Abstract
Increasing evidence suggests that neutrophils may participate in the regulation of adaptive immune responses, and can reach draining lymph nodes and cross-prime naive T cells. The aim of this study was to identify the mechanism(s) involved in the migration of neutrophils to the draining lymph nodes. We demonstrate that a subpopulation of human and mouse neutrophils express CCR7. CCR7 is rapidly expressed at the membrane upon stimulation. In vitro, stimulated human neutrophils migrate in response to the CCR7 ligands CCL19 and CCL21. In vivo, injection of complete Freund adjuvant induces a rapid recruitment of neutrophils to the lymph nodes in wild-type mice but not in Ccr7−/− mice. Moreover, intradermally injected interleukin-17–and granulocyte-macrophage colony-stimulating factor–stimulated neutrophils from wild-type mice, but not from Ccr7−/− mice, migrate to the draining lymph nodes. These results identify CCR7 as a chemokine receptor involved in the migration of neutrophils to the lymph nodes.
Introduction
Polymorphonuclear neutrophils are the most abundant immune cells in human blood (60% of leukocytes). Every day, 1011 neutrophils transit through the adult human circulation.1 They are the first type of leukocytes recruited at the site of infection, where they represent the major infiltrating cells. Their migration and subsequent activation are controlled by chemokines and cytokines such as chemokine (C-X-C motif) ligand 8 (CXCL8) and tumor necrosis factor-α (TNF-α). At the inflammatory site, microorganisms are phagocytosed by neutrophils, destroyed via oxygen-dependent or oxygen-independent mechanisms, or sequestered in extracellular traps.1,2 Neutrophils also contain an important arsenal of microbicidal mediators.3
Neutrophils are short-lived, terminally differentiated cells that have long been considered to be the prototypic innate immune cells and to have a restricted number of properties.4,5 Nevertheless, recent studies have shown that the biologic properties of neutrophils are more complicated than was previously thought. Neutrophils exhibit characteristic features classically dedicated to professional antigen-presenting cells.6,7 Under certain circumstances, neutrophils display a dendritic cell (DC)–like phenotype, as evidenced by the expression of the DC markers CD83, CD80, CD86, and major histocompatibility complex II (MHC-II) molecules. Neutrophils may present antigens in an MHC-II–dependent manner and induce the proliferation of antigen-specific T cells.6-8 More recently, we reported that neutrophils cross-prime naive CD8+ T lymphocytes.9 Finally, neutrophils may also influence the polarization of antigen-specific T-cell responses.10-12
Interestingly, previous studies have reported that, after the capture of antigens in the periphery, neutrophils may migrate to the lymph nodes.13-15 In a murine model of Toxoplasma gondii infection, parasites are transported by neutrophils from the infection site to draining lymph nodes.15 Similarly, after an injection of ovalbumin (Ova), some neutrophils gain access to secondary lymphoid organs, where they produce TNF-α.14 Furthermore, the ability of neutrophils to cross-prime naive CD8+ T cells suggests that they have reached the lymph nodes.9 To date, the mechanisms responsible for the migration of neutrophils from the periphery to the draining lymph nodes remain unknown.
Immature DCs, which are located in peripheral tissues, are specialized in the recognition and capture of microbes. After the capture, DCs migrate to the draining lymph nodes for antigen presentation to naive T cells. The CC-chemokine receptor 7 (CCR7) plays a critical role in the migration of DCs to secondary lymphoid organs.16 The expression of CCR7 by DCs is induced, among other stimuli, by microbes and microbial moieties, and characterizes a mature phenotype. The CCR7 ligands CC-motif chemokine ligand 19 (CCL19) and CCL21 are constitutively expressed by fibroblastic reticular cells of the T-cell zones of secondary lymphoid organs. Likewise, CCL19 can be produced by activated DCs.17 CCL21 is also expressed by endothelial cells in lymphatic vessels and in mice by high endothelial venules.16 CCR7 induces the entry of DCs into the lymphatics, their migration through lymphatic vessels, and their final positioning in T-cell areas of the lymph nodes.18,19 In addition to migratory DCs, naive T cells constitutively express CCR7 and migrate from the blood to the peripheral lymph nodes via high endothelial venules in a multistep sequence of events that includes interactions with CCR7 ligands. In addition to naive T cells, regulatory and central memory T cells migrate to lymph nodes via CCR7, whereas CCR7− effector memory cells are excluded from lymph nodes.16 These data underline the pivotal role played by CCR7 in the migration of immune cells into draining lymph nodes.
The aim of this study was to evaluate the potential involvement of CCR7 in the migration of neutrophils from the periphery to the lymph nodes. Our results showed that neutrophils express functional CCR7 and that the expression of CCR7 by neutrophils is required for their migration to secondary lymphoid organs.
Methods
Animals
C57BL/6 and Ccr7−/− mice of the C57BL/6 background (Charles River Laboratories and The Jackson Laboratory, respectively), 6-10 weeks old, were bred and housed in a pathogen-free environment. Experiments were conducted according to institutional guidelines, and were approved by the ethics committee of Région Pays de la Loire (agreement 2009.18).
Murine bone marrow neutrophil purification
Single-cell bone marrow preparations were placed on top of a Percoll (Sigma-Aldrich) step gradient (52%, 65%, and 75% Percoll in phosphate-buffered saline [PBS]) and the enriched neutrophil population was recovered at the interface between 65% and 75% Percoll. Neutrophils were then purified by positive selection based on Ly-6G expression using phycoerythrin (PE)–labeled anti–Ly-6G monoclonal antibody (mAb; BD Pharmingen) and anti-PE mAb magnetic microbeads (Miltenyi Biotec). Cell purity, determined by fluorescence-activated cell sorting (FACS) using anti–Ly-6G and anti-neutrophils (clone 7/4; Serotec) mAbs, was > 99% (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Neutrophils were incubated overnight in RPMI-1640 medium (BioWhittaker Cambrex) supplemented with 2mM l-glutamine (Life Technologies), 50 U/mL of penicillin (Life Technologies), 50 μg/mL of streptomycin (Life Technologies), 10mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Life Technologies), 0.1mM nonessential amino acids (cRPMI; Life Technologies), 50μM β-mercaptoethanol (Sigma-Aldrich), and 5% fetal calf serum (FCS; Biowest) before use.
Human neutrophil purification and induction of cell death
Blood from healthy subjects (Blood Collection Center, Angers, France) was obtained with written informed consent in accordance with the Angers University Hospital ethics committee. Neutrophils were isolated from peripheral blood under endotoxin-free conditions following the protocol described by Boyum,20 allowing the isolation of pure and minimally activated cells.21,22 Briefly, after Ficoll-Paque centrifugation of peripheral blood, neutrophils were separated from erythrocytes by 3% dextran (Amersham Biosciences) density-gradient sedimentation. Purity was determined by FACS analysis on forward scatter/side scatter parameters and with specific mAbs (see “Flow cytometric analysis”) and was determined to be > 99%. Spontaneous apoptosis of neutrophils was obtained by culturing cells (1 × 106 cells/mL) in RPMI-1640 medium containing 1% heat-inactivated FCS for 72 hours.23 Apoptosis was evaluated by annexin V and 7-amino-actinomycin D (7-AAD) labeling. Briefly, cells were incubated with allophycocyanin-labeled annexin V (BD Pharmingen) for 15 minutes at room temperature according to the manufacturer's instructions before the addition of 7-AAD. Viable cells, early apoptotic cells, and late apoptotic cells are characterized as annexin V−/7-AAD−, annexin V+/7-ADD−, and annexin V+/7-ADD+, respectively. After washing, membrane expression of CCR5 and CCR7 was evaluated using PE-labeled anti-CCR5 or anti-CCR7 mAb (BD Pharmingen), respectively.
Flow cytometric analysis
The phenotype of murine cells was determined using FITC or Alexa Fluor 647–labeled anti-neutrophils (rat IgG2a, clone 7/4), FITC-labeled MHC-I H2-Kb, -MHC-II I/Ab, -CD3, -CD11b, -CD11c, -CD62L, -CD44, -CD80, and -CD86 mAbs, and with PE-labeled anti–Ly-6G, -CD8α, -CCR7 (all from BD Pharmingen) and anti-F4/80 mAbs (Dako Cytomation). FITC- and PE-labeled isotype controls were from Serotec and BD Pharmingen. Human neutrophil purity and phenotype analysis were determined using FITC-labeled anti-CD13, -CD3, -CCR5, and -CD14 mAbs and with PE-labeled and biotin-labeled anti-CCR7 mAb (BD Pharmingen). Allophycocyanin-labeled streptavidin (BD Pharmingen) was used to detect biotin-labeled mAbs. Isotype control mAbs were also from BD Pharmingen. For intracellular staining, neutrophils were fixed and permeabilized in 1% paraformaldehyde/0.2% saponin in PBS (Sigma-Aldrich) before incubation with the mAbs.24 Fluorescence was analyzed using a FACSCalibur or a FACSaria cytofluorometer (BD Biosciences).
Confocal microscopy
Neutrophils (3 × 105) were seeded on poly-L-lysine round glasses (Sigma-Aldrich) and fixed with 4% paraformaldehyde (Euromedex). Cells were washed with Ca2+/Mg2+ PBS and maintained in Ca2+/Mg2+ PBS or permeabilized with 0.3% Triton X-100 in Ca2+/Mg2+ PBS for 5 minutes. Aspecific ligands were blocked with 5% goat serum (Dako Cytomation) in Ca2+/Mg2+ PBS containing 2% bovine serum albumin and 0.1% glycine for 1 hour at room temperature. Cells were then incubated with the following primary antibodies: rat anti–human CCR7 mAb (clone 552176; BD Biosciences), mouse anti–CXCR2/CD182 mAb (clone 5E8; BioLegend), rabbit anti–myeloperoxidase (MPO) polyclonal antibody (pAb; Dako Cytomation), mouse anti–lactoferrin mAb (clone 2651K1; Hbt Biotechnology), and mouse anti–gelatinase (MMP9) mAb (clone 697; Hbt Biotechnology). Irrelevant immunoglobulins G (IgGs) were used as the isotypic control. After 1 hour of incubation at room temperature, cells were washed and incubated with 1 μg/mL of Alexa Fluor 488–conjugated goat anti–rat IgG, Alexa Fluor 647–conjugated goat anti–mouse IgG, or Alexa Fluor 647–conjugated goat anti–rabbit IgG (Invitrogen) for 1 hour at room temperature. 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) was used to stain DNA. After each step, cells were extensively washed with Ca2+/Mg2+ PBS + 0.05% Tween 20 (Merck). Specimens were finally mounted with the reagent FluorSave (Merck) and analyzed with a laser scanning confocal microscope (FluoView FV1000, Olympus) using a fine focusing lens (60× or 100×; numerical aperture 1.4).
Analysis of CCR7 expression by reverse transcriptase-polymerase chain reaction
Total RNA from mouse bone marrow neutrophils was extracted using TRIzol reagent (Life Technologies). cDNA was synthesized from 1 μg of total RNA using an oligo-dT primer and reverse transcriptase (GE Healthcare). Polymerase chain reaction amplification was performed with an amount of cDNA corresponding to 25 ng of the starting total RNA using specific oligonucleotides (Ccr7, 5′-ATGGACCCAGGGAAACCCAGGA-3′ and 5′-RTGAGCCTCTTGAAATAGATGTA-3′). RNA integrity was assessed by GAPDH (glyceraldehyde 3-phosphate dehydrogenase) cDNA amplification. The polymerase chain reaction products were analyzed on a 1% agarose gel by electrophoresis and visualized with ethidium bromide.
Neutrophil chemotaxis
Human neutrophil chemotaxis was performed using Transwell (0.4-μm pore size) in 24-well plates (Costar; Corning). CCL19 and/or CCL21 (R&D Systems) at optimal concentrations (300 and 250 ng/mL, respectively; supplemental Figure 2) or 50 ng/mL of interleukin-8 (IL-8; Immunotools) were added in the lower chambers. Neutrophils (1 × 106) were resuspended in RPMI-1640 medium containing 20 ng/mL of IL-17 (R&D Systems), 50 ng/mL of TNF-α (R&D Systems), 20 ng/mL of granulocyte macrophage colony-stimulating factor (GM-CSF; R&D Systems), 10% (v:v) complete Freund adjuvant (CFA; Sigma-Aldrich), 500 ng/mL of lipopolysaccharide (LPS; Sigma-Aldrich), 5 μg/mL of T gondii antigen extract (TAg; kindly provided by I. Dimier-Poisson, University of Tours, France), or 1 μg/mL of prostaglandin E2 (PGE2; Sigma-Aldrich) and added in the upper chambers. After 30 minutes, 1 hour, or 3 hours of incubation at 37°C, migrated neutrophils were collected in the lower chambers and counted in a blind manner by 3 independent observers.
In vivo neutrophil migration
Wild-type (WT) and Ccr7−/− mice were injected intradermally with 25 μL of CFA or PBS. In other experiments, neutrophils from WT or Ccr7−/− mice were labeled with PKH26 (Sigma-Aldrich) and stimulated or not with 50 ng/mL of GM-CSF (R&D Systems) and 20 ng/mL of IL-17 before injection into the footpads of WT mice. Popliteal lymph nodes were harvested 6 hours later, and the number and percentage of migrated neutrophils were determined by FACS.
Statistical analysis
Data are shown as mean ± standard error of the mean (SEM) and were analyzed by the 2-tailed Wilcoxon matched pairs test, with P values of .05 or less considered significant.
Results
Human and mouse neutrophils express CCR7
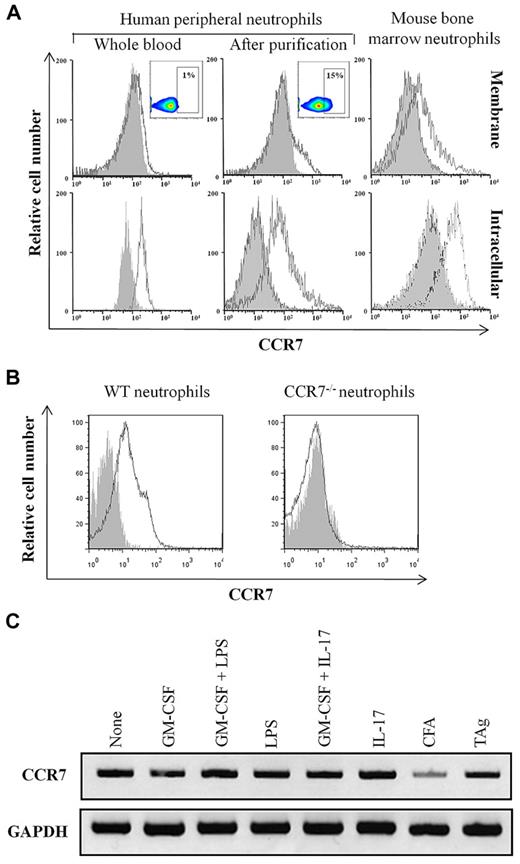
The membrane and intracellular expression of CCR7 by human neutrophils was evaluated by flow cytometry using the anti-CCR7 mAb 3D12 before and after purification from fresh whole human blood. Although CCR7 expression was undetectable on neutrophils in fresh whole human blood (Figure 1A), it was expressed on a fraction of neutrophils (ranging from 10%-30%, according to the donor) after purification (Figure 1A). Similar results were obtained using the clones 150503 and FR11-11E8 (supplemental Figure 3). The expression of CCR7 was also evaluated in highly purified (supplemental Figure 1) mouse bone marrow neutrophils. Results showed that these cells constitutively express CCR7 (Figure 1A). No staining of bone marrow neutrophils isolated from Ccr7−/− mice was observed (Figure 1B), confirming the specificity of the detection. The expression of CCR7 was equivalent on peripheral blood and bone marrow mouse neutrophils (data not shown), and the levels of CCR7 detected in permeabilized neutrophils (Figure 1A bottom panels) were higher than that detected at the surface of neutrophils (Figure 1A top panels). The expression of CCR7 on human neutrophils was not modulated in response to proinflammatory cytokines (supplemental Figure 4). Similar results were obtained using mouse bone marrow neutrophils (data not shown and Figure 6A).
CCR7 is expressed by neutrophils. (A) Membrane CCR7 expression was analyzed in human fresh whole blood neutrophils and in purified human and mouse neutrophils (top histograms). Inserts correspond to dot-plot analysis. Intracellular expression of CCR7 was analyzed in human fresh whole blood and in purified human and mouse neutrophils after permeabilization (bottom histograms). Results are representative of 1 of 5 independent experiments. (B) Bone marrow neutrophils isolated from WT and Ccr7−/− mice were incubated with PE-labeled anti-CCR7 (white histogram) and control mAbs (gray histogram). Results are representative of 1 of 5 experiments. (C) The expression of CCR7 mRNA was analyzed in mature murine bone marrow neutrophils isolated from WT mice. Cells were not stimulated or stimulated for 3 hours by GM-CSF, GM-CSF + LPS, LPS, GM-CSF + IL-17, CFA, and TAg. RNA integrity and cDNA synthesis were verified by amplifying GAPDH. Results are representative of 1 of 3 experiments.
CCR7 is expressed by neutrophils. (A) Membrane CCR7 expression was analyzed in human fresh whole blood neutrophils and in purified human and mouse neutrophils (top histograms). Inserts correspond to dot-plot analysis. Intracellular expression of CCR7 was analyzed in human fresh whole blood and in purified human and mouse neutrophils after permeabilization (bottom histograms). Results are representative of 1 of 5 independent experiments. (B) Bone marrow neutrophils isolated from WT and Ccr7−/− mice were incubated with PE-labeled anti-CCR7 (white histogram) and control mAbs (gray histogram). Results are representative of 1 of 5 experiments. (C) The expression of CCR7 mRNA was analyzed in mature murine bone marrow neutrophils isolated from WT mice. Cells were not stimulated or stimulated for 3 hours by GM-CSF, GM-CSF + LPS, LPS, GM-CSF + IL-17, CFA, and TAg. RNA integrity and cDNA synthesis were verified by amplifying GAPDH. Results are representative of 1 of 3 experiments.
As described for other molecules preformed in neutrophils,25 and in agreement with the fact that neutrophils are terminally differentiated cells, CCR7 mRNA was undetectable in circulating human and murine neutrophils (data not shown). In contrast, CCR7 mRNA was evidenced in bone marrow murine neutrophils (Figure 1C) and was not modulated by proinflammatory molecules and/or microbial moieties (Figure 1C).
The absence of CCR7 mRNA in circulating neutrophils and the rapid mobilization of CCR7 at the surface of neutrophils upon stimulation suggested the existence of an intracellular stock of preformed CCR7.
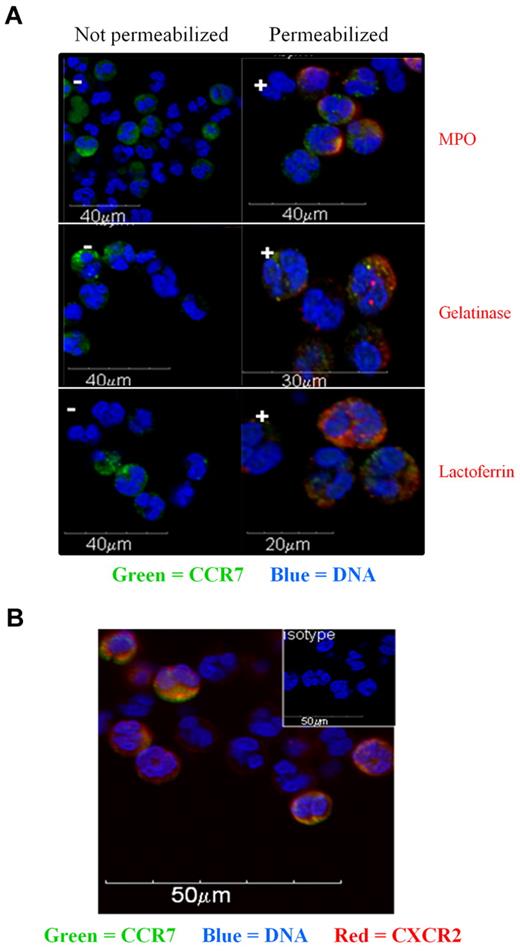
Confocal microscopy on nonpermeabilized cells confirmed the presence of CCR7 at the cell surface in 10%-30% of human neutrophils (Figure 2A). In agreement with an expression at the cell surface, CCR7 colocalized with the membrane chemokine receptor CXCR2 (Figure 2B). Moreover, intracellular CCR7 was evidenced after cell permeabilization (Figure 2A). No CCR7 was detected in the primary (MPO+), specific (lactoferrin+), or tertiary (gelatinase+) granules (Figure 2A). Neutrophils are short-lived cells that are highly sensitive to cell death. Moreover, some intracellular molecules can relocalize at the membrane of apoptotic neutrophils.23 We therefore had to exclude the possibility that the membrane expression of CCR7 may result from the apoptotic process. Apoptosis was evaluated by annexin V and 7-AAD staining. Results showed that the expression of CCR7 was restricted to live cells (Figure 3A-B). No membrane expression of CCR7 was detected on early and late apoptotic neutrophils (Figure 3A). In contrast, CCR5 was detected at the surface of late apoptotic cells (Figure 3A), as previously reported by Ariel et al.26 These results suggest that membrane CCR7 expression was not associated with cell death.
Localization of CCR7 in neutrophils by confocal microscopy. (A) Confocal microscopy analysis of CCR7 in freshly isolated human neutrophils. Cells were not permeabilized (−; left panels) or permeabilized (+; right panels) and stained for CCR7 and MPO, CCR7 and gelatinase, or CCR7 and lactoferrin. Nuclei were stained with DAPI. (B) Neutrophils were fixed and stained for CCR7 (green) and CXCR2 (red) expression. Nuclei were stained with DAPI.
Localization of CCR7 in neutrophils by confocal microscopy. (A) Confocal microscopy analysis of CCR7 in freshly isolated human neutrophils. Cells were not permeabilized (−; left panels) or permeabilized (+; right panels) and stained for CCR7 and MPO, CCR7 and gelatinase, or CCR7 and lactoferrin. Nuclei were stained with DAPI. (B) Neutrophils were fixed and stained for CCR7 (green) and CXCR2 (red) expression. Nuclei were stained with DAPI.
CCR7 is not expressed at the surface of apoptotic neutrophils. (A) Human neutrophils were cultured for 72 hours in RPMI-1640 medium containing 1% FCS before analysis of CCR5 and CCR7 expression. Neutrophil apoptosis was determined by annexin V and 7-AAD staining and analyzed by cytometry. Histograms show membrane CCR5 and CCR7 expression by live (annexin V− 7-AAD−; R1), early apoptotic (annexin V+ 7-AAD−; R2), and late apoptotic (annexin V+ 7-AAD+; R3) neutrophils. Results are representative of 1 of 5 independent experiments. (B) Analysis of CCR7 and CCR5 expression by live (■) and apoptotic (□) human neutrophils. Results are expressed in mean fluorescence intensity values (mean ± SEM, n = 5). *P < .05. PMN indicates polymorphonuclear neutrophil.
CCR7 is not expressed at the surface of apoptotic neutrophils. (A) Human neutrophils were cultured for 72 hours in RPMI-1640 medium containing 1% FCS before analysis of CCR5 and CCR7 expression. Neutrophil apoptosis was determined by annexin V and 7-AAD staining and analyzed by cytometry. Histograms show membrane CCR5 and CCR7 expression by live (annexin V− 7-AAD−; R1), early apoptotic (annexin V+ 7-AAD−; R2), and late apoptotic (annexin V+ 7-AAD+; R3) neutrophils. Results are representative of 1 of 5 independent experiments. (B) Analysis of CCR7 and CCR5 expression by live (■) and apoptotic (□) human neutrophils. Results are expressed in mean fluorescence intensity values (mean ± SEM, n = 5). *P < .05. PMN indicates polymorphonuclear neutrophil.
These results show that murine and human neutrophils constitutively express CCR7, which is stored intracellularly and rapidly mobilized at the cell surface upon activation.
CCR7 expressed by neutrophils is functional
To assess whether CCR7 expressed by human neutrophils is functional, we analyzed the in vitro migration of neutrophils in response to the CCR7 ligands CCL19 and CCL21 in a Transwell chemotaxis assay. A preliminary dose-dependent analysis showed that 300 ng/mL of CCL19 and 250 ng/mL of CCL21 induced an optimal migration of neutrophils (supplemental Figure 2). The maximal migration was induced when both chemokines were combined (supplemental Figure 2), so this experimental condition was used in the following experiments.
To determine the optimal timing of in vitro neutrophil migration, a kinetic experiment was performed using GM-CSF- and LPS-stimulated neutrophils (Figure 4A). Results showed that CCL19 + CCL21 induced the migration of neutrophils compared with the spontaneous migration in the absence of chemokines. The number of migrated neutrophils was significantly increased at 60 minutes (530 × 102 ± 121 × 102 migrated cells; mean ± SEM, n = 5) and maximal at 3 hours (697 × 102 ± 144 × 102 migrated cells; Figure 4A). Low or no migration of neutrophils was observed using unprimed neutrophils (Figure 4A), showing that the migration of neutrophils in response to CCL19 and CCL21 required their priming by GM-CSF and LPS. Finally, although CCL19 and CCL21 induced neutrophil migration, the number of migrated neutrophils was lower than that induced by IL-8, which was used as a positive control (Figure 4A).
CCL19 and CCL21 induce the in vitro migration of human neutrophils. (A) Purified human neutrophils were first incubated or not with 20 ng/mL of GM-CSF and 500 ng/mL of LPS. Neutrophil chemotaxis in response to 300 ng/mL of CCL19 and 250 ng/mL of CCL21 or to 50 ng/mL of IL-8 was evaluated at 30 minutes, 60 minutes, and 3 hours in a Transwell assay. Results are expressed as the number of migrated cells (mean ± SEM, n = 5). *P < .05. (B) Human neutrophils were treated or not with 20 ng/mL of IL-17, 50 ng/mL of TNFα, 20 ng/mL of GM-CSF, 1 μg/mL of PGE2, 500 ng/mL of LPS, 20 μL of CFA, or 5 μg/mL of TAg. Chemotaxis was assessed using Transwell in response to a 3-hour stimulation with 300 ng/mL of CCL19 and 250 ng/mL of CCL21. Results are expressed as the number of migrated neutrophils (mean ± SEM, n = 5). *P < .05.
CCL19 and CCL21 induce the in vitro migration of human neutrophils. (A) Purified human neutrophils were first incubated or not with 20 ng/mL of GM-CSF and 500 ng/mL of LPS. Neutrophil chemotaxis in response to 300 ng/mL of CCL19 and 250 ng/mL of CCL21 or to 50 ng/mL of IL-8 was evaluated at 30 minutes, 60 minutes, and 3 hours in a Transwell assay. Results are expressed as the number of migrated cells (mean ± SEM, n = 5). *P < .05. (B) Human neutrophils were treated or not with 20 ng/mL of IL-17, 50 ng/mL of TNFα, 20 ng/mL of GM-CSF, 1 μg/mL of PGE2, 500 ng/mL of LPS, 20 μL of CFA, or 5 μg/mL of TAg. Chemotaxis was assessed using Transwell in response to a 3-hour stimulation with 300 ng/mL of CCL19 and 250 ng/mL of CCL21. Results are expressed as the number of migrated neutrophils (mean ± SEM, n = 5). *P < .05.
Because in vivo infiltrating neutrophils are in contact with different stimuli, we also evaluated the impact of inflammatory mediators other than GM-CSF and LPS on neutrophil responsiveness to CCL19 and CCL21. We evaluated IL-17, TNFα, PGE2, and microbial components (CFA and TAg). With the exception of IL-17 and LPS, all of these stimuli induced the spontaneous migration of neutrophils. Interestingly, the migration of these activated neutrophils was increased in the presence of CCL19 and CCL21 (Figure 4B). The most potent migration was induced using neutrophils primed with GM-CSF + LPS or GM-CSF + IL-17. However, none of these stimuli modulated the expression of CCR7 (data not shown and supplemental Figure 4).
These results show that priming renders neutrophils responsive to CCR7 ligands.
CCR7 is involved in the in vivo migration of neutrophils
We next evaluated the in vivo role of CCR7 in neutrophil migration. With the aim of analyzing neutrophil traffic into WT and Ccr7−/− mice, we first evaluated whether both mouse strains have the same numbers of neutrophils. The densities of bone marrow and of circulating neutrophils were equivalent in WT (68% ± 10% and 10% ± 2.5%, respectively; mean ± SEM, n = 5) and in Ccr7−/− mice (69% ± 12.5% and 8.75% ± 2.5%, respectively; Figure 5A).
Role of CCR7 in the in vivo migration of neutrophils. (A) The density of neutrophils in blood and bone marrow from WT and Ccr7−/− mice was determined by FACS. Results are expressed as density of neutrophils (mean ± SEM, n = 3). (B) WT and Ccr7−/− mice were injected intradermally with PBS or CFA. The presence of neutrophils in the draining lymph nodes was analyzed 6 hours after injection by FACS using PE-labeled anti-Ly6G and FITC-labeled anti-neutrophil mAbs. Left, results are expressed as the number of neutrophils in the draining lymph nodes (mean ± SEM, n = 5). *P < .05. Right, dot-plot representative of 1 of 5 experiments.
Role of CCR7 in the in vivo migration of neutrophils. (A) The density of neutrophils in blood and bone marrow from WT and Ccr7−/− mice was determined by FACS. Results are expressed as density of neutrophils (mean ± SEM, n = 3). (B) WT and Ccr7−/− mice were injected intradermally with PBS or CFA. The presence of neutrophils in the draining lymph nodes was analyzed 6 hours after injection by FACS using PE-labeled anti-Ly6G and FITC-labeled anti-neutrophil mAbs. Left, results are expressed as the number of neutrophils in the draining lymph nodes (mean ± SEM, n = 5). *P < .05. Right, dot-plot representative of 1 of 5 experiments.
To analyze the role of CCR7 in the in vivo migration of neutrophils, WT and Ccr7−/− mice were intradermally injected with CFA or PBS. After 6 hours, the frequency and total number of neutrophils in the draining lymph nodes were determined. CFA induced the migration of neutrophils in the draining lymph nodes in both strains. However, the total number of neutrophils was significantly reduced in Ccr7−/− mice compared with WT mice (1535 ± 651 and 3907 ± 977, respectively; mean ± SEM, n = 5; Figure 5B left panel). Similarly, the frequency of neutrophils in the draining lymph nodes was lower in Ccr7−/− mice compared with WT mice (0.34% and 1.13%, respectively; Figure 5B right panel). No migration of neutrophils was observed in response to PBS (Figure 5B).
These results demonstrate the role of CCR7 in the migration of neutrophils into the lymph nodes.
In a second set of experiments, neutrophils from WT and Ccr7−/− mice were labeled with PKH26 and stimulated or not with IL-17 and GM-CSF before intradermal injection in WT mice. As mentioned above, the levels of CCR7 expression were not modulated by IL-17 and/or GM-CSF (Figure 6A). Kinetic experiments showed that the maximal number of migrated neutrophils was detected 6 hours after cell injection in the draining lymph nodes of WT mice (data not shown). Results showed that the frequency of activated WT neutrophils migrated to the draining lymph nodes was higher than that observed with activated Ccr7−/− neutrophils (3.8% ± 1.5% and 1.3% ± 0.6%, respectively; mean ± SEM, n = 4). The frequencies of nonactivated neutrophils from WT and Ccr7−/− mice were equivalent (2.2% ± 1% and 1.5% ± 0.8%, respectively), whatever the time point analyzed (from 1 to 24 hours; data not shown).
CCR7 is involved in the in vivo migration of neutrophils to the draining lymph nodes. (A) The expression of CCR7 on WT mouse neutrophils, stimulated or not with 50 ng/mL of GM-CSF and/or 20 ng/mL of IL-17, was evaluated by FACS using anti-CCR7 mAb (white histograms) compared with a control mAb (gray histograms). Results are representative of 1 of 5 experiments. (B) Neutrophils isolated from WT and Ccr7−/− mice were labeled with PKH26 and activated with GM-CSF and IL-17 before injection in WT mice. Lymph nodes cells were collected 6 hours after injection and incubated with an anti-neutrophil mAb. Results are expressed as the number of PKH26-labeled neutrophils in the draining lymph nodes (mean ± SEM, n = 5). *P < .05.
CCR7 is involved in the in vivo migration of neutrophils to the draining lymph nodes. (A) The expression of CCR7 on WT mouse neutrophils, stimulated or not with 50 ng/mL of GM-CSF and/or 20 ng/mL of IL-17, was evaluated by FACS using anti-CCR7 mAb (white histograms) compared with a control mAb (gray histograms). Results are representative of 1 of 5 experiments. (B) Neutrophils isolated from WT and Ccr7−/− mice were labeled with PKH26 and activated with GM-CSF and IL-17 before injection in WT mice. Lymph nodes cells were collected 6 hours after injection and incubated with an anti-neutrophil mAb. Results are expressed as the number of PKH26-labeled neutrophils in the draining lymph nodes (mean ± SEM, n = 5). *P < .05.
These results show that CCR7 is required for the migration of neutrophils into the lymph nodes.
Discussion
Recent studies have shown that neutrophils migrate from the periphery to lymph nodes, where they may induce and/or modulate adaptive immune responses. However, the mechanisms responsible for their migration remained undetermined. In addition to reinforcing data showing that neutrophils migrate to draining lymph nodes, we report here that CCR7 plays a major role in this process.
Among leukocytes, neutrophils are the first and the most abundant cells to migrate to the site of infection. Until recently, neutrophils were considered to be prototypic innate cells with the main recognized function of clearance of invading microorganisms. However, in vivo studies showed that neutrophils migrate from the peripheral tissues to the lymph nodes. Neutrophils rapidly shuttled subcutaneously injected Ova and intradermally injected bacille Calmette-Guérin to the T-cell zone of draining lymph nodes via lymphatics.13,14 We have previously shown that subcutaneously injected Ova-pulsed neutrophils cross-prime naive CD8+ T cells, suggesting that injected neutrophils might have encountered naive T cells in the draining lymph nodes.9 In this study, we confirmed that neutrophils can migrate to the draining lymph nodes and that this process is dependent on the expression of CCR7. Naive T cells, central memory T cells, and mature DCs express CCR7, which is involved in their migration to the T-cell area of the lymph nodes. The chemokines CCL19, which is constitutively produced by stromal cells and DCs in the lymph nodes, and CCL21, which is constitutively produced by lymphatic endothelial cells, are the ligands of CCR7 and are involved in the migration of CCR7-expressing cells to the lymph nodes.27,28
Circulating neutrophils do not express CCR7 at the membrane. However, CCR7 was detected at the surface of a fraction of neutrophils upon stimulation, suggesting the existence of a preformed stock that can be rapidly mobilized at the membrane in response to a stimulus. Supporting the hypothesis of a preformed stock, confocal microscopy confirmed the presence of CCR7 intracellularly. Interestingly, the level of membrane CCR7 expression was not modulated by microbes, microbial moieties, or proinflammatory cytokines.
Preformed molecules, such as enzymes (eg, MPO, proteinase 3 [PR3], elastase), costimulatory molecules (eg, CD80, CD86), and human leukocyte antigen-DR,29 are contained in primary, secondary, and tertiary granules or in secretory vesicles (eg, albumin).30 The translocation at the cell surface of some of these intracellular proteins, present either in granules (eg, MPO, CD86, elastase, CD63, PR3, and pentraxin 3) or in secretory vesicles (eg, CD10), may occur upon activation, as has been reported for MPO and CD86.29 In some cases, the purification protocol by itself is sufficient to induce the membrane expression of intracellular molecules such as N-formyl-methionyl-leucyl-phenylalanine-binding molecules, CD10, or CD63.31 This activation may also induce the release of different molecules such as MPO and the production of reactive oxygen species.32,33 Even though the use of dextran to isolate neutrophils has been reported to minimize cell activation,22 the purification step was sufficient by itself to induce the translocation of CCR7 at the cell surface, suggesting that CCR7 is localized in highly mobilizable vesicles, presumably secretory vesicles.22 Confocal microscopy showed that CCR7 is localized in intracellular vesicles distinct from primary, secondary, and tertiary granules. Additional evidence is therefore required to determine precisely the nature of the CCR7-containing vesicles.
Neutrophils are terminally differentiated cells in which most proteins are synthesized and stored before the cells leave the bone marrow. Accordingly, the mRNA encoding most of these proteins is not expressed in peripheral neutrophils and is not induced upon activation. Granules are produced sequentially during the differentiation from precursors and their content is driven by the “targeting by timing” process.34 Moreover, granules and secretory vesicles are secreted in a targeted manner, with a timing hierarchy in exocytosis. CCR7 mRNA was expressed in mouse mature bone marrow neutrophils but not in peripheral blood neutrophils, suggesting that, according to the targeting by timing hypothesis, CCR7 may be rapidly mobilized upon stimulation. Indeed, results confirmed the rapid expression of CCR7 at the membrane upon stimulation. The apoptosis of neutrophils may be also associated with translocation at the cell surface of intracellular proteins such as PR3,35 pentraxin 3,23 and the chemokine receptor CCR5.26 CCR7 was not detected at the surface of apoptotic neutrophils.
The CCR7 ligands CCL19 and CCL21 induced the in vitro migration of human neutrophils, and the role of CCR7 in the in vivo migration of neutrophils was apparent in Ccr7−/− mice. Indeed, neutrophils from WT mice, but not from Ccr7−/− mice, migrated to the draining lymph nodes. Interestingly, even though CCR7 was expressed by purified neutrophils, their migration in response to CCL19 and CCL21 required their priming by microbial moieties or proinflammatory cytokines. Some functional properties of neutrophils, such as cytokine production and/or degranulation, required priming. GM-CSF, TNF-α, LPS, and chemotactic factors are potent in vitro priming molecules for neutrophils.36-38 It is generally accepted that inflammation and/or microbes are involved in the priming of neutrophils in vivo. This hypothesis is supported by the observation that neutrophils are constitutively primed in patients suffering from prodromal infection or chronic inflammation.39 Similarly, we observed that intradermally injected murine neutrophils have to be primed in vitro, before injection, to acquire the capacity to migrate to the lymph nodes. Supporting this observation, studies showed that migration of neutrophils from the periphery to the lymph nodes was induced by the injection of a proinflammatory stimulus (CFA or bacille Calmette-Guérin)13,14,40 able to prime or to induce locally the production of priming molecules. However, to our knowledge, the molecular mechanism responsible for the priming of neutrophils remains undetermined. It is interesting that the activation of neutrophils (by microbial moieties and/or proinflammatory cytokines) may also enhance their survival.
Neutrophils modulate DC functions in vitro5,41 and under some conditions can present antigens to T cells,9 suggesting that they may influence the adaptive immune response through interaction with DC and/or T cells.5,42 Supporting this hypothesis, neutrophil depletion induced a Th2-biased immune response during Candida albicans infection.12 Moreover, after Ova injection, neutrophils shuttled Ova to the secondary lymphoid organs, where they produced TNF-α and polarized the Ova-specific T-cell response.14 Recently, natural killer cells, another innate immune cell type, have been also found to access the T-cell zone of draining lymph nodes,43 where they produce the Th1-priming factor interferon-γ. These data suggest that innate immune cells that have reached the lymph nodes may modulate the amplitude and the nature of adaptive immune responses.
Our results showed that only a fraction of neutrophils express CCR7. Although we cannot exclude the possibility that this phenotype corresponds to neutrophils at different stages of differentiation, it is tempting to speculate that 2 subpopulations may exist. CCR7+ neutrophils may migrate to the lymph nodes and modulate adaptive immune response, whereas CCR7− neutrophils may be involved in innate host defense. Additional experiments are required to determine the phenotypical and functional characteristics of CCR7+ versus CCR7− neutrophils.
In conclusion, this study shows that a subpopulation of neutrophils constitutively expresses CCR7 and that CCR7 is involved in the migration of neutrophils from the periphery to the lymph nodes. These results highlight a novel mechanism involved in the immunoregulatory properties of neutrophils.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Alberto Mantovani (Istituto Clinico Humanitas, Rozzano, Milan, Italy) for helpful discussion. We are grateful to Prof I. Dimier-Poisson (UMR 483, INRA/University of Tours, France) for providing the T gondii antigen extract. We thank Prof P. Asfar, Jérôme Leroux, and Pierre Legras (Service Commun d'Animalerie Hospitalo-Universitaire, University of Angers, Angers, France) for their help.
We acknowledge La Ligue contre le Cancer (équipe labellisée 2008-2010) for grant support. This work was also supported by institutional grants from Inserm and the University of Angers. P.C. received a grant from the Ligue contre le Cancer.
Authorship
Contribution: C.B. designed and performed the experiments and wrote the paper; P.C., A.D., M.S., M.-L.L., and S.J. performed experiments; G.M. and K.M. provided reagents; A.C. supervised experiments; and Y.D. and P.J. supervised the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pascale Jeannin, Inserm Unité 892, CHU d'Angers, Bâtiment IBS-IRIS, 4 rue Larrey, 49933 Angers, France; e-mail: pascale.jeannin@univ-angers.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal