Abstract
Activation of the adaptive Ire1-XBP1 pathway has been identified in many solid tumors and hematologic malignancies, including multiple myeloma (MM). Here, we report the identification of STF-083010, a novel small-molecule inhibitor of Ire1. STF-083010 inhibited Ire1 endonuclease activity, without affecting its kinase activity, after endoplasmic reticulum stress both in vitro and in vivo. Treatment with STF-083010 showed significant antimyeloma activity in model human MM xenografts. Similarly, STF-083010 was preferentially toxic to freshly isolated human CD138+ MM cells compared with other similarly isolated cell populations. The identification of this novel Ire1 inhibitor supports the hypothesis that the Ire1-XBP1 axis is a promising target for anticancer therapy, especially in the context of MM.
Introduction
Multiple myeloma (MM) is a B-cell neoplasm associated with unregulated/uncontrolled differentiation and proliferation of mature B cells to plasma cells. Despite significant therapeutic advances in recent years, MM remains an incurable disease in most patients.1
Because of high production of secreted antibodies, MM cells display chronic endoplasmic reticulum (ER) stress, and their survival is dependent on the adaptive Ire1-XBP1 branch of the unfolded protein response pathway.2 Investigators have shown that perturbing the unfolded protein response with proteasome inhibitors can sensitize MM cells to apoptosis.3 The Food and Drug Administration has approved the proteasome inhibitor bortezomib as the first example of an unfolded protein response modulating chemotherapy for the treatment of MM.4
Recent in vitro Ire1 kinase and RNase assays have yielded insight into determining the mechanism of Ire1 activation.5,6 Mutant Ire1 proteins with amino acid substitutions at conserved positions in the kinase domain identified nucleotide binding and kinase-domain phosphorylation as necessary for Ire1 RNase activation.7-9 However, a report found that an adenosine triphosphate (ATP) competitive inhibitor, 1NM-PP1, could activate Ire1 that contained a mutation in the ATP-binding site. In this context, kinase activity was not required for Ire1 function, suggesting that Ire1 activity may be modulated through an allosteric mechanism.10 The crystal structure of the cytosolic portion of activated yeast Ire1 revealed a back-to-back configuration of the kinase domain within the Ire1 dimer.11 This structure supported a model in which dimerization (or oligomerization) of Ire1 juxtaposes kinase domains, which facilitates trans-autophosphorylation of the protein, resulting in a competent nuclease pocket and enhanced RNase activity.8,9 More recently, the flavanoid quercetin was shown to activate Ire1 through a newly described ligand-binding pocket along the Ire1 dimer interface.12 These data suggest the pharmacologic potential for multiple ligands to selectively modulate either Ire1 kinase or RNase activity.
We hypothesize that MM cells exist under inherent ER stress and that targeting the adaptive Ire1-XBP1 response could be a promising therapeutic strategy. We report the identification of a novel compound that specifically blocks the endonuclease activity of Ire1 without affecting its kinase activity. In support of our hypothesis, we show that this small molecule demonstrated significant single-agent activity in MM xenograft and human MM ex vivo studies.
Methods
Chemical library screening
The screen was performed in the Stanford High-Throughput Bioscience Center. A detailed protocol is described in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell free Ire1 assays
hIre1α protein, containing both Ire1 cytoplasmic kinase and RNase domains, was expressed and purified from baculovirus as described.13 Autophosphorylation activity was determined by the addition of 32P-γATP. Endonuclease activity was determined by the addition of radiolabeled HAC1 508-nt RNA substrate synthesized in vitro using α32P-UTP.5 STF083010 was incubated with recombinant hIRE1α protein, radiolabeled HAC1 508 nt RNA, and appropriate buffers. Kinase activity and RNAse cleavage products were quantitated by polyacrylamide gel electrophoresis and 32P-γATP or 32P-UTP autoradiography, respectively.
Human specimen isolation and assays
Bone marrow aspirates were obtained from MM patients after obtaining informed consent in accordance with the Declaration of Helsinki with approval from the Institutional Review Board of the University of Ghent, Ghent, Belgium. CD138+ plasma cells were selected by positive magnetic bead selection (StemCell Technologies) either after isolation of nucleated cells (patients 1-4) or directly (patients 5-8). Peripheral blood cells were obtained by Ficoll-Hypaque density centrifugation from separate control patients.
Cell culture, reporter assays, reverse-transcription polymerase chain reaction, Western blotting, and bioluminescent imaging
Results and discussion
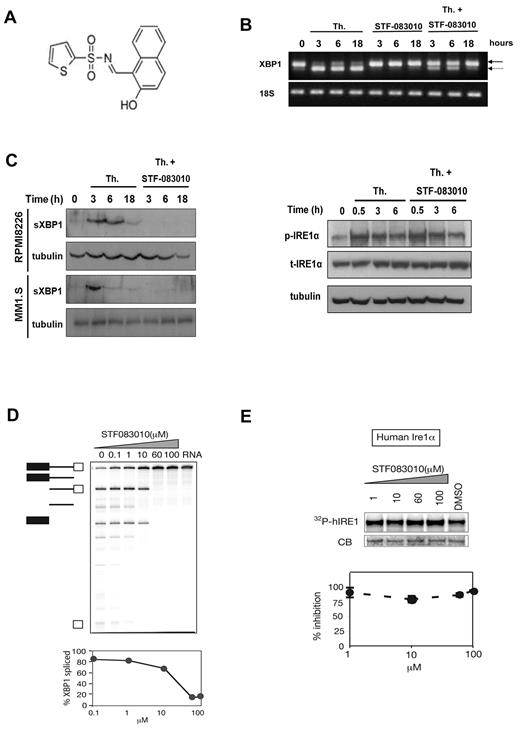
STF-083010 was identified in our cell-based reporter gene, high-throughput screen, and its structure is shown in Figure 1A. To confirm the molecular target, we analyzed the effect of STF-083010 on endogenous XBP1 mRNA splicing levels in control and ER-stressed RPMI 8226 human MM cells (Figure 1B). Incubation with thapsigargin, an inhibitor of ER calcium flux that induces ER stress, resulted in XBP1 splicing. However, with the addition of STF-083010, splicing was almost completely blocked. STF-083010 also inhibited XBP1 splicing activated by other types of ER stress, including tunicamycin treatment, glucose deprivation, or severe hypoxia (data not shown). As expected, inhibiting XBP1 mRNA splicing also inhibited production of active sXBP1 protein (Figure 1C left panel). However, STF-083010 did not inhibit Ire1α kinase activity, as shown in the immunoblot of autophosphorylated Ire1α (Figure 1C right panel). To establish that this effect was the result of a direct interaction between STF-083010 and Ire1α, we analyzed Ire1α enzymatic activity in a cell-free system. STF-083010 blocked Ire1α's endonuclease activity (Figure 1D) without affecting its kinase activity (Figure 1E).
Identification of an Ire1α endonuclease inhibitor. (A) Chemical structure of inhibitor STF-083010. (B) STF-083010 inhibited endogenous XBP1 mRNA splicing. RPMI 8226 cells were treated with 300nM thapsigargin (Th), 60μM STF-083010, or both for the indicated amount of time, and the relative XBP1 splicing was determined by reverse-transcribed polymerase chain reaction. Solid arrow indicates the unspliced form; and broken arrow, the spliced form. (C) STF-083010 inhibits the production of sXBP1 protein but not the autophosphorylation of Ire1α. The indicated MM cell lines were treated for the indicated times with 300nM Th and 60μM STF-083010, and sXBP1 protein was detected by immunoblotting (left panel). RPMI 8226 cells were treated with 300nM Th and 60μM STF-083010 for the indicated times, and the levels of phosphorylated and total Ire1α were detected using specific antibodies (right panel). (D) STF-083010 effect on cell-free Ire1α RNase (endonuclease) activity. Upper panel: hIre1 was incubated with uniformly labeled (32P) HAC1 508-nt transcript for 30 minutes in the presence of increasing concentrations of STF-083010 (1-100μM). HAC1 mRNA cleavage reaction was analyzed by separation of products on denaturing polyacrylamide gels, followed by autoradiography. Lower panel: Quantitation of HAC1 mRNA processing showing half-maximal inhibition at approximately 25μM. Error bars represent SEM of 3 independent experiments. (E) Effect of STF-083010 on cell-free Ire1α kinase activity. Upper panel: hIre1α was incubated with 32P-γATP and increasing concentrations (0-100μM) of STF-083010. Ire1α autophosphorylation was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by autoradiography to determine the amount of 32P incorporation (32P-hIRE1). Lower panel: Kinase activity showed no significant change during coincubation with STF-083010. Error bars represent SEM of 3 independent experiments.
Identification of an Ire1α endonuclease inhibitor. (A) Chemical structure of inhibitor STF-083010. (B) STF-083010 inhibited endogenous XBP1 mRNA splicing. RPMI 8226 cells were treated with 300nM thapsigargin (Th), 60μM STF-083010, or both for the indicated amount of time, and the relative XBP1 splicing was determined by reverse-transcribed polymerase chain reaction. Solid arrow indicates the unspliced form; and broken arrow, the spliced form. (C) STF-083010 inhibits the production of sXBP1 protein but not the autophosphorylation of Ire1α. The indicated MM cell lines were treated for the indicated times with 300nM Th and 60μM STF-083010, and sXBP1 protein was detected by immunoblotting (left panel). RPMI 8226 cells were treated with 300nM Th and 60μM STF-083010 for the indicated times, and the levels of phosphorylated and total Ire1α were detected using specific antibodies (right panel). (D) STF-083010 effect on cell-free Ire1α RNase (endonuclease) activity. Upper panel: hIre1 was incubated with uniformly labeled (32P) HAC1 508-nt transcript for 30 minutes in the presence of increasing concentrations of STF-083010 (1-100μM). HAC1 mRNA cleavage reaction was analyzed by separation of products on denaturing polyacrylamide gels, followed by autoradiography. Lower panel: Quantitation of HAC1 mRNA processing showing half-maximal inhibition at approximately 25μM. Error bars represent SEM of 3 independent experiments. (E) Effect of STF-083010 on cell-free Ire1α kinase activity. Upper panel: hIre1α was incubated with 32P-γATP and increasing concentrations (0-100μM) of STF-083010. Ire1α autophosphorylation was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by autoradiography to determine the amount of 32P incorporation (32P-hIRE1). Lower panel: Kinase activity showed no significant change during coincubation with STF-083010. Error bars represent SEM of 3 independent experiments.
Next, we used transgenic mice harboring the same XBP1-luciferase reporter gene15 to determine the in vivo efficacy of STF-083010. Treatment of these reporter mice with bortezomib induced ER stress and a commensurate increase in XBP1-luciferase bioluminescent activity. However, when STF-083010 was injected with bortezomib, there was no increase in bioluminescent signal (Figure 2A-B). This inhibition was not the result of cellular toxicity in the animals, as we did not find any histologic effects of STF-083010 after treatment at doses used in these studies (60 mg/kg one time and 30 mg/kg 2 times; supplemental Figure 1 and supplemental Table 1).
In vivo and ex vivo effects of STF-083010. (A) STF-083010 blocks bortezomib-induced XBP1 activity in vivo. Transgenic XBP1-luc mice were injected intraperitoneally with drug vehicle (16% chremophor), 1 mg/kg bortezomib, or 1 mg/kg bortezomib and 60 mg/kg STF-083010, and bioluminescence was measured after 24 hours. Graph represents the average change in the number of photons per animal 24 hours after treatment. Error bars represent SEM of at least 4 animals. (B) Images of representative animals before and after treatment. (C) In vitro cytotoxicity of STF-083010. RPMI 8226, MM.1S, and MM.1R MM cells were treated with 0, 30, or 60μM of STF-083010, and viable cell number was measured daily by the trypan blue exclusion method. (D) Antitumor activity of STF-083010 in vivo. RPMI 8226 MM cells were established as subcutaneous tumor xenografts in NOD/SCID/IL2Rγ null mice. When tumors reached an average volume of 150 mm3, 2 groups of 5 mice each were treated with 30 mg/kg STF-083010 or drug vehicle once weekly for 2 weeks. (E) STF-083010 is preferentially cytotoxic against human MM cells. MM cells were obtained by CD138+ selection from bone marrow samples from MM patients; lymphocytes were obtained by Ficoll density-gradient centrifugation of peripheral blood samples from control patients, followed by staining with anti-CD3, anti-CD19, and anti-CD56 monoclonal antibodies to differentiate between T, B, and NK cells. Cells were cultured with the indicated concentrations of STF-083010 for 24 hours, and cell viability was measured by flow cytometric analysis of annexin V/propidium iodide (MM) or 7-amino-actinomycin D (peripheral blood lymphocytes)-stained samples.
In vivo and ex vivo effects of STF-083010. (A) STF-083010 blocks bortezomib-induced XBP1 activity in vivo. Transgenic XBP1-luc mice were injected intraperitoneally with drug vehicle (16% chremophor), 1 mg/kg bortezomib, or 1 mg/kg bortezomib and 60 mg/kg STF-083010, and bioluminescence was measured after 24 hours. Graph represents the average change in the number of photons per animal 24 hours after treatment. Error bars represent SEM of at least 4 animals. (B) Images of representative animals before and after treatment. (C) In vitro cytotoxicity of STF-083010. RPMI 8226, MM.1S, and MM.1R MM cells were treated with 0, 30, or 60μM of STF-083010, and viable cell number was measured daily by the trypan blue exclusion method. (D) Antitumor activity of STF-083010 in vivo. RPMI 8226 MM cells were established as subcutaneous tumor xenografts in NOD/SCID/IL2Rγ null mice. When tumors reached an average volume of 150 mm3, 2 groups of 5 mice each were treated with 30 mg/kg STF-083010 or drug vehicle once weekly for 2 weeks. (E) STF-083010 is preferentially cytotoxic against human MM cells. MM cells were obtained by CD138+ selection from bone marrow samples from MM patients; lymphocytes were obtained by Ficoll density-gradient centrifugation of peripheral blood samples from control patients, followed by staining with anti-CD3, anti-CD19, and anti-CD56 monoclonal antibodies to differentiate between T, B, and NK cells. Cells were cultured with the indicated concentrations of STF-083010 for 24 hours, and cell viability was measured by flow cytometric analysis of annexin V/propidium iodide (MM) or 7-amino-actinomycin D (peripheral blood lymphocytes)-stained samples.
To determine antimyeloma activity of STF-083010, first we treated a panel of MM cell lines with increasing concentrations of the compound in vitro and measured viability by trypan blue exclusion (Figure 2C). STF-083010 showed cytostatic and cytotoxic activity in a dose- and time-dependent manner. Next, we treated RPMI 8226 human MM cells grown as tumor xenografts in NSG mice. Intraperitoneal injection of STF-083010 alone (day 1, day 8) significantly inhibited the growth of these tumors (Figure 2D). In addition, we treated fresh CD138+ cells isolated from MM patients and compared the ex vivo toxicity to that of cells from 7 control patients (Figure 2E; supplemental Tables 2-3). STF-083010 was selectively cytotoxic to CD 138+ cells compared with B (CD19+), T (CD3+), and NK (CD56+) cells.
In conclusion, we have identified a novel compound with the unique property that specifically blocks the endonuclease activity of Ire1 without affecting its kinase function. This small molecule represents a new class of targeted agents with significant anticancer activity in various in vitro and in vivo models of MM. Future studies are necessary to determine how best to integrate Ire1-XBP1-targeted therapies with existing treatments for MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant PO1 CA67166, A.C.K. and N.C.D.) and the American Cancer Society (M.N.).
National Institutes of Health
Authorship
Contribution: I.P., M.O., H.V.M., S.L., and A.T. performed experiments; I.P., N.C.D., and A.C.K. conceived the overall study design; and all authors contributed to manuscript writing and editing and helped design experiments and analyze data.
Conflict-of-interest disclosure: A.C.K. is one of the cofounders of Ruga. The other authors declare no competing financial interests.
The current affiliation for M.O. is Edna Williams Cancer Center, Jacksonville, FL.
Correspondence: Albert C. Koong, Department of Radiation Oncology, Stanford School of Medicine, 269 Campus Dr, Rm 1245, Stanford, CA 94305; e-mail: akoong@stanford.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal