Abstract
Extranodal NK/T-cell lymphoma, nasal type, is a rare and highly aggressive disease with a grim prognosis. No therapeutic strategy is currently identified in relapsing patients. We report the results of a French prospective phase II trial of an L-asparaginase-containing regimen in 19 patients with relapsed or refractory disease treated in 13 centers. Eleven patients were in relapse and 8 patients were refractory to their first line of treatment. L-Asparaginase–based treatment yielded objective responses in 14 of the 18 evaluable patients after 3 cycles. Eleven patients entered complete remission (61%), and only 4 of them relapsed. The median overall survival time was 1 year, with a median response duration of 12 months. The main adverse events were hepatitis, cytopenia, and allergy. The absence of antiasparaginase antibodies and the disappearance of Epstein-Barr virus serum DNA were significantly associated with a better outcome. These data confirm the excellent activity of L-asparaginase–containing regimens in extranodal NK/T-cell lymphoma. L-Asparaginase–based treatment should thus be considered for salvage therapy, especially in patients with disseminated disease. First-line L-asparaginase combination therapy for extranodal NK/T-cell lymphoma warrants evaluation in prospective trials. This trial is registered at www.clinicaltrials.gov as #NCT00283985.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma, nasal type, is a rare and severe malignancy. It is more frequent in Asia and Central/South America than in Europe and North America. It is thought to arise from NK cells or, occasionally, from a subset of γδ or αβ cytotoxic T cells, and shows a tight association with Epstein-Barr virus (EBV). It is classically characterized by a cytoplasmic CD3 ϵ phenotype, with no surface CD3 or T-cell receptor expression, no T-cell receptor gene rearrangements, an activated cytotoxic profile with perforin, granzyme B, and TIA-1 expression, and frequent CD56 expression.1-3
Extranodal NK/T-cell lymphoma is usually diagnosed in adults, with a median age in the fifth decade. The nasal cavity or other parts of the upper aerodigestive tract are primarily involved, but some cases occur at extranasal sites. The term “nasal-type” is used in the World Health Organization classification to describe forms arising both in the nasal cavity and in extranasal sites.3,4
Because disease incidence is rare even in prevalent areas, there has been no randomized controlled trial, and most treatment protocols are consensus-guided.5 Localized NK/T-cell lymphomas often respond to radiotherapy6,7 or to concurrent radiation and chemotherapy,8 but relapse is common. Chemotherapy protocols used for lymphomas of other histologic subtypes are poorly effective, at least in part, because of frequent multidrug resistance gene expression by tumor cells.9 Patients with disseminated or relapsing disease have a very poor outcome,4,10,11 and there is no standard management for relapsed or refractory disease. We and others, in small retrospective studies,12-16 have observed very good response and survival rates in patients treated with L-asparaginase, a drug with an original antitumoral mechanism not affected by MDR. NK cells lack asparagine synthase activity, and asparaginase has been shown to induce apoptosis of tumoral NK cells in vitro.17 These findings provided the rationale for this open-label phase 2 study of an L-asparaginase–containing regimen for patients with relapsed and/or refractory extranodal NK/T-cell lymphoma. We chose to combine L-asparaginase with methotrexate, a drug insensitive to the multidrug resistance pathway, because of its well-known synergistic effect with asparaginase in acute lymphoblastic leukemia and its ability to prevent central nervous system involvement. Dexamethasone was added because T-cell lymphomas are usually sensitive to corticosteroids and dexamethasone seems to be associated with a lower risk of thrombosis when given with L-asparaginase.18
Methods
Patients
The patients were at least 18 years of age, with relapsed or refractory extranodal NK/T-cell lymphoma. Eligibility criteria included biopsy-proven diagnosis of NK/T-cell lymphoma, nasal-type, irrespective of the anatomic site. Malignant cells in all cases had a CD3ϵ+, CD20− phenotype, a cytotoxic profile, and evidence of EBV infection (EBV-encoded small nuclear RNA by in situ hybridization, or latent membrane protein-1 by immunohistochemistry). Most cases (15 among 18 with this marker available) were CD56+. All the cases were centrally reviewed by 3 expert hematopathologists (B.P., L.d.L., and P.G.). Exclusion criteria included previous asparaginase administration, pregnancy or breastfeeding, contraindication to one of the trial drugs (uncontrolled diabetes mellitus, progressive phlebitis, renal or hepatic impairment contraindicating methotrexate or L-asparaginase), and another unrelated severe progressive disease or malignancy. There was no upper age or performance status limit for inclusion; central nervous system involvement was not mentioned as an exclusion criterion.
Staging consisted of a complete history taking and physical examination, as well as routine blood tests and serum chemistry before treatment, including lactate dehydrogenase and ferritin assay. EBV serum DNA, which is predictive of the treatment response and outcome in this setting,19 was centrally monitored by polymerase chain reaction-based assay. We define high and low levels as values more than or less than the median value of 26 900 copies/mL. The patients were routinely screened for antiasparaginase antibodies, with an antibody-capture enzyme-linked immunosorbent assay,20 before treatment, before the second and third cycles, 2 days after the end of cycle 3, and 1 month after the end of the study treatment.
Computed tomography, magnetic resonance imaging, and whole-body positron electron tomography (PET), when possible, were used to determine the extent of the primary lesion, to assess the response after 3 cycles and at the end of the study treatment, and to document relapses.
All the patients gave their written informed consent before entering the study, which conformed to the Declaration of Helsinki and was approved by our institutional review board (CPP Limousin).
Study design and treatment
The patients received 3 21-day cycles of the asparaginase, methotrexate, dexamethasone (AspaMetDex) protocol, consisting of Escherichia coli L-asparaginase (Kidrolase, Eusa Pharma) 6000 units/m2 of body surface area on days 2, 4, 6, and 8, intramuscularly (unless contraindicated), plus methotrexate 3 g/m2 on day 1, and oral dexamethasone 40 mg from day 1 to 4. The methotrexate and dexamethasone doses were reduced to 2 g/m2 and to 20 mg for 4 days, respectively, in patients more than 70 years of age.
Patients who had allergic reactions to the L-asparaginase injections subsequently received Erwinia asparaginase (20 000 U/m2, Erwiniase, Eusa Pharma) with the same schedule. Antithrombin and fibrinogen serum levels were measured before each injection of asparaginase and 48 hours after the last one of each cycle. Patients with serum antithrombin levels less than 60% of normal or fibrinogen levels less than 0.5 g/L received replacement therapy. During a short hospitalization, all the patients received alkaline hydration and leucoverin rescue with methotrexate, and anti-infectious prophylaxis with trimethoprim-sulfamethoxazole (which was stopped during methotrexate treatment) and valaciclovir prophylaxis were given.
After 3 cycles, patients with localized disease who had never been irradiated received radiotherapy. Patients with good performance status and disseminated disease received intensive treatment with the BEAM regimen (carmustine 300 mg/m2 intravenously on day 1, etoposide 400 mg/m2 intravenously on days 2-5, cytarabine 400 mg/m2 by continuous infusion on days 2-5, and melphalan 140 mg/m2 intravenously on day 6), followed by peripheral blood stem cell infusion within 36 to 48 hours after melphalan administration. Other responder patients could receive up to 6 cycles of the AspaMetDex regimen.
Assessment of efficacy
The primary endpoint was the complete response rate after 3 cycles of AspaMetDex, Secondary endpoints were overall response and progression rate, overall survival (OS), progression-free survival (PFS), tolerability, and toxicity.
Responses were assessed by computed tomography scan, MRI, and/or PET scan (depending on abnormal results at baseline) with an adaptation of the Revised Response Criteria for Lymphoma,21 taking into account the rarity of lymph node involvement in this type of lymphoma and the frequency of PET false-positives resulting from residual inflammatory and infectious lesions, particularly in the nasal cavity. Complete remission (CR) was defined as no evidence of residual disease; a partial response as at least a 50% reduction in tumor burden compared with the beginning of treatment; and “no response” as less than a 50% reduction in tumor burden, or disease progression.
Responses were assessed 3 weeks after the third cycle of the AspaMetDex protocol and 1 month after the end of treatment.
Assessment of safety and other secondary endpoints
Asparaginase toxicity was monitored by complete blood cell counts, coagulation tests, and antithrombin, amylase, glycemia, bilirubin, aspartate aminotransferase, and alanine aminotransferase assays. Serious adverse events were assessed at each cycle and graded according to the National Cancer Institute Common Toxicity Criteria, Version 3, from the first injection of methotrexate until 1 month after the last study treatment.
Statistical methods
Sample size.
The trial based on a one single stage Fleming plan22 was designed to have a power of 80% to test the following one-sided hypothesis as regards the actual probability of complete response (p); H0: P less than or equal to 20% (20% being the usual probability of response while using conventional therapy) versus H1: P ≥ 40% (40% being the response proportion that would imply the treatments warrants further investigation), with an α risk of 5%. An estimated 29 patients needed to be enrolled to obtain 10 complete responses. The trial was halted by the independent monitoring committee after inclusion of 20 patients because 11 complete responses had been obtained.
Statistical analysis.
Quantitative variables were described using median, range, and interquartile range. Qualitative variables were described using frequencies. Proportion of complete response rate was calculated as well as the lower bound of the one-sided 95% confidence interval (95 CI) of this percentage. A one-sided Yates χ2 test was used to compare actual response rate to 20%.
All efficacy analyses were conducted in the intention-to-treat population. All the patients were included in the safety analysis.
Survival analyses for OS and PFS were performed using Kaplan-Meier method. Median survival time and their 95% CI were assessed. Censoring times are displayed on curves. Baseline time was the delay between the date of inclusion to the event or censoring date. For PFS, dying of an unrelated cause was censored. Comparisons of OS or PFS between groups were performed using log-rank test. P values less than 5% were considered statistically significant.
Results
Patients and treatment
From March 2006 to October 2008, 13 centers prospectively enrolled a total of 20 patients. One patient was excluded after pathologic review. Table 1 shows the characteristics of the remaining 19 patients (4 women and 15 men). Median age was 60 years (range, 45-77 years), 16 patients were of European white origin, and 3 patients were one each from Cambodia, Algeria, and Haiti. Eleven patients were in relapse, a median of 10 months after their last treatment, and 8 patients were refractory to their initial treatment. Before inclusion, 18 patients had received chemotherapy, including Cytoxan, hydroxyrubicin, Oncovin, prednisone (CHOP) or CHOP-like regimens in 17 patients, and 9 patients had received irradiation. The median number of chemotherapy lines was 1 (range, 1-4).
Patient characteristics, treatment and outcome
| Sex . | Median age, y (range) . | Stage/site at inclusion . | Median cycle number (range) . | Consolidation treatment after L-asparaginase . | Response after 3 cycles and outcome . | Median follow-up, months for all patients and surviving patients (range) . |
|---|---|---|---|---|---|---|
| Male: 15 | 60 (45-76) | IE /IIE: 12 | 3 (1-6) | ASCT (BEAM): 5 | CR: 11, PR: 3 | 12.3 (1-48) |
| Female: 4 | IV: 7 | RT: 1 | NR: 4, NE: 1 | 26.2 (17-48) | ||
| NOD: 8 | ||||||
| DOD: 10 | ||||||
| DUD: 1 |
| Sex . | Median age, y (range) . | Stage/site at inclusion . | Median cycle number (range) . | Consolidation treatment after L-asparaginase . | Response after 3 cycles and outcome . | Median follow-up, months for all patients and surviving patients (range) . |
|---|---|---|---|---|---|---|
| Male: 15 | 60 (45-76) | IE /IIE: 12 | 3 (1-6) | ASCT (BEAM): 5 | CR: 11, PR: 3 | 12.3 (1-48) |
| Female: 4 | IV: 7 | RT: 1 | NR: 4, NE: 1 | 26.2 (17-48) | ||
| NOD: 8 | ||||||
| DOD: 10 | ||||||
| DUD: 1 |
ASCT indicates autologous stem cell transplantation; BEAM, BCNU, etoposide, Ara-C, melphalan; RT, radiotherapy; CR, complete response; PR, partial response; NR, no response; NE, not evaluated; NOD, no evidence of disease; DOD, died of disease; and DUD, dead unrelated to disease.
At inclusion, 7 patients were in stage IE, 5 were in stage IIE (with node involvement in 4 patients), and 7 were in stage IV (with involvement of the liver in 2 patients, bone marrow in 3 patients, muscle in 2 patients, and skin in 2 patients). Only one patient had no apparent nasal involvement.
Performance status was 0 or 1 in 15 patients and 2 or 3 in 4 patients. Systemic B symptoms were present in 9 patients. Nine patients had a high lactate dehydrogenase serum level. One patient had a hemophagocytic syndrome, with fever, hepatosplenomegaly, cytopenia, hyperferritinemia, hypertriglyceridemia, and hypofibrinogenemia.
Three patients who rapidly progressed or died received only one or 2 cycles of the study treatment, 10 patients received 3 or 4 cycles (with intensive treatment in 5 cases), and 6 patients received 6 cycles. Only one patient, with localized disease, received radiotherapy.
Response and relapse rates
The response rate was assessed in 18 patients among whom 14 patients were responders with 11 complete responses (61%). The lower bound of the one-sided 95% CI of this percentage was 42%. The CR rate was significantly different from 20% (P < .001, Yates χ2 test). One patient was not evaluated at 3 cycles because he died of an unrelated cause and received only 2 cycles of the study treatment. This patient, a 70-year-old man who had extranasal disease with muscle and lymph node involvement, which had progressed after CHOP, had an excellent partial response documented by PET after the first cycle of the AspaMetDex protocol. Of the 4 nonresponder patients, one had stable disease and progressed after one year, whereas 3 patients progressed during the first 3 cycles and died rapidly.
Six of the 14 patients who had responded after 3 cycles relapsed, 1 to 9 months (median, 3 months) after the end of treatment, composing 2 of the 3 patients with a partial response and 4 of the 11 patients with a complete response. Three of the 5 patients who received intensive treatment relapsed, 2 of whom were in partial remission before intensive treatment.
Survival
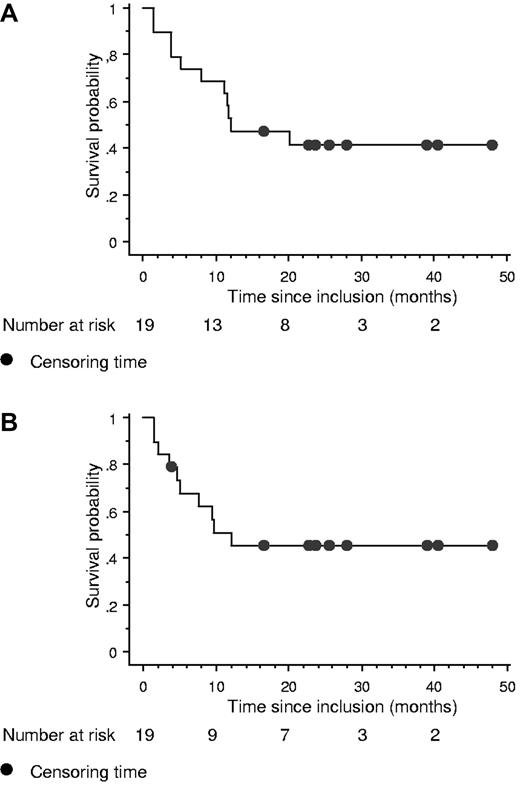
The median survival time was 12.2 months 95% CI (8.0 months to not calculated) in the entire cohort and the median follow-up among surviving patients was 26.2 months interquartile range (16.9-48.6 months; Figure 1A). The median PFS was 12.2 months 95% CI (5.0 months to not calculated; Figure 1B). Eight patients are alive in persistent complete remission. The median survival times after inclusion for nonresponding patients and after relapse for responding patients who relapsed were only 4.2 months (range, 1-12 months) and 3.6 months (2.5-8 months), respectively.
Prognostic factors
In univariate analysis, the only clinical factor predictive of poor survival was refractoriness at inclusion (P = .019 for OS and P = .053 for PFS).
Age, poor performance status (Eastern Cooperative Oncology Group performance status 2-4), advanced Ann Arbor stage (stage III/IV), an elevated lactate dehydrogenase level, regional lymphadenopathy, and B symptoms were not predictive of OS or PFS.
Antiasparaginase antibodies
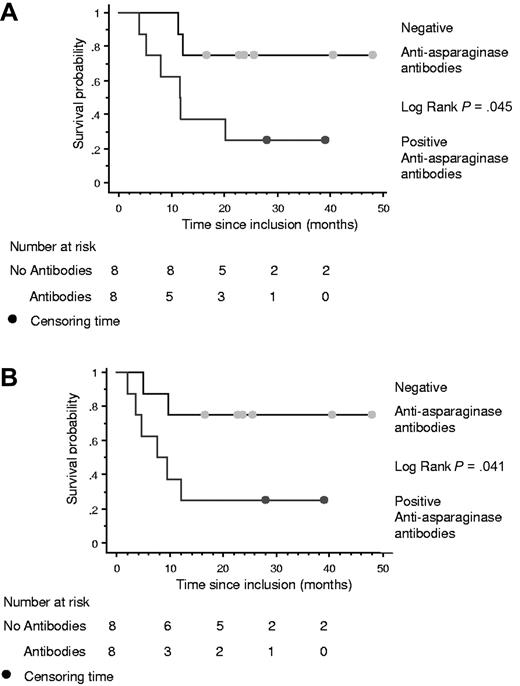
The 16 patients who received 3 or more chemotherapy cycles had serial antiasparaginase antibody assays. No antibodies were found before treatment. Eight patients became positive (5 after 1 cycle, 1 after 2 cycles, and 2 after 6 cycles), including all 4 patients with allergic reactions to asparaginase infusion, and 6 of these patients progressed (n = 2) or relapsed (n = 4). By comparison, only 2 of the 8 patients without detectable antibodies relapsed. Survival and PFS were significantly better among patients without antibodies (P = .045 and P = .041, respectively; Figure 2).
Kaplan-Meier curves among the 16 patients who received at least 3 cycles, according to antiasparaginase antibody status. (A) OS. (B) PFS.
Kaplan-Meier curves among the 16 patients who received at least 3 cycles, according to antiasparaginase antibody status. (A) OS. (B) PFS.
EBV serum DNA
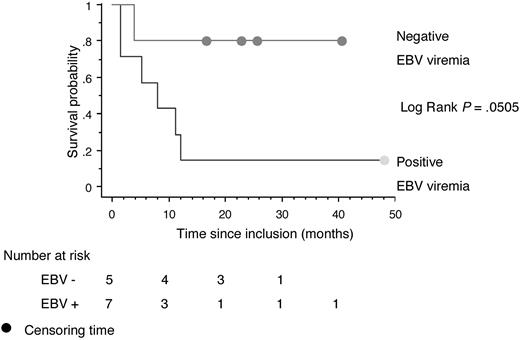
Before treatment, 13 patients (68%) had detectable EBV serum DNA. There was no difference in survival between positive and negative patients (P = .60) or between patients with low and high EBV serum DNA (P = .46). Survival and PFS were markedly better (P = .0505) in patients in whom serum EBV DNA was detectable at inclusion and became undetectable after one cycle (5 patients, 4 CR) compared with those in whom serum DNA was still detectable after the first cycle (7 patients, 3 CR; Figure 3). All patients whose EBV DNA never became undetectable at any stage of their illness died.
Kaplan-Meier survival curve for OS among 12 patients with positive EBV serum DNA at inclusion in whom EBV serum DNA became undetectable after the first cycle of the AspaMetDex regimen (n = 5) or not (n = 7).
Kaplan-Meier survival curve for OS among 12 patients with positive EBV serum DNA at inclusion in whom EBV serum DNA became undetectable after the first cycle of the AspaMetDex regimen (n = 5) or not (n = 7).
Safety
The main adverse events are shown in Table 2. Fifteen patients had a fall in the antithrombin level to less than 60% of normal and thus received antithrombin infusions (Aclotine). Two grade 2 thromboembolic events occurred. Eleven patients developed hepatitis (severe in 3 cases) and 4 had anaphylactic reactions to asparaginase infusion. Eleven patients developed neutropenia (grade 4 in one patient), 5 patients had reversible renal impairment probably resulting from methotrexate (grade 1 in 3 patients and grade 2 in 2 patients), and 5 patients developed infections that were grade 2 in 3 patients, grade 3 and 4 (Pseudomonas aeruginosa septicemia) in one patient each. No diabetes or pancreatitis occurred.
Toxicity profiles: all grades and grade 3/4
| Toxicity . | All grades . | Grades 3/4 . |
|---|---|---|
| Liver | 11 | 3 |
| Allergy | 4 | 1 |
| Infection | 5 | 2 |
| Renal failure | 5 | 0 |
| Thrombosis | 2 | 0 |
| Neutropenia | 11 | 8 |
| Febrile neutropenia | 3 | 2 |
| Anemia | 12 | 4 |
| Thrombopenia | 7 | 1 |
| Prolonged (or additional) hospitalizations resulting from toxicity | 8 (1) | 0 |
| Toxicity . | All grades . | Grades 3/4 . |
|---|---|---|
| Liver | 11 | 3 |
| Allergy | 4 | 1 |
| Infection | 5 | 2 |
| Renal failure | 5 | 0 |
| Thrombosis | 2 | 0 |
| Neutropenia | 11 | 8 |
| Febrile neutropenia | 3 | 2 |
| Anemia | 12 | 4 |
| Thrombopenia | 7 | 1 |
| Prolonged (or additional) hospitalizations resulting from toxicity | 8 (1) | 0 |
Discussion
In a recent international multicenter series reported by the International Peripheral T-cell Lymphoma Project, the median survival time for patients with de novo NK/T-cell lymphoma was only 7.8 months, the worst survival time among all the peripheral T-cell lymphoma categories included in this project.23 Importantly, the authors emphasized that the failure-free survival and OS curves were similar at one year, as most relapses were not salvageable.
The results obtained here in a high-risk population of relapsing and/or refractory patients with NK/T-cell lymphoma are therefore remarkable. Almost 80% of the patients responded to asparaginase-containing regimen, and the response was complete in 11 of 18 cases (61%) after 3 cycles.
The complete response rate is twice the response rate obtained elsewhere with first-line CHOP23 and also in a recent study for refractory/relapsing patients with a salvage regimen (etoposide, ifosfamide, methotrexate, and prednisolone).11 The complete response rate to conventional chemotherapy in patients with stage III or IV disease is less than 15%,24,25 whereas 6 of our 7 patients with stage IV disease responded, with 4 of them entering complete remission (50%). These results, obtained in a prospective trial, confirm those obtained by the Chinese group who first reported the use of asparaginase to treat NK/T-cell lymphoma, in 4 retrospective series.13-16 However, in these studies, the patients had localized disease and received irradiation after asparaginase, making it impossible to determine whether asparaginase or irradiation was responsible for the excellent response rate. Because our 19 patients had already received radiation or had disseminated disease, only one patient received irradiation after 3 cycles of asparaginase-based therapy. The persistent complete responses obtained in the other 7 patients were therefore the result of the chemotherapy alone (AspaMetDex in 5 patients and AspaMetDex plus intensive treatment with stem cell support in 2 patients). These results are in keeping with those of a recent small prospective study26 and several case reports showing the remarkable efficacy of asparaginase in NK/T-cell neoplasms.27-33
The responses occurred very rapidly, usually after the first cycle as shown in responding patients by the rapid drop in EBV serum DNA, which was, as already published,19 a good marker of outcome.
Why NK/T tumor cells are so sensitive to asparagine privation is not completely understood, but the usual absence of asparagine synthetase gene expression in this setting probably plays a role. Further work will be critical to decipher the mechanisms responsible for asparaginase resistance, which is found in only 20% of patients.
The precise contribution of methotrexate and dexamethasone to the efficacy of the AspaMetDex protocol is not known, but the better survival of responding patients who did not develop antiasparaginase antibodies points to a prominent role of L-asparaginase. These antibodies inhibit asparaginase activity and may affect clinical outcome of acute lymphoblastic leukemia.34 In our study, these antibodies, which were associated with allergic reactions in approximately half the patients concerned, were associated with more frequent relapse or progression (6 of 8 patients with antibodies vs 2 of 8 patients without antibodies). Several strategies may be used to overcome this problem, including early antibody detection, a routine switch between asparaginase molecules after 2 cycles, or the use of pegylated asparaginase, which is reported to be less immunogenic. In case of relapse after a response to one form of asparaginase, another form should be tested if antibodies are present because no other effective salvage regimen is available, as shown by the international study and confirmed by the very short survival of nonresponding and relapsing patients in our trial.
One striking result of our study is the difference in outcome between relapsing and refractory patients as 7 of 11 of the former are in persistent remission, whereas only one of 8 of the latter is still alive. This might be ascribed to a higher frequency of disseminated disease in the refractory group (5 of 8 stage IV patients) than in the relapsing group (2 of 11 stage IV patients). Another possibility might be that tumor cells from refractory patients have developed specific mechanisms of resistance to chemotherapy, including asparaginase. Further work is warranted to identify these mechanisms at the molecular level.
In conclusion, the results of this phase 2 trial confirm the efficacy of L-asparaginase in patients with extranodal NK/T-cell lymphoma. Patients with NK/T-cell lymphoma must not be placed on supportive care without first trying an asparaginase-containing regimen, even if their disease is disseminated, refractory to previous treatments, or in relapse.
Data from clinical and laboratory studies may already justify trials of first-line L-asparaginase combination therapy for extranodal NK/T-cell lymphoma, combined with irradiation for patients with localized disease and high-dose treatment with stem cell support for patients with disseminated disease.
Presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 8, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fabienne Auroy for excellent clinical data management, Florence Bosselut for excellent biologic data management, Abdeslam Bentaleb for administrative work, and Eusa pharma for providing Erwinia asparaginase.
This work was supported by Direction de la Recherche Clinique, Centre Hospitalier Universitaire, Limoges, and the French “program hospitalier de recherche clinique” and Limousin Association pour la Recherche en Hématologie Clinique.
Authorship
Contribution: A.J., N.G., B.M., S.R., P.G., J.F., and O.H. designed the research; A.J., B.M., P.G., and O.H. wrote the paper; A.J., N.G., S.R., M.A., F.S., H.T., F.M., C.T., L.Y., A.D., D.B., and O.H. performed research; B.P., L.d.L., and P.G. did the pathologic review; and A.J., B.M., and O.H. analyzed results and made the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of participating clinicians, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Arnaud Jaccard, Service d'Hématologie et de Thérapie Cellulaire, 1 av ML-King, CHU Limoges, France; e-mail: arnaud.jaccard@chu-limoges.fr; and Olivier Hermine, Service d'Hématologie, Hôpital Necker, 149–161 rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: ohermine@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal