Abstract
The human Fcγ receptor (FcγR) system is composed of 2 opposing families, the activating FcγRs (FcγRI, FcγRIIa, and FcγRIII) and the inhibitory FcγR (FcγRIIb). The disturbed balance of the activating and inhibitory FcγRs has been implicated in the pathogenesis of many autoimmune diseases. In this study, the expression of FcγRs on monocytes was determined in 23 patients with primary immune thrombocytopenia (ITP) before and after high-dose dexamethasone (HD-DXM) treatment. The FcγRI expression was significantly higher in ITP patients and decreased after HD-DXM treatment. The ratio of FcγRIIa/IIb mRNA expression on monocytes was significantly higher in untreated patients than in healthy controls. After HD-DXM therapy, the ratio decreased and the increased expression of FcγRIIb mRNA and protein coincided with a remarkable decrease in the expression of FcγRIIa, FcγRI, and monocyte phagocytic capacity. There was no significant difference in FcγRIII expression on monocytes between patients and controls. In vitro cell-culture experiments showed that DXM could induce FcγRIIa and FcγRIIb expression in monocytes from ITP patients, with FcγRIIb at higher amplitudes. These findings suggested that the disturbed FcγR balance might play a role in the pathogenesis of ITP, and that HD-DXM therapy could shift monocyte FcγR balance toward the inhibitory FcγRIIb in patients with ITP.
Introduction
The human Fcγ receptor (FcγR) system is composed of 2 opposing families, the activating FcγRs (FcγRI, FcγRIIa, and FcγRIII) and the inhibitory FcγR (FcγRIIb), the balance of which determines the magnitude of the inflammatory response. FcγRIIb is the only FcγR that has an inhibitory function, and is expressed by a variety of immune cells, including B cells, monocytes, macrophages, dendritic cells, mast cells, and basophils. FcγRIIb can decrease antibody production by raising the activation threshold of B cells if cross-linked to the B-cell receptor,1,2 and inhibits activating FcγR functions such as phagocytosis and pro-inflammatory cytokine release by monocytes and dendritic cells. The disturbed balance of the activating and inhibitory FcγRs has been found in many autoimmune diseases such as systemic lupus erythematosus3,4 and rheumatoid arthritis,5,6 and therapeutic response in these 2 diseases is often associated with the restoration of the balance between activating and inhibitory FcγRs.7,8
Primary immune thrombocytopenia (ITP) is an immune-mediated bleeding disorder in which platelets are opsonized by autoantibodies directed against platelet surface membrane glycoproteins and prematurely cleared by FcγR-bearing macrophages in the reticuloendothelial system.9,10 Previous studies have shown that leukocyte immunoglobulin G (IgG)–FcγRs such as FcγRII and FcγIII play an important role in the phagocytosis of autoantibody-coated platelets.11 McKenzie et al demonstrated that human FcγRIIa was necessary for antibody-mediated platelet clearance by using a murine model transgenic for human FcγRIIa and lacking murine FcγRI and FcγRIII.12 Samuelsson et al showed that decreased expression of murine inhibitory FcγRIIb was associated with increased platelet destruction.13 In a multivariate logistic regression analysis of data from 60 children with ITP, the presence of the FcγRIIbT232 variant predicted a chronic disease course.14 Recently, it was reported that Helicobacter pylori eradication could induce a significant platelet count increase in a subset of H pylori–infected ITP patients, and this effect was shown to be mediated by shifting the monocyte Fcγ receptor balance toward the inhibitory FcγRIIb.15 Whether there was a difference in the expression of FcγRs in ITP patients compared with healthy controls was unknown.
The therapeutic regimen for ITP includes glucocorticosteroids, intravenous immunoglobulin, splenectomy, thrombopoietin receptor agonists, and other immunosuppressive drugs. Intravenous immunoglobulin could induce a beneficial response in ITP patients by up-regulating FcγRIIb on monocytes/macrophages,16 and the same results were found in mouse models of ITP.13,17 High-dose dexamethasone (HD-DXM) has been widely recognized as the first-line therapy for ITP patients in need of management.18-20 However, the effects of HD-DXM on FcγR regulation remain unelucidated in ITP patients. In the present study, we demonstrated decreased expression of FcγRIIb and elevated expression of FcγRIIa and FcγRIII on monocytes in ITP patients before HD-DXM therapy. HD-DXM treatment may induce a shift in the FcγR balance toward the inhibitory FcγRIIb, thus providing new insights into the mechanism of HD-DXM in the treatment of ITP.
Methods
Patients and controls
Twenty-three newly diagnosed primary ITP patients (16 female and 7 male; age range 16-68 years, median 43 years) were enrolled in this study. Enrollment took place between March 2009 and April 2010 at the Department of Hematology, Qilu Hospital, Shandong University, Jinan, China. Patients were diagnosed according to recently published criteria,21 including history, physical examination, complete blood count, and peripheral blood smear examination consistent with ITP. The patients' platelet counts ranged between 2 and 19 × 109/L, with a median count of 10 × 109/L (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). None of them had been treated with glucocorticosteroids before the first sampling. Cases that were complicated by diabetes, hypertension, cardiovascular diseases, pregnancy, active infection, or connective tissue diseases such as systemic lupus erythematosus were excluded. In vitro monocyte culture was performed in 14 newly diagnosed ITP patients before HD-DXM treatment (9 female and 5 male; age range 21-58 years, median 40.5 years; platelet count range 3-18 × 109/L, median 11 × 109/L; supplemental Table 2), and samples from 10 of them were also used in assays for the phagocytic capacity of monocyte-derived macrophages.
The healthy control group consisted of 20 healthy adult volunteers (13 female and 7 male; age range 21-49 years, median 32 years). Platelet counts ranged from 162 to 274 × 109/L, with a median count of 196.5 × 109/L. The phagocytic capacity of monocytes was analyzed in an additional 10 healthy controls (7 female and 3 male; age range 21-52 years, median 38 years).
This study was approved by the Medical Ethical Committee of Qilu Hospital, Shandong University. Informed consent was obtained from all patients before enrollment in the study in accordance with the Declaration of Helsinki.
Treatment regimen
All patients received HD-DXM 40 mg/d for 4 consecutive days. Initial response evaluation was made at the end of the second week after treatment initiation. The response was evaluated according to the following criteria21 : a complete response was defined as a platelet count ≥ 100 × 109/L and absence of bleeding; a response was defined as a platelet count between 30 and 100 × 109/L, at least a doubling of the baseline counts, and absence of bleeding; and no response was defined as any platelet count less than 30 × 109/L and less than doubling of the baseline counts or bleeding.
Preparation of peripheral blood mononuclear cells, monocytes, B cells, and plasma
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by gradient centrifugation (400g, 20 minutes) on Ficoll-Paque (Pharmacia Diagnostics), washed twice, and resuspended for magnetic separation and cell culture. Plasma was obtained from all subjects by centrifugation of heparinized peripheral blood and stored at −80°C until being analyzed for cytokines.
Circulating CD14+ monocytes were isolated from PBMCs as follows: PBMCs were resuspended in AutoMACS sample buffer and 20 μL of anti-CD14–coated magnetic beads (Miltenyi Biotec) per 107 cells for 25 minutes with constant rotation before purification in a miniMACS Separator. The unlabeled cells were collected for further B-cell isolation with anti-CD19–coated magnetic beads (Miltenyi Biotec).
Monocytes from an additional 14 newly diagnosed ITP patients before treatment were adjusted to 1 × 106/mL in RPMI 1640 culture medium supplemented with 10% heat-inactivated human pooled AB serum at a density of 2 × 106 cells/well in a 6-well culture plate and incubated in humidified air in 5% CO2 at 37°C in the presence of different concentrations of DXM. After 16 hours, the monocyte-derived macrophages were collected for real-time reverse-transcription polymerase chain reaction (RT-PCR) analysis of FcγRIIa/IIb mRNA expression.
Cell-surface staining for flow cytometric determination of FcγRs
Antibodies were purchased from BD Pharmingen or Jingmei Biotech. Heparinized whole blood (100 μL) was stained with fluorescein isothiocyanate (FITC)–conjugated anti–CD14 monoclonal antibodies (mAbs) (clone M5E2), PE-Cy5–conjugated anti–CD14 (clone 4AW14), or FITC-conjugated anti–CD19 (clone HIB19) mAbs, and in combination with FITC-conjugated anti–CD16/FcγRIII (clone 3G8), PE-conjugated anti–CD32/FcγRII (clone FLI8.26), or CD64/FcγRI (clone CT101) mAbs for 30 minutes in the dark. The same-species, same-isotype IgG was used as an isotype control. The cross-reactivity between anti–CD16/FcγRIII mAb (clone 3G8) and anti–CD32/FcγRIII mAb (clone FLI8.26) was determined and shown to be lower than 8% (supplemental Figure 1). After lysis of erythrocytes, cells were washed with phosphate-buffered saline (PBS)/1% bovine serum albumin/0.01% NaN3 and fixed in 1% paraformaldehyde PBS. Analysis was performed with a FACSCalibur with CellQuest Pro software (BD Biosciences). Gates were set around monocytes and B cells based on their forward/sideward light scatter pattern and CD14 or CD19 expression. Surface-marker expression of FcγRI, FcγRII, and FcγRIII on gated CD14+ monocytes and FcγRII on CD19+ B cells were expressed as geometrical mean fluorescence intensity (MFI), which was calculated based on the intensity of the cells incubated with appropriate isotype-matched control mAbs as a reference.
Real-time RT-PCR of FcγRIIa and FcγRIIb on monocytes and B cells
TRIzol reagent (Invitrogen) was used to isolate total RNA of monocytes or B cells. RNA was converted into cDNA using the PrimeScript RT reagent kit (Perfect Real Time; Takara) according to the manufacturer's instructions. Multiplex real-time RT-PCR was performed for FcγRIIa, FcγRIIb, and the endogenous control GAPDH (glyceraldehyde-3-phosphate dehydrogenase) on a PRISM_7500 Sequence Detection System (Applied Biosystems) and SYBR Green (Applied Biosystems). The primers for all mRNA assays were intron spanning. The PCR reactions were cycled 40 times after the initial denaturation and DNA polymerase activation (95°C, 10 minutes) with the following parameters: denaturation 95°C, 15 seconds; annealing 62°C for FcγRIIa and 65°C for FcγRIIb, 15 seconds; extension 72°C, 45 seconds. The primers for FcγRIIa, FcγRIIb, and GAPDH are as follows: FcγRIIa-F, ATCATTGTGGCTGTGGTCATTG; FcγRIIa-R, TGTTTCATAGTCATTGTTGGTTTCTTC; FcγRIIb-F, ATCCCACTAATCCTGATGAGGCTG; FcγRIIb-R, ACGGTTCTGGTCATCAGGCTC; GAPDH-F, GCACCGTCAAGGCTGAGAAC; GAPDH-R, TGGTGAAGACGCCAGTGGA.
The comparative Ct method using arithmetic formulas was used for relative quantification of FcγRIIa and FcγRIIb mRNA according to relative expression software tool (REST).22 The amplification efficiency between the target (FcγRIIa and FcγRIIb) and the reference control (GAPDH) were compared in order to use the delta Ct (ΔΔCt) calculation.
Immunoprecipitation and Western blotting of FcγRIIa and FcγRIIb
Monocytes (2 × 107 cells/sample) were solubilized in lysis buffer (1% Nonidet P-40, 10% glycerol, 16mM Na2HPO4, 4mM NaH2 PO4, 70mM NaCl, 50mM NaF, 5mM EDTA, 0.4mM Na3VO4, 10 μg/mL each of aprotinin, leupeptin, soybean trypsin inhibitor, and pepstatin A, and 500 μg/mL of Pefabloc, pH 7.4) for 1 hour at 4°C. The lysates were incubated overnight with a mixture of anti–FcγRII AT10 and FUN-2 antibodies (Santa Cruz Biotechnology), and 20 μL of protein G-agarose beads (Beyotime Institute of Biotechnology, Nantong, China) were added and incubated for 2 hours at 4°C. The immunoprecipitates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and electrophoretically transferred to nitrocellulose membranes. Membranes were blocked and incubated with goat anti–human FcγRIIa (R&D Systems) and FcγRIIb (Abcam), followed by polyclonal rabbit anti–goat Abs conjugated with horseradish peroxidase (ZSGB-BIO). The reaction was developed by enhanced chemiluminescence. In addition, monocyte lysates were blotted with goat polyclonal antibodies specific for human β-actin (Santa Cruz Biotechnology) as a protein loading control. Analysis of radiograms was performed by densitometry. Background values from each gel were subtracted to normalize measurements. Western blots were scored for relative densitometry normalized against β-actin.
ELISA for IFN-γ and IL-4
Plasma interferon-γ (IFN-γ) and interleukin 4 (IL-4) from ITP patients and healthy controls were also determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Jingmei Biotech) according to the manufacturer's instructions. The detection limit for this assay was 2 pg/mL.
Isolation, CMFDA labeling, and opsonization of platelets
Isolation and 5-chloromethylfluorescein diacetate (CMFDA) labeling of platelets for phagocytic assays were as described previously.23,24 Briefly, peripheral blood was obtained by venipuncture into trisodium citrate from the healthy volunteer. Platelet-rich plasma was prepared, and platelets were adjusted to 109/mL in the presence of 5μM prostaglandin E1 (Cayman Chemical). CMFDA (GM-G; Invitrogen) was added to the platelets at a final concentration of 20μM, incubated in the dark for 2 hours at 37°C, washed, and resuspended in PBS. For opsonization, GM-G–labeled platelets were incubated with 5 μg of murine IgG2a anti–human major histocompatibility complex class I monoclonal antibody (W6/32; Abcam) for 30 minutes at room temperature. Platelets were washed once and used in the phagocytic assay.
Evaluation of phagocytic capacity of monocyte-derived macrophages
In vitro phagocytosis of IgG-opsonized platelets by macrophages was carried out according to a method described previously 24,25 with slight modification. The assay was performed in 10 healthy controls and in 10 patients before and after HD-DXM therapy. PBMCs were obtained by Ficoll-Paque density gradient centrifugation, washed, and further purified by centrifugation on a hypotonic Percoll density gradient (1.129 g/mL; 400g, 30 minutes). Two interphases were found and the upper phase with the enriched monocytes was collected. Monocytes were cultured in RPMI 1640 medium in a 5% CO2/95% air atmosphere at 37°C for 2 hours, and washed twice with PBS. The purity of the enriched monocytes was > 90% as assessed by flow cytometric analysis. The adherent cells were further cultured for 1 hour in Iscove modified Dulbecco medium supplemented with 10% heat-inactivated human pooled AB serum in the presence of 50 ng/mL of phorbol 12-myristate 13-acetate. The cells were then washed twice with PBS, incubated with opsonized CMFDA-labeled platelets (macrophages: platelets, 1:5), centrifuged at 200g for 1 minute to establish contact between macrophages and platelets, and further incubated for 1 hour on ice or at 37°C. To remove free platelets, the macrophages were treated with 0.5mM EDTA and 0.05% trypsin in PBS for 5 minutes at 37°C, followed by detachment of macrophages using PBS/5mM EDTA at 4°C. Extracellular fluorescence was then quenched by the addition of 0.1% Trypan blue. The mixture was centrifuged at 200g for 10 minutes at 4°C, the supernatant discarded, and the macrophages were incubated with DNA stain LDS-751 (FL3; Molecular Probes). The macrophages were then washed and resuspended for flow cytometric analysis. Intracellular FL1 GM-G platelet fluorescence in the nucleated events was determined. The phagocytic index was calculated as the MFI obtained at 37°C divided by the MFI at 0°C.
Statistical analysis
Data were expressed as means ± SD. Statistical significance among patients before and after HD-DXM therapy and healthy groups was determined by analysis of variance (ANOVA), and the difference between 2 groups was determined by Student-Newman-Keuls test unless the data were not normally distributed, in which case the Kruskal-Wallis test and Nemenyi test or Mann-Whitney U test was used. P values < .05 were considered statistically significant.
Results
Clinical response to HD-DXM
Complete response was observed in 16 (69.57%), response in 4 (17.39%), and no response in 3 (13.04%) of the 23 patients. Median platelet count 2 weeks after the initial treatment was 137 × 109/L (range, 16-242 × 109/L; supplemental Table 1). No bleeding or other obvious complications were observed throughout the treatment.
Effect of HD-DXM treatment on the expression of FcγRI, FcγRII, and FcγRIII on monocytes and FcγRII on B cells in ITP patients
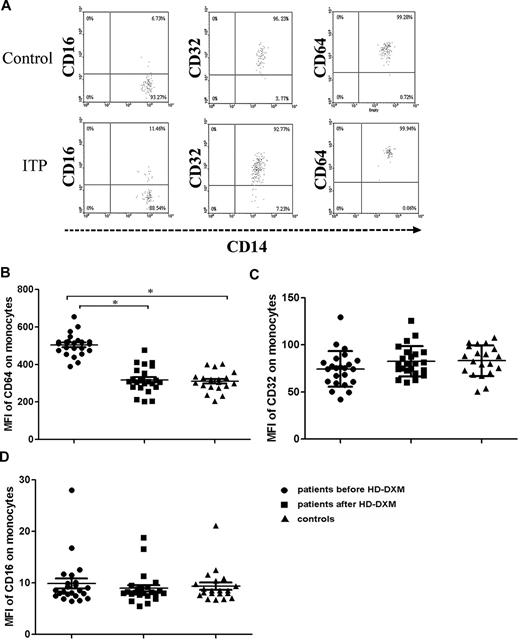
To investigate the effect of 4-day HD-DXM administration on FcγR regulation, the surface expression of FcγRs on circulating monocytes and B cells from ITP patients and healthy controls were determined. Blood samples for flow cytometric analysis of FcγRs were collected before and 2 weeks after HD-DXM initiation. Quantification of FcγRI expression on monocytes revealed a significantly higher MFI in ITP patients (504.6 ± 58.6) compared with healthy controls (309.8 ± 52.9; P < .001), and after 4 days of HD-DXM therapy, FcγRI expression showed a significant decrease (317.5 ± 69.4; P < .001) compared with the levels before treatment (Figure 1A-B).
Expression of FcγRs on monocytes from ITP patients before or after therapy and from healthy controls. (A) Representative scattergrams of surface expression of FcγRIII/CD16, FcγRII/CD32, and FcγRI/CD64 on CD14+ monocytes from a healthy control and an ITP patient before HD-DXM therapy. CD14+ monocytes were gated based on their forward/sideward scatter. (B-D) Expression (MFI) of FcγRI/CD64 (B), FcγRII/CD32 (C), and FcγRIII/CD16 (D) on monocytes from ITP patients before and after HD-DXM therapy and from healthy controls. Significance among patients before and after HD-DXM therapy and healthy groups was determined by ANOVA, and differences between 2 groups were compared by Student-Newman-Keuls test. Bars represent SD *P < .001.
Expression of FcγRs on monocytes from ITP patients before or after therapy and from healthy controls. (A) Representative scattergrams of surface expression of FcγRIII/CD16, FcγRII/CD32, and FcγRI/CD64 on CD14+ monocytes from a healthy control and an ITP patient before HD-DXM therapy. CD14+ monocytes were gated based on their forward/sideward scatter. (B-D) Expression (MFI) of FcγRI/CD64 (B), FcγRII/CD32 (C), and FcγRIII/CD16 (D) on monocytes from ITP patients before and after HD-DXM therapy and from healthy controls. Significance among patients before and after HD-DXM therapy and healthy groups was determined by ANOVA, and differences between 2 groups were compared by Student-Newman-Keuls test. Bars represent SD *P < .001.
FcγRII (IIa + IIb) expression on monocytes from ITP patients showed a slight increase after HD-DXM therapy compared with the levels before treatment (82.6 ± 16.0 vs 74.5 ± 18.9), but this increase did not achieve statistical significance. There was no difference between the ITP group and the control individuals (83.2 ± 16.3; P = .947, ANOVA; Figure 1A-C). After 4 days of HD-DXM administration, monocytes from 17 patients (73.9%) showed increased FcγRII expression, and 6 patients (26.1%) had decreased FcγRII expression. There were no significant changes in FcγRIII expression on monocytes detected by fluorescence-activated cell sorting (FACS) with anti–FcγRIII mAb after HD-DXM administration, and no difference was found among patients before or after therapy and the healthy control group (9.9 ± 4.6, 8.9 ± 3.0, and 9.3 ± 3.2, respectively; P = .694, ANOVA; Figure 1A-D).
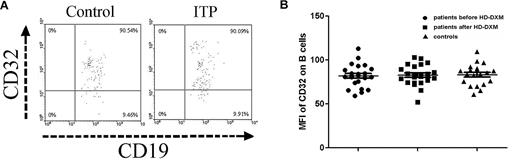
The FcγR expression on B cells was also determined by flow cytometry in ITP patients and controls. As shown in Figure 2A-B, the expression of FcγRII on B cells in ITP patients before HD-DXM administration was similar to healthy controls (82.0 ± 13.0 vs 83.2 ± 12.1), and HD-DXM treatment had no effect on B-cell FcγRII expression (82.7 ± 11.8; P = .954, ANOVA; Figure 2A-B).
Expression of FcγRII/CD32 on B cells from ITP patients before and after therapy and from healthy controls. (A) Representative scattergrams of surface expression of FcγRII/CD32 on CD19+ B cells from a healthy control and an ITP patient before HD-DXM therapy. CD19+ B cells were gated based on their forward/side scatter. (B) Expression of FcγRII/CD32 (MFI) on B cells from ITP patients before and after HD-DXM therapy and from healthy controls.
Expression of FcγRII/CD32 on B cells from ITP patients before and after therapy and from healthy controls. (A) Representative scattergrams of surface expression of FcγRII/CD32 on CD19+ B cells from a healthy control and an ITP patient before HD-DXM therapy. CD19+ B cells were gated based on their forward/side scatter. (B) Expression of FcγRII/CD32 (MFI) on B cells from ITP patients before and after HD-DXM therapy and from healthy controls.
Gene and protein expression of FcγRIIa and IIb on monocytes
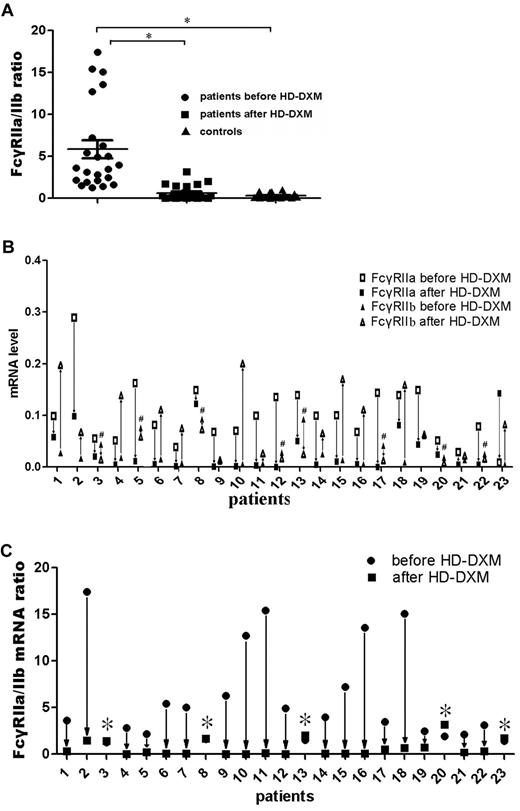
Two types of FcγRII were expressed on monocytes: an activating receptor, FcγRIIa, and an inhibitory receptor, FcγRIIb.26 To evaluate the effect of HD-DXM on FcγRIIa/IIb mRNA expression of monocytes, real-time RT-PCR was performed on sorted monocytes before or after HD-DXM therapy using specific primers for FcγRIIa and IIb. The results showed that the ratio of FcγRIIa/IIb mRNA expression on monocytes was significantly higher in untreated ITP patients than in healthy controls (5.88 ± 5.12 vs 0.31 ± 0.29; P < .001). After HD-DXM therapy, the ratio of FcγRIIa/IIb mRNA decreased significantly (0.61 ± 0.86; P < .001) in comparison with the level before treatment (Figure 3A). Of the 23 patients, 22 showed decreased FcγRIIa mRNA expression, and 15 patients had increased FcγRIIb mRNA expression levels on monocytes after HD-DXM administration (Figure 3B). Decreased ratios of FcγRIIa/IIb mRNA expression were observed in 18 of the 23 patients, whereas increased FcγRIIa/IIb ratios were found in 5 patients, including 3 nonresponders and 2 responsive cases (Figure 3C). However, no difference in FcγRIIb on B cells was found among patients either before or after therapy and healthy controls (data not shown).
FcγRIIa/IIb mRNA ratio on monocytes from ITP patients and controls, and changes in FcγRIIa/IIb mRNA expression levels in ITP patients before and after treatment. (A) FcγRIIa and FcγRIIb mRNA expression ratio on monocytes from ITP patients before and after treatment and controls was determined by quantitative PCR. The differences among these 3 groups were analyzed using Kruskal-Wallis test and the differences between 2 groups were analyzed using the Nemenyi test. *P < .001. (B) FcγRIIa and FcγRIIb mRNA expression levels on monocytes from ITP patients were measured before and after HD-DXM treatment. #Patients with decreased FcγRIIb mRNA expression after HD-DXM treatment. (C) After administration of HD-DXM, 18 of 23 patients had reduced FcγRIIa/IIb mRNA expression ratios, while the other 5 had similar or higher FcγRIIa/IIb mRNA expression ratios compared with those before treatment. *Patients with increased FcγRIIa/IIb mRNA ratios after HD-DXM treatment.
FcγRIIa/IIb mRNA ratio on monocytes from ITP patients and controls, and changes in FcγRIIa/IIb mRNA expression levels in ITP patients before and after treatment. (A) FcγRIIa and FcγRIIb mRNA expression ratio on monocytes from ITP patients before and after treatment and controls was determined by quantitative PCR. The differences among these 3 groups were analyzed using Kruskal-Wallis test and the differences between 2 groups were analyzed using the Nemenyi test. *P < .001. (B) FcγRIIa and FcγRIIb mRNA expression levels on monocytes from ITP patients were measured before and after HD-DXM treatment. #Patients with decreased FcγRIIb mRNA expression after HD-DXM treatment. (C) After administration of HD-DXM, 18 of 23 patients had reduced FcγRIIa/IIb mRNA expression ratios, while the other 5 had similar or higher FcγRIIa/IIb mRNA expression ratios compared with those before treatment. *Patients with increased FcγRIIa/IIb mRNA ratios after HD-DXM treatment.
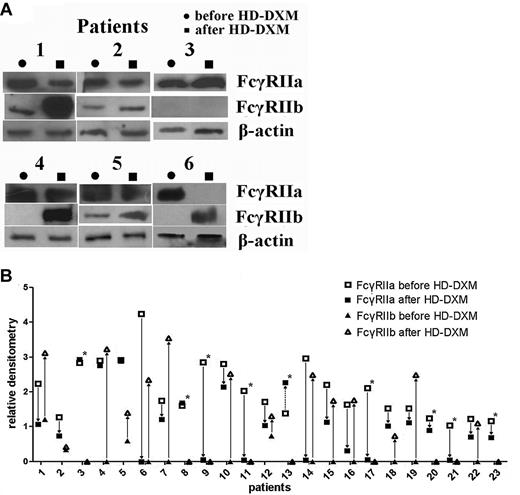
To further evaluate the effects of HD-DXM on protein expression levels of FcγRIIa and IIb, immunoprecipitation and Western blotting were performed. After therapy, the patients had a marked down-regulation of FcγRIIa protein expression on monocytes compared with the levels before treatment (2.05 ± 0.81 vs 1.07 ± 0.96; P < .001, Student t test; Figure 4A). At the same time, the decreased FcγRIIa protein levels were associated with up-regulated FcγRIIb levels. The relative densitometry of monocyte FcγRIIb in the ITP group was 0.13 ± 0.31, which increased significantly after HD-DXM therapy (1.23 ± 1.25; P < .001, Mann-Whitney U test). Nineteen of the 23 patients had undetectable FcγRIIb expression before therapy, and 2 weeks after HD-DXM initiation, FcγRIIb expression was detectable in 14 patients (Figure 4B). These results indicate that HD-DXM can modulate FcγRIIa/IIb expression, shifting the balance of the 2 opposing FcγR isoforms from an activating phenotype to an inhibitory phenotype on monocytes in patients with ITP.
Modulation of FcγRIIa/IIb protein expression on monocytes before treatment and 2 weeks after initiation of HD-DXM therapy. (A) Representative Western blotting of monocyte lysates immunoprecipitated with anti–FcγRII cocktail and blotted with goat anti–human FcγRIIa, FcγRIIb, and β-actin are shown. (B) Changes of FcγRIIa and FcγRIIb protein expression on monocytes in patients after HD-DXM treatment. Fourteen of the 23 patients had increased FcγRIIb expression. *Patients who still showed no FcγRIIb expression after HD-DXM therapy.
Modulation of FcγRIIa/IIb protein expression on monocytes before treatment and 2 weeks after initiation of HD-DXM therapy. (A) Representative Western blotting of monocyte lysates immunoprecipitated with anti–FcγRII cocktail and blotted with goat anti–human FcγRIIa, FcγRIIb, and β-actin are shown. (B) Changes of FcγRIIa and FcγRIIb protein expression on monocytes in patients after HD-DXM treatment. Fourteen of the 23 patients had increased FcγRIIb expression. *Patients who still showed no FcγRIIb expression after HD-DXM therapy.
Effects of HD-DXM therapy on monocyte/macrophage phagocytic capacity in ITP patients
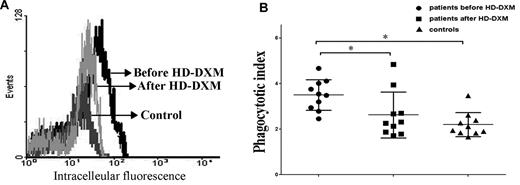
The balance of activating and inhibitory FcγRs determines the magnitude of the cellular response in monocytes. To evaluate whether modulation of FcγRs by HD-DXM, as shown in Figures 1, 3, and 4, has an effect on monocyte phagocytic capacity, monocytes from ITP patients before and after HD-DXM treatment were incubated with opsonized CMFDA-labeled platelets for FcγR-mediated phagocytosis assay. Monocytes from untreated ITP patients demonstrated higher phagocytic capacity compared with healthy controls (MFI ratio: 3.5 ± 0.7 vs 2.2 ± 0.5; P < .05). After HD-DXM therapy, the phagocytic capacity of monocytes decreased significantly (2.6 ± 1.0; P < .05; Figure 5A-B).
Phagocytic capacity of monocyte-derived macrophages from ITP patients and healthy controls. Platelets were isolated, labeled with the cell tracker CMFDA, opsonized with W6/32 antibody, incubated with monocyte-derived macrophages for 1 hour, and analyzed by flow cytometry. (A) Representative histogram of macrophage intracellular fluorescence from one ITP patient before and after treatment. Platelets were labeled with the cell tracker CMFDA, opsonized with W6/32, and coincubated with macrophages at 37°C for 1 hour. Negative controls were performed at 4°C for 1 hour. The phagocytic index was calculated as the MFI obtained at 37°C divided by the MFI at 0°C. (B) Phagocytic capacity of macrophages from ITP patients before and after HD-DXM therapy and from healthy controls. *P < .05.
Phagocytic capacity of monocyte-derived macrophages from ITP patients and healthy controls. Platelets were isolated, labeled with the cell tracker CMFDA, opsonized with W6/32 antibody, incubated with monocyte-derived macrophages for 1 hour, and analyzed by flow cytometry. (A) Representative histogram of macrophage intracellular fluorescence from one ITP patient before and after treatment. Platelets were labeled with the cell tracker CMFDA, opsonized with W6/32, and coincubated with macrophages at 37°C for 1 hour. Negative controls were performed at 4°C for 1 hour. The phagocytic index was calculated as the MFI obtained at 37°C divided by the MFI at 0°C. (B) Phagocytic capacity of macrophages from ITP patients before and after HD-DXM therapy and from healthy controls. *P < .05.
Changes of plasma IFN-γ and IL-4 after HD-DXM therapy
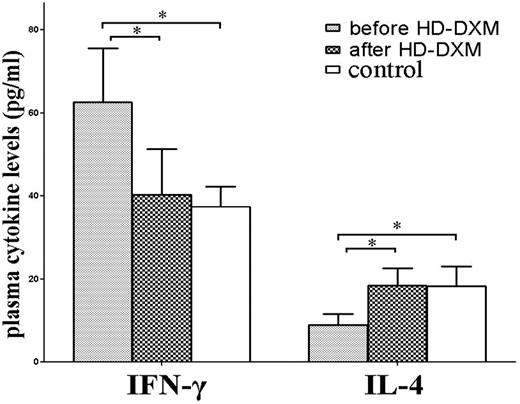
To examine the potential role of HD-DXM therapy on T-helper 1/T-helper 2 (Th1/Th2) regulation, plasma IFN-γ and IL-4 levels in ITP patients and healthy controls were determined. Consistent with previous reports,27,28 significantly higher levels of plasma IFN-γ and lower IL-4 were found in untreated patients compared with healthy controls (IFN-γ: 62.7 ± 13.0 vs 37.4 ± 4.8 pg/mL, P < .001; and IL-4: 8.9 ± 2.7 vs 18.3 ± 4.7 pg/mL, P < .001, respectively). Plasma IFN-γ levels in untreated patients decreased significantly after HD-DXM administration (40.3 ± 11.0 pg/mL, P < .001), while posttreatment levels of IL-4 increased significantly compared with the levels before treatment (18.5 ± 4.2 pg/mL, P < .001; Figure 6).
Plasma levels of IFN-γ and IL-4 in untreated ITP patients, patients after HD-DXM therapy, and healthy controls. Levels of plasma IFN-γ and IL-4 were determined by ELISA. Elevated plasma IFN-γ level, and decreased IL-4 level was detected in untreated ITP patients compared with patients after HD-DXM therapy (IFN-γ: 62.7 ± 13.0 vs 40.3 ± 11.0 pg/mL, P < .001; IL-4: 8.9 ± 2.7 vs 18.5 ± 4.2 pg/mL, P < .001, respectively) or healthy controls (37.4 ± 4.8 pg/mL, P < .001; and 18.3 ± 4.7 pg/mL, P < .001, respectively). There was no significant difference in plasma IFN-γ or IL-4 levels between posttreatment ITP patients and healthy controls (P > .05). *P < .001.
Plasma levels of IFN-γ and IL-4 in untreated ITP patients, patients after HD-DXM therapy, and healthy controls. Levels of plasma IFN-γ and IL-4 were determined by ELISA. Elevated plasma IFN-γ level, and decreased IL-4 level was detected in untreated ITP patients compared with patients after HD-DXM therapy (IFN-γ: 62.7 ± 13.0 vs 40.3 ± 11.0 pg/mL, P < .001; IL-4: 8.9 ± 2.7 vs 18.5 ± 4.2 pg/mL, P < .001, respectively) or healthy controls (37.4 ± 4.8 pg/mL, P < .001; and 18.3 ± 4.7 pg/mL, P < .001, respectively). There was no significant difference in plasma IFN-γ or IL-4 levels between posttreatment ITP patients and healthy controls (P > .05). *P < .001.
In vitro effects of DXM on regulation of FcγRIIa/IIb mRNA expression in cultured monocytes/macrophages
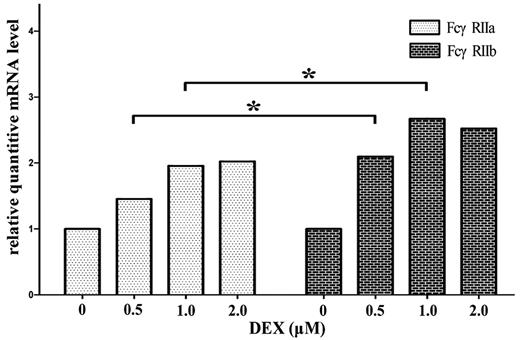
Because the mRNA ratio of FcγRIIa/IIb was increased in untreated ITP patients and decreased significantly after HD-DXM therapy, we investigated whether DXM is able to act directly on monocyte/macrophage FcγRIIa/IIb regulation. We cultured magnetically isolated monocytes from untreated ITP patients in the presence of different concentrations of DXM and after 16 hours, the cells were harvested for real-time RT-PCR. As shown in Figure 7, DXM could increase the mRNA expression of both FcγRIIa and FcγRIIb, with FcγRIIb at higher amplitudes. This was a little different from the in vivo effect of HD-DXM (Figure 3A-B), which demonstrated that most of the ITP patients (22/23) had down-regulated FcγRIIa mRNA expression after HD-DXM administration. Further studies are needed to elucidate the discrepancy between in vivo and in vitro effects of DXM on FcγRII regulation.
DXM up-regulates the mRNA expression of FcγRIIa and FcγRIIb on in vitro–cultured monocytes. Magnetically isolated monocytes from untreated ITP patients were cultured in the presence of different concentrations of DXM for 16 hours and harvested for real-time RT-PCR. β-actin mRNA was used as a control. Relative mRNA level represents the FcγRIIa or FcγRIIb expression normalized by β-actin (FcγRIIa/β-actin, FcγRIIb/β-actin) in each cell culture with respect to the expression of FcγRIIa/β-actin or FcγRIIb/β-actin measured in untreated cells. *P < .05.
DXM up-regulates the mRNA expression of FcγRIIa and FcγRIIb on in vitro–cultured monocytes. Magnetically isolated monocytes from untreated ITP patients were cultured in the presence of different concentrations of DXM for 16 hours and harvested for real-time RT-PCR. β-actin mRNA was used as a control. Relative mRNA level represents the FcγRIIa or FcγRIIb expression normalized by β-actin (FcγRIIa/β-actin, FcγRIIb/β-actin) in each cell culture with respect to the expression of FcγRIIa/β-actin or FcγRIIb/β-actin measured in untreated cells. *P < .05.
Discussion
In this study, oral HD-DXM was used in a single 4-day course as the initial treatment schedule in previously untreated ITP patients with active disease. As shown in supplemental Table 1, HD-DXM resulted in a good initial response in ITP patients, which was in agreement with previous reports.18-20 In the present study, FcγRs were investigated in ITP patients before and after HD-DXM treatment, and it was demonstrated that HD-DXM therapy could down-regulate FcγRI expression on monocytes. The FcγRII and III expression showed no change after HD-DXM therapy. Because FcγRIIa could not be discriminated from the only inhibitory FcγRIIb by FACS, real-time RT-PCR and western immunoblotting were performed. The data demonstrated that the FcγRIIb mRNA and protein expression levels on monocytes increased significantly after HD-DXM treatment. The up-regulation of FcγRIIb occurred in parallel with a remarkably decreased expression of FcγRIIa; however, the mRNA expression level of FcγRIIb on B cells was not affected by HD-DXM therapy.
Functional properties of monocytes or macrophages, such as phagocytosis and antigen presentation, were controlled by the balance of the activating FcγRs and the inhibitory receptor FcγRIIb.29-31 Our results demonstrated that circulating monocytes from untreated ITP patients exhibited enhanced FcγR-mediated phagocytic capacity compared with healthy controls, and this trend was consistent with the up-regulated FcγRI expression and increased FcγRIIa/IIb mRNA ratio. These abnormalities of FcγRs were corrected after HD-DXM therapy, suggesting the alteration of monocytes from an activating phenotype to an inhibitory phenotype. However, these changes were not found in nonresponders to HD-DXM and in 2 of 4 responders. Several studies demonstrated that IFN-γ, one of the Th1 cytokines, was up-regulated,27 while IL-4 was down-regulated in active ITP.28 Studies by Pricopl et al showed that IFN-γ, one of the Th1 cytokines, could down-regulate FcγRIIb mRNA and cell-surface expression on monocytes.26 On the contrary, IL-4, which belongs to the family of Th2 cytokines, had the opposite effect on monocyte FcγRIIb expression.26,32
In accordance with our previous report,28 the present data on plasma IFN-γ/IL-4 further suggested that Th1/Th2 balance in ITP was disturbed and that HD-DXM could resume the balance. This prompted us to study whether the function of HD-DXM on monocyte FcγR regulation was a direct effect or a secondary one mediated by cytokines secreted by T cells. In vitro monocyte culture experiments showed that DXM could up-regulate mRNA expression of both FcγRIIa and FcγRIIb, which was slightly different from previous results.33 In their work, Comber et al showed that treatment with DXM had no significant effect on the expression of FcγRII transcripts in in vitro–cultured monocytes.33 Disparity in cell-preparation procedures or mRNA-detecting methods might have contributed to this discrepancy.
ITP is an autoimmune disorder in which platelets are opsonized by antiplatelet autoantibodies and phagocytosed by macrophages via FcγRs.34 Anti–glycoprotein IIb/IIIa (GPIIb/IIIa) antibodies are one of the primary autoantibodies found in ITP.35,36 It has been demonstrated that anti–GPIIb/IIIa autoantibodies are predominant in the IgG1 subclass,37 which has the highest affinity for FcγRII.38 FcγRs expressed on macrophages in the reticuloendothelial system play 2 roles in ITP: platelet destruction and sustained autoimmune responses to platelet antigens.39,40 An increase in the expression of inhibitory FcγRIIb relative to the activating FcγRs could attenuate the dysfunction of macrophages in ITP.34 Recently, a single HD-DXM course was administered as first-line therapy in adult patients with ITP.18-20 HD-DXM increases the number of CD4+Foxp3+ Treg cells.41 The Th1 cytokine dominance in ITP27 can also be corrected by HD-DXM.28 Our results showed that DXM could shift the FcγR balance toward the inhibitory FcγRIIb on monocytes in ITP and correct the enhanced phagocytic capacity of monocytes, thus providing a new mechanism for the therapeutic effect of HD-DXM on ITP.
The regulation of FcγRIIb expression is complex and differs depending on the cell type. In addition to the above-mentioned cytokines IL-4 and IFN-γ,26,32 activation of complement may also affect FcγRIIb expression. Shushakova et al demonstrated that the complement component C5a could directly up-regulate FcγRIII and down-regulate FcγRIIb expression by alveolar macrophages.42 ITP is a heterogeneous and complex autoimmune disorder. In addition to our findings regarding the effect of HD-DXM on FcγRIIb regulation, recent studies by Pels suggested that the therapeutic effects of intravenous immunoglobulin on ITP was possibly mediated by up-regulation of FcγRIIb on macrophages.16 Asahi demonstrated that H pylori eradication could induce the recovery of platelet counts in a certain part of H pylori–positive ITP patients, which was associated with a change in FcγR balance toward the inhibitory FcγRIIb on monocytes.10 Thus, it appears that FcγRIIb up-regulation in ITP might be a common phenomenon in the recovering process induced by immune-modulating agents, and the precise mechanism of FcγRIIb modulation in ITP awaits further investigation.
In summary, decreased FcγRIIb expression and increased FcγRIIa and FcγRI expression in untreated ITP patients suggest the possible role of the disturbed FcγR balance in the pathogenesis of ITP. HD-DXM may reduce inflammation in ITP by restoring the balance of activating and inhibitory FcγRs, raising the threshold for monocyte activation and decreasing the phagocytic capacity of monocytes. The role of FcγRs remains to be elucidated, but they are possibly involved in the efficacy of HD-DXM in ITP treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 30971278, 81070411, 30570779, 30770922, 81070407, 81070408, and 81070396); the 973 Program (no. 2009 CB521904); the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (no. 200561); the Program for New Century Excellent Talents in University (NCET-07-0514); the Key Project of Chinese Ministry of Education (no. 109097); the Key Clinical Research Project of Public Health Ministry of China 2010-2012; the Outstanding Young Scientist Research Award Foundation of Shandong Province (no. BS2010YY024); the Natural Science Foundation of Shandong Province (nos. ZR2009CM001 and ZR2010HQ002); the Clinical Medicine Center Foundation of Shandong Province, Leading Medical Professionals Foundation of Shandong Province, Tai Shan Scholar Foundation, Independent Innovation Foundation of Shandong University (no. 2009TS053); the State Program of National Natural Science Foundation of China for Innovative Research Group (no. 81021001); and Commonweal Trade for Scientific Research (200802031).
Authorship
Contribution: X.-g.L. and J.P. designed and performed research, analyzed data, and wrote the paper; S.-h.M., J.-z.S., L.S., X.-y.D., C.-s.G., and M.H. performed research and analyzed data; and J.R., Y.S., and P.Q. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, Jinan 250012, China; e-mail: junpeng88@sina.com.cn; or Ming Hou, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Jinan 250012, China; e-mail: houming@medmail.com.cn.
References
Author notes
X.-g.L., S.-h.M., and J.-z.S. contributed equally to this work.