to their clinical status (stable/improved or rituximab-unresponsive disease) a median of 25 months after the first rituximab dose. Patient groups did not differ in terms of the total doses of rituximab received or type of active immune-suppressive agents given as assessed on day of sample analysis.
Characteristics of patients with cGVHD 2 years after rituximab
| . | Stable/improved (n = 11) . | Unresponsive (n = 9) . | P . |
|---|---|---|---|
| Age, y (range) | 39 (20-61) | 41 (30-58) | .45 |
| Sex | |||
| Female | 5 (45) | 5 (56) | > .99 |
| Male | 6 (55) | 4 (44) | |
| Disease | |||
| ALL | 0 (0) | 1 (11) | |
| AML | 3 (27) | 3 (33) | |
| CLL | 1 (9) | 0 (0) | |
| CML | 4 (36) | 4 (44) | |
| MDS | 3 (27) | 0 (0) | |
| NHL | 0 (0) | 1 (11) | |
| Type of transplant | |||
| RIC | 2 (18) | 3 (33) | .62 |
| STD | 9 (82) | 6 (67) | |
| Cell source | |||
| BM | 3 (27) | 3 (33) | > .99 |
| PBSC | 8 (73) | 6 (67) | |
| T cell-depleted | 3 (27) | 3 (33) | > .99 |
| Non–T cell-depleted | 8 (73) | 6 (67) | |
| No. of rituximab doses | |||
| 4 | 2 (18) | 3 (33) | .44 |
| 6 | 0 (0) | 1 (11) | |
| 8 | 9 (82) | 5 (56) | |
| Steroids | |||
| < 30 mg | 11 (100) | 6 (67) | .07 |
| ≥ 30 mg | 0 (0) | 3 (33) | |
| Thalidomide | 0 (0) | 1 (11) | .45 |
| Sirolimus | 4 (36) | 4 (44) | .17 |
| Tacrolimus | 4 (36) | 3 (33) | .26 |
| MMF | 0 (0) | 3 (33) | .07 |
| ECP | 2 (18) | 4 (44) | .34 |
| Grade II-IV aGVHD | 5 (45) | 2 (22) | .37 |
| Time from post-HSCT to onset of cGVHD (range) | 9.5 (6-60) | 10.0 (4-39) | .97 |
| Time from post-HSCT to date of sample (range) | 10.0 (4-39) | 9.0 (6-60) | .91 |
| cGVHD type | |||
| Sclerodermatous | 5 (45) | 8 (89) | .07 |
| Lichenoid | 1 (9) | 2 (22) | .57 |
| Mouth | 3 (27) | 5 (56) | .36 |
| Lung | 1 (9) | 0 (0) | > .99 |
| Liver | 5 (45) | 2 (22) | .37 |
| Marrow | 1 (9) | 0 (0) | > .99 |
| Muscle | 3 (27) | 0 (0) | .22 |
| Eye | 2 (18) | 1 (11) | > .99 |
| . | Stable/improved (n = 11) . | Unresponsive (n = 9) . | P . |
|---|---|---|---|
| Age, y (range) | 39 (20-61) | 41 (30-58) | .45 |
| Sex | |||
| Female | 5 (45) | 5 (56) | > .99 |
| Male | 6 (55) | 4 (44) | |
| Disease | |||
| ALL | 0 (0) | 1 (11) | |
| AML | 3 (27) | 3 (33) | |
| CLL | 1 (9) | 0 (0) | |
| CML | 4 (36) | 4 (44) | |
| MDS | 3 (27) | 0 (0) | |
| NHL | 0 (0) | 1 (11) | |
| Type of transplant | |||
| RIC | 2 (18) | 3 (33) | .62 |
| STD | 9 (82) | 6 (67) | |
| Cell source | |||
| BM | 3 (27) | 3 (33) | > .99 |
| PBSC | 8 (73) | 6 (67) | |
| T cell-depleted | 3 (27) | 3 (33) | > .99 |
| Non–T cell-depleted | 8 (73) | 6 (67) | |
| No. of rituximab doses | |||
| 4 | 2 (18) | 3 (33) | .44 |
| 6 | 0 (0) | 1 (11) | |
| 8 | 9 (82) | 5 (56) | |
| Steroids | |||
| < 30 mg | 11 (100) | 6 (67) | .07 |
| ≥ 30 mg | 0 (0) | 3 (33) | |
| Thalidomide | 0 (0) | 1 (11) | .45 |
| Sirolimus | 4 (36) | 4 (44) | .17 |
| Tacrolimus | 4 (36) | 3 (33) | .26 |
| MMF | 0 (0) | 3 (33) | .07 |
| ECP | 2 (18) | 4 (44) | .34 |
| Grade II-IV aGVHD | 5 (45) | 2 (22) | .37 |
| Time from post-HSCT to onset of cGVHD (range) | 9.5 (6-60) | 10.0 (4-39) | .97 |
| Time from post-HSCT to date of sample (range) | 10.0 (4-39) | 9.0 (6-60) | .91 |
| cGVHD type | |||
| Sclerodermatous | 5 (45) | 8 (89) | .07 |
| Lichenoid | 1 (9) | 2 (22) | .57 |
| Mouth | 3 (27) | 5 (56) | .36 |
| Lung | 1 (9) | 0 (0) | > .99 |
| Liver | 5 (45) | 2 (22) | .37 |
| Marrow | 1 (9) | 0 (0) | > .99 |
| Muscle | 3 (27) | 0 (0) | .22 |
| Eye | 2 (18) | 1 (11) | > .99 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; RIC, reduced-intensity conditioning; STD, standard; BM, bone marrow; PBSC, peripheral blood stem cell; MMF, mycophenolate mofetil; and ECP, extracorporeal photopheresis.
Processing of patient plasma and peripheral blood cells
Whole blood was drawn into standard ethylenediaminetetraacetic acid-containing collection tubes. Plasma was separated from whole blood cells by centrifugation at 600g. Plasma was stored in aliquots at −80°C and used after first thaw for BAFF measurements. Whole blood for flow cytometry studies was also collected on the day of use in ethylenediaminetetraacetic acid-containing tubes.
BAFF ELISA
Soluble BAFF in patient plasma samples was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) and the manufacturer's recommended procedures (R&D Systems).
Antibodies
Antibodies used for flow cytometry were as follows: CD19 ECD or CD19 PC7 (both clone J4.119), CD20 ECD (clone B9E9), CD38 phycoerythrin (PE; clone LS198), CD27 PCD5 (1A4), and CD80 PE (MAB104) (Beckman Coulter). IgD fluorescein isothiocyanate (clone IA6-2) and IgM PE-Cy5 (G20-127) were both from BD Biosciences PharMingen; BR3 PE (clone 8A7) was from eBioscience.
Flow cytometric analysis of peripheral B cells
Whole blood or viable frozen Ficoll peripheral blood mononuclear cells were processed for flow cytometry using the Prep Plus 2 system (Beckman Coulter). Lymphocytes were gated by size using forward and side scatter criteria. A total of 50 000 lymphocytes were collected for all samples to ensure adequate total numbers of B cells for subset analysis. Peripheral B-cell subsets (IgD+CD38lo “naive” and IgD+ mem, IgD+CD38hi “transitional” and pre-GC vs the IgD−CD38Lo “post-GC mem,” and IgD−CD38Hi PB populations) were delineated as follows. CD19+ expression was analyzed to exclude CD3+ cells from the analysis of CD38 or CD27 expression (not shown). Gates positive for CD19, IgD, CD38, and CD27 were first set according to isotype controls and healthy control staining with each marker as previously described.16 CD27+ B-cell subsets analyzed included IgDLo post-GC memory and “plasmablast-like” (PB/PC) populations. CD27+ IgD+ B-cell populations include “IgD+ Memory” V region-mutated memory B cells. In addition, the presence of CD27 on IgD+ CD38Hi expressing B cells allows distinction of human peripheral transitional B cells from pre-GC B cells. Pre-GC cells (also called BM2′, GC founder, or preplasmablast cells) are IgD+CD38HiCD27+. Anti-CD19 PC7 (Beckman Coulter) was used per the manufacturer's instructions. BR3 fluorescein isothiocyanate (clone 8A7; R&D Systems) was used to detect surface expression of BAFF-receptor (BAFF-R). Red blood cells were lysed and leukocytes were fixed using the Beckman Coulter TQPrep system before fluorescence-activated cell sorter. Cells were analyzed using a Cytomics FC 500 instrument and CXP Analysis, Version 2.0 software (Beckman Coulter).
Measurement of Ro-52 autoantibodies and Epstein-Barr virus–specific antibodies
The 96-well ELISA plates (NUNC Scientific) were coated with either Ro-52 protein (ProSpec-Tany Technogene) or EBNA-1 (Advanced Biotechnologies) at 0.125 μg/well and 0.25 μg/well, respectively, in carbonate binding buffer (15mM Na2CO3, 30mM NaHCO3, 0.02% NaN3, pH 9.6) overnight at 4°C. Wells were washed with phosphate-buffered saline with 0.05% Tween 20 (PBST, pH 7.2) 5 times before blocking with 2% nonfat milk in PBST for 2 hours at 4°C. After washing once with PBST, 50 μL of patient plasma was added at a 1:50 dilution in 2% nonfat milk/PBST overnight at 4°C. Plates were washed 5 times with PBST and once with PBS before addition of 1:1000 diluted goat-antihuman IgG alkaline phosphatase (Pierce Chemical) for 1 hour at room temperature. Wells were washed with PBST 5 times and once with PBS before adding 75 μL of Tris-para-nitrophenyl phosphatase and developing for 25 minutes. Absorbance was measured at 405 nm.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics. The Wilcoxon rank-sum test and Fisher exact test were used for 2-sample comparisons. The Wilcoxon signed rank test was used for paired sample test. Spearman rank correlation coefficient was used for correlation analysis. All tests were 2-sided.
Results
Recovery of naive B cells occurs in patients with stable/improved cGVHD after rituximab
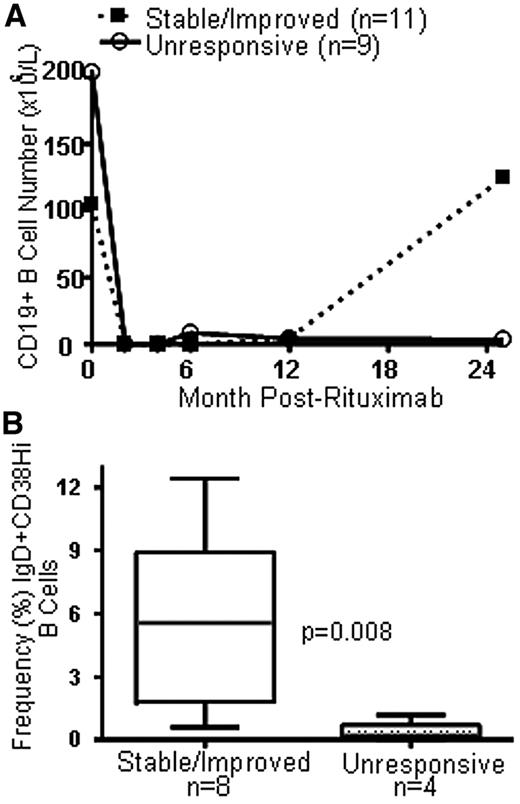
Previous studies in the first year after HSCT showed that patients with persistently elevated BAFF levels and poor naive B-cell reconstitution were more likely to develop cGVHD. We studied a group of cGVHD patients treated with rituximab after the majority of patients had recovered B-cell numbers. The patients were characterized according to clinical severity of cGVHD: 9 patients had clinically progressed and 11 patients had stable or improved cGVHD. As shown in Table 1, other patient characteristics were not significantly different between the 2 groups. Figure 1A shows the total B-cell number over time before and after rituximab treatment in the 2 groups. Median CD19+ B-cell number did not significantly differ immediately before rituximab treatment (104.5 × 106/L vs 203.7 × 106/L, P = .60, in the stable/improved vs the rituximab-unresponsive group). All patients had B-cell depletion to less than 0.3% B cells of total lymphocytes from the second to the 12th month (as previously reported by Cutler et al2 for the majority of these patients).
B-cell reconstitution in patients with stable/improved cGVHD after rituximab. (A) Serial measurement of total CD19+ B-cell number in patients with cGVHD who received rituximab at time 0 in 2 cGVHD groups (stable/improved = patients who required no further treatment 2 years after rituximab vs unresponsive = patients who required additional treatment 2 years after rituximab treatment for active cGVHD). (B) Comparison of the relative frequency of IgD+CD38Hi “transitional” B cells before rituximab in the 2 cGVHD groups.
B-cell reconstitution in patients with stable/improved cGVHD after rituximab. (A) Serial measurement of total CD19+ B-cell number in patients with cGVHD who received rituximab at time 0 in 2 cGVHD groups (stable/improved = patients who required no further treatment 2 years after rituximab vs unresponsive = patients who required additional treatment 2 years after rituximab treatment for active cGVHD). (B) Comparison of the relative frequency of IgD+CD38Hi “transitional” B cells before rituximab in the 2 cGVHD groups.
The median number of B cells in the majority of patients reached the prerituximab number by approximately 2 years (a median of 25 months) after the first dose of rituximab. At this time, 11 patients had stable/improved cGVHD group with a significantly higher median total B-cell number of 124.7 × 106/L versus 3.8 × 106/L in the rituximab-unresponsive cGVHD group (P = .002, Figure 1A). The notable persistent profound B lymphopenia after rituximab in the unresponsive cGVHD group (median total B-cell number) was not attributable to immunosuppressive agents at 2 years after rituximab alone because patients in both groups were receiving immune-suppressive agents. Table 1 shows the immune-suppressive agents taken by each cGVHD group 2 years after rituximab. Total lymphocyte and T-cell numbers were similar at one year in the 2 groups, but rituximab-unresponsive cGVHD patients had a decreased median number of both total lymphocyte and total T cells 2 years after rituximab (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), with median counts below expected in the post-HSCT setting.17 Importantly, immune-suppressive therapy, such as extracorporeal photopheresis therapy, did not appear to result in lower proportions of B-cell numbers because the proportion of B cells was at least as high in patients receiving as it was in those not receiving extracorporeal photopheresis (supplemental Figure 1). Thus, rituximab resulted in profound B-cell depletion in all patients, but this effect was transient in patients with stable/improved cGVHD.
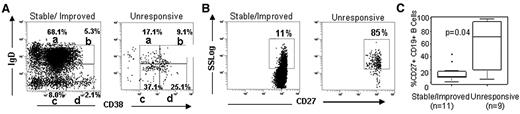
Because we previously found that patients who never developed cGVHD had a significantly higher frequency of IgD+CD38Hi transitional B cells compared with patients who later developed disease after HSCT, the relative composition of peripheral B-cell pool preceding this time point was determined as previously described16 in all available prerituximab and 12-month post-rituximab banked samples. As shown in Figure 1B, patients with rituximab-unresponsive cGVHD at 25 months after rituximab had a median prerituximab transitional cell frequency of 0.2% (range, 0.0%-1.2%), whereas patients who later had stable/improved cGVHD had a median of 5.6% (range, 0.6%-12.4%). No other IgD versus CD38 stained B-cell subset was significantly different between the 2 groups before rituximab or at 12 months after rituximab. In most stable/improved patients at 25 months after rituximab, we found a pattern of IgD versus CD38 staining typical of a healthy person (representative example, Figure 2A), with a predominance of IgD+ cells16,18 Thus, increased numbers of B cells in the stable/improved cGVHD group were probably attributable to increased B-cell reconstitution.
CD27+ B cells predominate in the cGVHD group with progressive disease 2 years after rituximab. (A) Representative flow cytometric profile of B-cell subsets characterized by relative IgD versus CD38 staining in a patient with stable/improved cGVHD versus unresponsive cGVHD 2 years after rituximab. (B) Box plots showing the differences in frequencies of B-cell subsets a and b between the stable/improved and rituximab-unresponsive cGVHD groups. (C) Representative CD27 flow cytometric analysis of CD27 positivity of gated CD19+ B cells.
CD27+ B cells predominate in the cGVHD group with progressive disease 2 years after rituximab. (A) Representative flow cytometric profile of B-cell subsets characterized by relative IgD versus CD38 staining in a patient with stable/improved cGVHD versus unresponsive cGVHD 2 years after rituximab. (B) Box plots showing the differences in frequencies of B-cell subsets a and b between the stable/improved and rituximab-unresponsive cGVHD groups. (C) Representative CD27 flow cytometric analysis of CD27 positivity of gated CD19+ B cells.
In contrast, patients with rituximab-unresponsive cGVHD, who all had low numbers of B cells, had a distinctly different pattern of IgD versus CD38. Table 2 shows both numbers and frequency of the B-cell subsets measured. We previously demonstrated that CD27+ B cells in cGVHD were in vivo activated compared with CD27+ from healthy persons.16 BAFF is known to preferentially promote CD27+ B-cell survival.19 Strikingly, the B-cell pool in the patients with rituximab-unresponsive cGVHD was predominantly CD27+. Figure 2B depicts a representative example of CD27+ staining on B cells between these 2 patient groups. CD27+ staining was specific, revealing positive and negative subsets within each of the IgD and CD38+ subsets identified (supplemental Figure 2). The median frequency of total CD27+ B cells was significantly higher in unresponsive disease at 70.9% (range, 1%-44.2%) versus 9% (range, 4.6%-99%, P = .04; Figure 2C). Taken together, these data suggest that more robust recovery of B cells is associated with stable/improved cGVHD after induction of B lymphopenia with rituximab. Thus, in the absence of naive B cells, CD27+ B cells are potentially promoted in patients with rituximab-resistant cGVHD.
Total number and frequency of IgD versus CD38 B-cell subsets in patients with cGVHD 2 years after rituximab
| . | Stable/improved cGVHD (n = 11) . | Unresponsive cGVHD (n = 9) . | P . |
|---|---|---|---|
| Total no. of B-cell subsets, × 106/L | |||
| Naive (IgD+CD38Lo) | 28.28 (2.2-399.6) | 0.57 (0.1-45.4) | .002 |
| Transitional (IgD+CD38++) | 5.49 (0.7-138.5) | 0.23 (0.0-17.2) | .002 |
| Memory (IgDLoCD38Lo) | 27.02 (0.9-345.3) | 1.2 (0.76-7.7) | .005 |
| Plasmablast (IgD+CD38++) | 9.11 (0.9-31.9) | 0.36 (0.1-4.8) | .002 |
| Frequency of B-cell subsets, % | |||
| Naive (IgD+CD38Lo) | 39.8 (5.9-68.3) | 14.8 (2.2-73.5) | .13 |
| Transitional (IgD+CD38++) | 11.7 (0.5-35.9) | 6.6 (0.0-19.3) | .32 |
| Memory (IgDLoCD38Lo) | 12.9 (8.9-75.1) | 31.5 (8.4-61.3) | .08 |
| Plasmablast (IgD+CD38++) | 5.8 (2.6-18.3) | 18.7 (1.0-37.2) | .20 |
| . | Stable/improved cGVHD (n = 11) . | Unresponsive cGVHD (n = 9) . | P . |
|---|---|---|---|
| Total no. of B-cell subsets, × 106/L | |||
| Naive (IgD+CD38Lo) | 28.28 (2.2-399.6) | 0.57 (0.1-45.4) | .002 |
| Transitional (IgD+CD38++) | 5.49 (0.7-138.5) | 0.23 (0.0-17.2) | .002 |
| Memory (IgDLoCD38Lo) | 27.02 (0.9-345.3) | 1.2 (0.76-7.7) | .005 |
| Plasmablast (IgD+CD38++) | 9.11 (0.9-31.9) | 0.36 (0.1-4.8) | .002 |
| Frequency of B-cell subsets, % | |||
| Naive (IgD+CD38Lo) | 39.8 (5.9-68.3) | 14.8 (2.2-73.5) | .13 |
| Transitional (IgD+CD38++) | 11.7 (0.5-35.9) | 6.6 (0.0-19.3) | .32 |
| Memory (IgDLoCD38Lo) | 12.9 (8.9-75.1) | 31.5 (8.4-61.3) | .08 |
| Plasmablast (IgD+CD38++) | 5.8 (2.6-18.3) | 18.7 (1.0-37.2) | .20 |
BAFF/B-cell ratios and activated B cells decrease after rituximab in stable/improved cGVHD
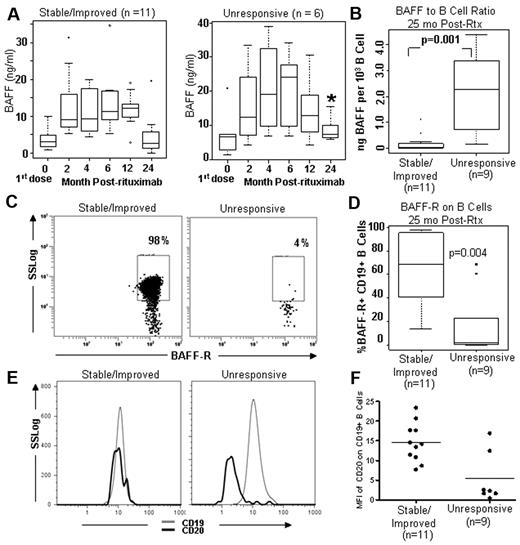
Because BAFF is also known to increase after rituximab in patients with autoimmune diseases, and altered B-cell homeostasis leading to excess BAFF per potentially autoreactive B-cell may contribute to cGVHD pathophysiology,16 we examined serial BAFF levels before and after rituximab in our 2 patient populations. As shown in Figure 3A, the distribution of BAFF levels before rituximab was similar, with the median values not significantly different between the 2 cGVHD groups (median, BAFF 2.94 ng/mL and 7.02 ng/mL in the subsequently stable/improved and rituximab-unresponsive groups, respectively, P = .17). Patients in both groups had increased BAFF after B-cell depletion in the first 12 months with a peak at 6 months after rituximab treatment, but the BAFF levels were different throughout the 2-year period. Thus, rituximab resulted in profound B-cell depletion in all patients and all patients had high BAFF levels for 12 months after rituximab. The BAFF levels were significantly different between the 2 groups at 25 months after rituximab when the unresponsive group had a BAFF level of 7.28 ng/mL compared with 2.58 ng/mL (P = .02) in the stable/improved group. The BAFF/B-cell ratio was significantly higher in patients with unresponsive cGVHD 2 years after rituximab, 2.28 × 10−3 versus 3.00 × 10−5 in the stable/improved group (Figure 3B). This represented a significant increase in the BAFF/B ratio from the prerituximab cGVHD ratio and over and above the previously reported cGVHD group not treated with rituximab.16 The BAFF/B-cell ratio before rituximab in the 2 groups revealed no significant difference, 4.49 × 10−5 versus 6.4 × 10−5 in the post-rituximab stable/improved and the unresponsive disease groups, respectively (data not shown). Those patients who did not progress maintained the same BAFF/B ratio before and after rituximab (data not shown). The results at 25 months after HSCT were unlikely affected by immune suppression treatment between the 2 groups because, as shown in Table 1, both groups received comparable immune-suppressive therapy at the time of this analysis.
BAFF/B-cell ratio and BAFF-R detection after rituximab in cGVHD. (A) Serial BAFF measurements before and after rituximab in patients with stable/improved cGVHD compared with unresponsive cGVHD 2 years after rituximab. (B) Comparison of BAFF/B ratios in patients at 2 years with stable/improved disease or unresponsive cGVHD. (C) Representative flow cytometry showing BAFF-R expression on CD19+ B cells in stable/improved cGVHD versus unresponsive cGVHD. (D) Frequency of BAFF-R+ CD19+ B cells in the 2 groups. (E) Representative flow cytometric analysis showing that CD20 expression on CD19+ gated B cells is low in progressive cGVHD after rituximab. (F) Comparison of cell surface expression of CD20 (in MFI) between patients with stable/improved versus unresponsive cGVHD after rituximab.
BAFF/B-cell ratio and BAFF-R detection after rituximab in cGVHD. (A) Serial BAFF measurements before and after rituximab in patients with stable/improved cGVHD compared with unresponsive cGVHD 2 years after rituximab. (B) Comparison of BAFF/B ratios in patients at 2 years with stable/improved disease or unresponsive cGVHD. (C) Representative flow cytometry showing BAFF-R expression on CD19+ B cells in stable/improved cGVHD versus unresponsive cGVHD. (D) Frequency of BAFF-R+ CD19+ B cells in the 2 groups. (E) Representative flow cytometric analysis showing that CD20 expression on CD19+ gated B cells is low in progressive cGVHD after rituximab. (F) Comparison of cell surface expression of CD20 (in MFI) between patients with stable/improved versus unresponsive cGVHD after rituximab.
Other investigators have shown that BAFF results in B-cell activation and Ig secretion, and these cells have low BAFF-R and low CD20 expression.20 Decreased BAFF-R has been previously shown by others to be the result of occupancy of BAFF-R by soluble BAFF.21 To determine whether high levels of soluble BAFF affected B cells in cGVHD after rituximab, we measured BAFF-R on the surface of CD19+ B cells in both cGVHD patient groups 25 months after rituximab (Figure 3C). As shown in Figure 3D, the median frequency of BAFF-R+ CD19+ B cells was 2.2% versus 68.4% in the post-rituximab-unresponsive and stable/improved groups, respectively. The median fluorescence intensity (MFI) of BAFF-R expression was lower in the rituximab-resistant disease group compared with the stable/improved group (0.62 vs 1.67 MFI, respectively, data not shown). Corroborating that BAFF occupancy of BAFF-R accounted for low BAFF-R detection by antibody on CD19+ B cells in cGVHD, we assessed whether the addition of increasing doses of rBAFF to purified CD19+ B cells resulted in decreased BAFF-R detection. Incubation of whole blood peripheral blood mononuclear cells from a healthy person with increasing concentrations of recombinant BAFF (rBAFF) resulted in decreased BAFF-R detection by flow cytometry (supplemental Figure 2A).21 Because BAFF-R detection is low on cGVHD B cells,22 we also examined purified CD19+ B cells from cGVHD patients (n = 3, representative example shown in supplemental Figure 2B). After ex vivo incubation in excess volume of cell culture medium, but not in small volume of culture medium, resulted in increased detection of BAFF-R on the surface of B cells, suggesting that release of soluble BAFF from cGVHD B cells occurred. Low CD20 expression is found on plasma cells and plasmablasts and on B cells after in vitro activation by soluble BAFF. A representative flow example of relative CD20 expression (by MFI measurement) on gated CD19+ B cells is shown in Figure 3E. cGVHD patients who did not improve after rituximab had significantly lower cell surface CD20 on B cells compared with stable/improved cGVHD patients (median CD20 MFI: 14.5 in the unresponsive cGVHD group vs 5.4 in stable/improved disease group, P = .015, Figure 3F). Thus, the peripheral B-cell pool in patients with stable/improved cGVHD did not have a circulating B-cell pool predominantly composed of cells with an activated BAFF-RLoCD20Lo cell surface plasmablast-like phenotype.
One potential hypothesis for the reduced activity of rituximab in a subset of patients after therapy is changes in the expression of CD20 on the surface of B cells. Cell surface expression of CD20 of the total B-cell populations did not differ between groups before rituximab (data not shown). We previously examined peripheral B-cell pool composition in a cohort of patients and healthy persons. Because levels of CD20 on B-cell surface probably determine B-cell susceptibility to rituximab,23 we examined the CD27+ B-cell subsets for relative CD20 expression on IgD+ B cells subsets compared with the IgDLo populations. We found lower CD20 cell surface expression on IgDLo CD27+ B-cell subsets (supplemental Figure 4). In addition, IgDLoCD27+ B-cell subsets from healthy persons did not have the same level of CD20 cell surface expression decrease (data not shown). CD27− naive populations had higher CD20 expression, suggesting that these cells would be readily eliminated by rituximab, whereas activated IgDLoCD27+ B cells might be unaffected.
Decreased autoantibody responses before and after rituximab in stable/improved compared with unresponsive cGVHD
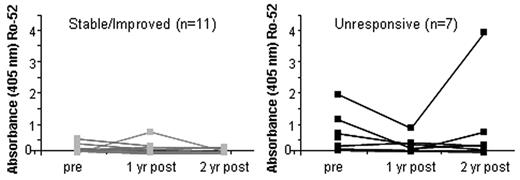
Next, we determined whether autoantibody production coincided with the presence of increased circulating activated B cells. Antibodies to the autoantigen complex Ro/SSA have been associated with shorter clinical responses to rituximab in patients with systemic lupus erythematosus (SLE).5 Although it has been difficult to assess autoantibody responses in cGVHD patients, we recently identified increased frequency of antibodies to Ro-52 (the recombinant 52-kDa Ro protein component of the ribonuclear protein complex Ro/SSA) antibodies in cGVHD.24 We examined anti-Ro-52 antibody responses using ELISA in the 2 rituximab-treated cGVHD patient groups. We found that the unresponsive cGVHD group had increased levels of anti–Ro-52 IgG both before rituximab and after rituximab (Figure 4). Anti–Ro-52 antibodies were present in higher amounts before rituximab in patients who later had unresponsive cGVHD 2 years after rituximab (n = 7). Although the average antibody response in Ro-52-positive patients decreased in all patients with cGVHD by 12 months and increased in the unresponsive cGVHD group, the anti-EBNA1 (Epstein-Barr virus) antibody response was not different between cGVHD groups before or after rituximab (data not shown). Interestingly, the anti-Ro-52 antibodies diminished after rituximab in these patients, but in at least one of the 4 patients who had prerituximab anti-Ro-52 antibodies, these autoantibody levels robustly returned after rituximab (Figure 4). Thus, autoantibody increase was coincident with clinical worsening of disease and increased circulating CD27+ B cells, whereas none of the 11 stable/improved cGVHD patients had detectable autoantibodies.
Autoantibody production in cGVHD patients before and after rituximab treatment. (A) Autoantibody (anti-Ro52) levels measured by direct binding ELISA in cGVHD patients before and after rituximab treatment. Patients are grouped by clinical status of cGVHD at 2 years after rituximab. (B) Average OD reading in an ELISA detecting anti-Ro-52 IgG in the cGVHD group with stable/improved compared with progressive disease before rituximab by ELISA. Average ELISA OD reading in an ELISA assessing IgG reactivity to Epstein-Barr virus protein (EBNA) in patients over time after rituximab treatment was not different between groups (data not shown).
Autoantibody production in cGVHD patients before and after rituximab treatment. (A) Autoantibody (anti-Ro52) levels measured by direct binding ELISA in cGVHD patients before and after rituximab treatment. Patients are grouped by clinical status of cGVHD at 2 years after rituximab. (B) Average OD reading in an ELISA detecting anti-Ro-52 IgG in the cGVHD group with stable/improved compared with progressive disease before rituximab by ELISA. Average ELISA OD reading in an ELISA assessing IgG reactivity to Epstein-Barr virus protein (EBNA) in patients over time after rituximab treatment was not different between groups (data not shown).
Discussion
Evaluation of cGVHD patients after specific B-cell elimination with rituximab allowed us to assess the effect of B-cell reconstitution on human cGVHD pathogenesis. Having previously shown that patients after allogeneic HSCT who do not develop cGVHD have supranormal numbers of naive B cells,16,25 we now demonstrate long-term clinical efficacy of rituximab in cGVHD patients who recover naive B cells (Figure 1). Our current findings are consistent with clinical responses to rituximab reported in patients with rheumatoid arthritis, SLE, and mixed cryoglobulinemia who recovered B-cell homeostasis.7,26-28 The patterns of B-cell homeostasis after rituximab treatment appear to recapitulate the events of reconstitution with donor B cells in the first year after HSCT.16 After rituximab, decreasing BAFF/B-cell ratios, along with a relative decrease in CD27+ activated B cells, were found in patients with stable/improved cGVHD. Because nonautoreactive B cells have been shown in murine models to outcompete autoreactive B cells for available soluble BAFF,14,29 our findings in cGVHD patients suggest that autoreactive B cells are deleted if naive B cells are present or provided. Taken together, our corroborating data suggest an important role for B-cell recovery both in human B-cell tolerance and avoidance of cGVHD.
Increased soluble BAFF levels have previously been found in patients with autoimmune disease after rituximab treatment, raising concern that high BAFF in this setting contributes to clinical relapse in these patients.3-6 We now demonstrate that altered B-cell homeostasis and excess BAFF are relevant for rituximab response in cGVHD. Significantly increased BAFF/B-cell ratios were found in rituximab-resistant cGVHD patients compared with patients who had stable/improved cGVHD 2 years after treatment. The decreased measurable BAFF after B-cell recovery in our rituximab-responsive cGVHD patients (Figure 3) was probably in part the result of BAFF-R occupancy on B cells by soluble BAFF (supplemental Figure 3).21 By contrast, the patients with rituximab-unresponsive cGVHD had a paucity of naive IgD+ B cells and significantly higher BAFF/B-cell ratios. In the current manuscript, we link high BAFF/B ratios after rituximab to perpetuation of CD27+ post-GC B cells in rituximab refractory patients.
In rituximab-unresponsive cGVHD, the B-cell pool had a distinct post-GC IgD versus CD38 cell surface expression profile found in rituximab-unresponsive SLE patients (Table 2; Figure 2A).8 A CD38+ post-GC IgD− population corresponds to a “plasmablast (PB)-like” cell capable of spontaneous IgG production,17 whereas the CD38−IgD− population corresponds to a post-GC memory cell that requires BAFF for survival and further differentation into PB-like cells.19 After rituximab treatment in patients with autoimmune disease, mature PB-like post-GC B cells are not diminished in number after rituximab.30-32 This circulating post-GC B-cell pool in rituximab-unresponsive cGVHD patients was predominantly CD27+ (Figure 2B). Increases in CD27+ B cells have also previously been found in rheumatoid patients with clinical relapse after rituximab.7,8,10,14,29 Because antimicrobial antibody responses are known to remain constant after rituximab,33,34 CD27+ B cells in autoimmune disease and in cGVHD are unlike memory B cells detected in healthy persons. We previously identified and characterized populations of in vivo-activated CD27+ B cells in cGVHD. These CD27+ B cells in cGVHD were B-cell receptor (BCR)-activated because they were able to produce IgG ex vivo without requiring “second signal,” BCR, or cytokine stimulation.16 These CD27+ B cells may have been unrecognized by rituximab because of low cell surface CD20 expression because we confirmed that IgDLo B cells have low CD20 expression (supplemental Figure 4). Human PB-like cells promoted by BAFF have low CD20 cell surface expression and secrete IgG, further suggesting that these cells were perpetuated by BAFF after rituximab treatment in unresponsive cGVHD patients.16,19 Within 3 months after B-cell depletion with rituximab in rheumatoid arthritis, CD27+ antigen experienced, BCR-mutated PB-like cells were detectable and found to persist.35
Alloantibody responses in rituximab-treated cGVHD patients were previously shown to decrease one year after rituximab, whereas antimicrobial antibody titers remain unchanged.2 It is possible that pre-GC IgD+CD38Hi populations in cGVHD that we now show have high CD20 cell surface expression (supplemental Figure 4) represent short-lived plasmablasts in these patients,36,37 potentially explaining why alloantibody and autoantibody levels decrease one year after rituximab (Figure 4). Although it has been difficult to measure frequent alloantibody and autoantibody responses in cGVHD,38,39 we recently found higher frequencies of autoantibodies to Ro-52 in patients with active cGVHD, allowing us to examine an autoantibody response in the current study.24 Thus, we were able to find that patients with rituximab-unresponsive cGVHD at 2 years were more likely to produce autoantibodies before and after rituximab, whereas antimicrobial antibody responses were unchanged (Figure 4). This finding is consistent with promotion of rare autoreactive B cells in patients with rituximab-unresponsive cGVHD. In addition, return of autoantibodies 2 years after rituximab suggests that post-GC cells also contribute to autoreactivity in cGVHD.
The profound, persistent naive B lymphopenia in patients with rituximab-unresponsive cGVHD contributed to the significantly higher BAFF/B-cell ratio in these patients. Immune-suppressive agents potentially contributed to the profound B lymphopenia because these patients received a higher number of immune-suppressive agents per patient compared with the stable/improved patients (2.7 vs 1.9 agents/patient). However, given the small numbers of patients studied and the fact that patients in both cGVHD groups were receiving immune-suppressive drugs, we could not attribute lack of B-cell reconstitution after rituximab to immune-suppressive agents alone. Importantly, B and total lymphocyte numbers were not different between the 2 cGVHD groups one year after rituximab (Figure 1; supplemental Figure 1). Despite receipt of immunosuppression, patients with stable/improved cGVHD who responded to rituximab had B-cell reconstitution. Also arguing against immune suppression as the sole contributor to B lymphopenia was the fact that median numbers of T cells at 12 months were higher, not lower, in the rituximab-unresponsive group at 12 months (supplemental Figure 1). Furthermore, relative to other lymphocytes, B cells were not decreased 2 years after rituximab because of extracorporeal photopheresis (supplemental Figure 1C-D), suggesting that the lymphopenia found in the rituximab-unresponsive group was potentially also a reflection of worsening cGVHD and an inability to produce B cells.
Having now demonstrated that B-cell compartment recovery and composition contribute to human cGVHD pathophysiology, the reason(s) for persistent, profound B lymphopenia in rituximab-unresponsive cGVHD can be addressed in future studies. Because significantly increased proportions of early bone marrow emigrants or transitional B cells in the patients were present during B-cell reconstitution after HSCT in patients who did not develop autoimmune disease or have active cGVHD (Figure 1B),12,16 bone marrow function was probably superior in these patients. Indeed, intrinsic host bone marrow defects are potentially present in cGVHD.40,41 Bone marrow suppression in the setting of chronic inflammation has been described,42 and emerging evidence suggests that GVHD itself is responsible for poor B-cell reconstitution in murine models and in patients.43,44 Others have also suggested that the donor stem cell product may also contribute to a decreased capacity for robust B-cell reconstitution in allo-HSCT.45 Finally, B-cell reconstitution after rituximab in the absence of autoantigen (such as is found in lymphoma patients or in autologous transplantation) occurs more rapidly, recapitulating B-cell ontogeny, suggesting that BCR specificity for autoantigens or alloantigens also contributes to B-cell pool composition during post-HSCT ontogeny.9,16
Despite the small number of patients in this study, our findings provide data that will inform future translational studies. We were unable to definitively identify predictive markers of rituximab response because of small numbers of patients studied, but our data suggest that numbers of transitional B cells at the time of planned rituximab may associate with full recovery of the naive B-cell pool and more durable clinical responses to rituximab. Thus, based on our data, studies aimed at identification of potential predictive B cell-related biomarkers for clinical responses to rituximab in cGVHD can now be performed. In addition, although we did not determine whether regulator T cells (Treg) numbers or frequency after rituximab in cGVHD associated with clinical response, we were able (in data not shown) to examine the relative proportion of CD4+CD25+ T cells before rituximab between the 2 rituximab-treated cGVHD groups. cGVHD patients who later had stable/improved cGVHD after rituximab had an increase in median CD4+CD25+ T-cell frequency before rituximab had 49.0 × 106/L versus 8.3 × 106/L in later rituximab-unresponsive cGVHD (P = .33). Given that evidence suggesting these cells contribute to human cGVHD pathophysiology is mounting, and data also suggest that SLE patients have increased Treg after rituximab,4,46 further studies of Treg should be pursued. Our results, together with recent evidence suggesting a potential role for Treg on B-cell survival and autoreactivity,47-49 will guide future studies addressing a potential Treg/B-cell interaction in cGVHD.
Our study also raises therapeutic questions involving timing and duration of B-cell depletion with rituximab relative to B-cell reconstitution and BAFF production.10 Administration of rituximab in the peri-HSCT period before cGVHD development may allow for improved naive B-cell reconstitution, a concept that is currently under active investigation in a clinical trial. Furthermore, given that naive B cells probably outcompete soluble BAFF, making it unavailable to CD27+ autoreactive B cells, properly timed, therapeutic infusion of naive donor B cells after HSCT may offer prophylaxis against cGVHD development. Finally, if perpetuation of cGVHD is in part mediated by lack of B-cell precursors, our current data further question whether agents that target BAFF would prove beneficial. To the extent that anti-BAFF agents would selectively deplete BAFF-dependent, activated B cells that are alloreactive and autoreactive, anti-BAFF agents might lead to initial clinical improvement in active cGVHD patients. We now also suggest that administration of anti-BAFF agents followed by or concomitantly with rituximab in active cGVHD might be clinically efficacious.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Daley, Suzan Lazo-Kallanian, and Sean McDonough for expert technical assistance, Doreen Hearsey for help obtaining clinical samples, and Drs Paul Armistead and George Fedoriw for critical review of the manuscript.
This work was supported by the National Marrow Donor Program Amy Strelzer Manasevit Award, the Jock and Bunny Adams Research and Education Endowment, the Ted and Eileen Pasquarello Research Fund, and the National Institutes of Health (grants AI29530, CA142106, and K12CA087723).
National Institutes of Health
Authorship
Contribution: S.S. conceived and designed the study, performed the research, analyzed and interpreted the data, and wrote the manuscript; K.E. S. and H.T.K. analyzed and interpreted data and participated in writing the paper; C.S.C., V.T.H., R.J.S., E.P.A., and J.H.A. provided vital patient samples and clinical information and edited the paper; W.S.W. and N.S.B. performed the research and analyzed the data; and J.R. supervised the work, contributed to design and interpretation of the study, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefanie Sarantopoulos, University of North Carolina Chapel Hill, Lineberger Comprehensive Cancer Center, CB 7295, Chapel Hill, NC 27599; e-mail: stefanie_sarantopoulos@med.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal