Abstract
Mucosal-associated invariant T (MAIT) cells are very abundant in humans and have antimicrobial specificity, but their functions remain unclear. MAIT cells are CD161hiIL-18Rα+ and either CD4−CD8− (DN) or CD8αβint T cells. We now show that they display an effector-memory phenotype (CD45RA−CD45RO+CD95hiCD62Llo), and their chemokine receptor expression pattern (CCR9intCCR7−CCR5hiCXCR6hiCCR6hi) indicates preferential homing to tissues and particularly the intestine and the liver. MAIT cells can represent up to 45% of the liver lymphocytes. They produce interferon-γ and Granzyme-B as well as high levels of interleukin-17 after phorbol myristate acetate + ionomycin stimulation. Most MAIT cells are noncycling cells (< 1% are Ki-67+) and express the multidrug resistance transporter (ABCB1). As expected from this phenotype, MAIT cells are more resistant to chemotherapy than other T-cell populations. These features might also allow MAIT cells to resist the xenobiotics potentially secreted by the gut bacteria. We also show that this population does not appear to have antiviral specificity and that CD8 MAIT cells include almost all the ABCB1+CD161hi CD8 T cells. Together with their already known abundance and antimicrobial specificity, the gut-liver homing characteristics, high expression of ABCB1, and ability to secrete interleukin-17 probably participate in the antibacterial properties of MAIT cells.
Introduction
Mainstream T cells display a very diverse repertoire of antigen receptors, which enable these cells to respond to a wide variety of antigens presented by polymorphic major histocompatibility complex (MHC) molecules. During development, interactions between the T cells and selecting MHC class II and I molecules on thymic epithelial cells lead to the specific features of CD4 and CD8 T-cell lineage. However, 2 “innate-like” T lymphocytes, the natural killer T (NKT) and mucosal-associated invariant T (MAIT) cells, follow different ontogenic pathways with selection by nonpolymorphic MHC class Ib molecules.1
Human MAIT cells express the semi-invariant T-cell receptor (TCR; iVα7.2-Jα33) and are selected by the MHC class Ib molecule, MR1 on hematopoietic cells, which confer peculiar features.2,3 Indeed, MAIT cells are enriched in the intestinal lamina propria and are numerous in the peripheral blood of healthy subjects. They are present in cord blood in small numbers with a naive phenotype, whereas in adults they represent approximately 10% of mature CD8 or CD4−CD8− (DN) T cells and display an effector-memory phenotype. We recently showed that MAIT cells specifically react against antigen-presenting cells (APCs), fed with a wide variety of bacteria and yeasts but not viruses, and display protective activity in experimental bacterial infection models.4,5 In humans, they are defined as CD161hiIL-18Rα+Vα7.2+γδ−CD3+ lymphocytes.3,4 Either CD161 or IL-18Rα expression at the cell surface, together with the Vα7.2 segment, allows for the unequivocal identification of MAIT cells in both peripheral blood and tissues.3,4
CD161 (KLRB1, NKRP1A) is a C-type lectin family member, part of the NK complex.6 Ligands for CD161 have been described only recently, and blocking or cross-linking CD161 may modulate T-cell functions.7-9 CD161 is also expressed by the majority of NK cells and diverse subsets of T lymphocytes that include TCR-γδ T cells and most NKT cells. CD161 expression is found on a significant proportion of tissue-infiltrating T cells, such as the intestine and liver.10-12 The frequency of these cells varies in certain pathologic conditions, such as cancer and viral or autoimmune diseases.12-15 Recently, it has been demonstrated that a subset of naive cord blood CD4 T cells express CD161 and are precursors of peripheral, mature, Th17 T cells.16 CD161 expression seems to correlate with interleukin-17 (IL-17) secretion by CD4 T cells.17 Among TCR-αβ T cells, most of the CD161+ T cells belong to the CD4 subset. However, CD8 T cells expressing CD161 can also be found, but their nature has just begun to be explored.
CD161+ CD8 T cells can be split into 2 different populations expressing intermediate or high levels of CD161. Two recent reports independently raised new findings on this latter subset. The first shows that CD161hi CD8 T cells represent self-renewing memory cells, which survive chemotherapy.18 These cells are absent from cord blood and display mostly an effector/memory phenotype in adults. They are in G0 and express high levels of the multidrug receptor ABCB1 (also called P-gp, MDR1, and PGY1), which can rapidly efflux xenobiotics. The authors suggest that these cells display heterogeneous antiviral specificities and reconstitute the antiviral memory T-cell pool after chemotherapy,18 leading to the idea of memory stem cells.19 The second report shows that CD161hi CD8 T cells express high levels of markers associated with Th17 functions, such as Rorc, and secrete high amounts of IL-17 after in vitro stimulation.11 However, to what extent these 2 subsets overlap and their relationship with MAIT cells are not known.
In the present study, we further characterize MAIT cell phenotype, location, and cytokine secretion ability. We found that MAIT cells are noncycling, tissue-targeted cells that secrete IL-17, express high levels of the multiple drug transporter protein ABCB1, arguing for resistance to xenobiotics and, accordingly, are resistant to chemotherapy. Thus, MAIT cells share many similarities with the 2 previously described CD161hi CD8 T-cell subsets: indeed, we demonstrate that MAIT cells include most, if not all, the CD161hi IL-17-secreting CD8 T cells that had been previously described.
Methods
Human samples
Fragments of human thymuses were surgical leftovers from children undergoing cardiac surgery. Thymuses were cut into small pieces in cold 0.5% bovine serum albumin/phosphate-buffered saline. The resulting cell suspension was centrifuged to exclude aggregates. The supernatant was recovered, and the cells were washed again with 0.5% bovine serum albumin/phosphate-buffered saline. Thymocytes were then counted and labeled.
Fragments of human lymph node, intestine, and liver were surgical leftovers from cancer patients treated at the Curie Institute undergoing surgery. In our institution, all patients were informed that pathologic specimens might be used for research purposes.
Blood samples from breast cancer patients treated at the Curie Institute were obtained on 3 occasions (before treatment, after 3 cycles, and 1 month after the last sixth cycle) during treatment with epirubicine, cyclophosphamide, and 5-fluorouracil.
Blood samples were obtained from healthy donors from the blood bank under a Curie Institute Institutional Review Board-approved protocol in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells were obtained after a standard Ficoll gradient (GE Healthcare). MAIT cells were isolated by magnetic cell sorting with biotinylated anti-Vα7.2 (3C10)3 and antibiotin magnetic beads according to the manufacturer's specifications (Miltenyi Biotec). Subsequently, the positive fraction was stained with anti-CD3-A700 (HIT3a, BioLegend), anti-CD161-APC (191B8, Miltenyi Biotec), and streptavidin phycoerythrin (PE)-Cy7 (BD Biosciences PharMingen). MAIT cells were fluorescence-activated cell sorter sorted on a BD Aria II.
Cells were stimulated with anti-CD3 and -CD28 Dynabeads (Invitrogen) or phorbol myristate acetate with ionomycin (2 ng/mL, 1μM, respectively) for 24 or 48 hours. Alternatively, T cells were stimulated by monocytes fed with bacteria as described.4
Flow cytometry
Flow cytometry was performed with directly conjugated antibodies according to standard techniques with analysis on FACSAria and LSRII flow cytometers (BD Biosciences). 4,6-Diamidino-2-phenylindole and a 405-nm excitation were used to exclude dead cells. The anti-Vα7.2 antibody was biotinylated and detected with streptavidin PE-Cy7 (BD Biosciences PharMingen). The following antibodies were used: anti-CD4-APC-Cy7 (RP4-T4, BioLegend), anti-CD3ϵ-Alexa-700 (HIT3a, BioLegend), anti-TCR-γδ-PE-Cy5 (IMMU510, Beckman Coulter), anti-CD8β-PE Texas Red (2ST8.5H7, Beckman Coulter), anti-CD45RO-FITC (UCHL1, Beckman Coulter), anti-CD45RA-PE (ALB11, Beckman Coulter), anti-CD27-FITC (M-T271, BD Biosciences PharMingen), anti-CD62L-PE-Cy5 (Dreg 56, Beckman Coulter), anti-CD95-PE-Cy5 (DX2, BioLegend), anti-CD161-APC (191B8, Miltenyi Biotec), anti-CD161-PE (191B8, Miltenyi Biotec), anti-CD25-PE (B1.49.9, Beckman Coulter), anti-CD26-PE (4EL-1C7, Beckman Coulter), anti-CD27-PE (M-T271, BD Biosciences PharMingen), anti-CD28-PE (L293, BD Biosciences PharMingen), anti-CD95-PE (DX2, BioLegend), anti-CD122-PE (CF1, Beckman Coulter) anti-CD127-PE (eBioRDR5, eBioscience), anti-CCR5-PE (2D7/CCR5, BD Biosciences PharMingen), anti-CCR6-PE (53103, R&D Systems), anti-CCR7-PE (3D12, BD Biosciences PharMingen), anti-CCR9-PE (242503, R&D Systems), anti-CXCR3-PE (1C6/CXCR3, BD Biosciences PharMingen), anti-CXCR4-PE (12G5, BD Biosciences PharMingen), anti-CXCR6-PE (56811, R&D Systems), anti-ICOS-PE (DX29, BD Biosciences PharMingen), anti-NKG2D-PE (BAT221, Miltenyi Biotec), anti-NKp80-PE (239127, R&D Systems), anti-CD150-PE (eBioA12, eBioscience), anti-CD244-PE (eBioC1.7, eBioscience), anti-IL18Rα-PE (70625, R&D Systems), anti-Ki67-PE (B56, BD Biosciences PharMingen) anti-interferon-γ (IFN-γ)-FITC (4S.B3, BD Biosciences PharMingen), anti-IL-17-PE (eBio64CAP17, eBioscience), and tetramer HLA-A*0201-EBV (gift from Dr Pierre Coulie, Université catholique de Louvain).
For quantification of cytokines, Cytometric Bead Array technology was used according to the manufacturer's specifications (BD Biosciences). For intracellular staining, Golgi Plug and Cytofix Cytoperm kit were used according to the manufacturer's specifications (BD Biosciences).
Analysis was performed with FlowJo Version 9 (TreeStar).
Immunohistochemistry
Immunohistochemistry for the detection of MAIT cells and conventional Vα12+ T cells was performed on acetone-fixed frozen sections. The antibody panel included CD3 (polyclonal rabbit, Dako France), IL-18Rα (polyclonal goat, R&D Systems), Vα7.2 and Vα12 (6D6.6, Endogen) detected by the respective secondary antibodies: donkey anti–rabbit-Cy5 (Jackson ImmunoResearch Laboratories), donkey anti–goat-Cy3 (Jackson ImmunoResearch Laboratories), and goat anti–mouse-A488 (Invitrogen). Slides were mounted with fluorescent mounting medium (ref S3023, Dako France). Images were acquired on a Nikon Eclipse 90i upright microscope with a 40× CFI Plan Fluor NA 1.3 objective and a CoolSNAP HQ2 CCD camera using Metamorph Version 7 software, which was also used for image processing.
The percentage of MAIT cells was calculated as follows: % MAIT = (number of CD3+IL18Rα+Vα7.2+ cells)/(number of CD3+ cells). At least 10 different fields were counted for each sample to enable to characterize at least 50 CD3+ cells per sample.
In vitro culture of MAIT cells and proliferation assay
Sort-purified (> 96% purity) MAIT cells were carboxyfluorescein succinimidyl ester (CFSE)-labeled (Invitrogen) at the concentration of 1μM and cultured in the presence of 5 μg/mL phytohemmaglutinin, 10 U/L IL-2, 1 ng/mL IL-7, and 5 ng/mL IL-15. After 10 days of culture, cells were washed and labeled with anti-CD3-peridinin chlorophyll protein-Cy5.5, anti-CD161-APC, and biotinylated anti-Vα7.2 (3C10) for 30 minutes at 4°C. Cells were subsequently washed and stained with streptavidin-PC7 for an additional 15 minutes, washed again, and analyzed by flow cytometry.
Quantitative RT-PCR
Quantitative RT-PCR for hVα7.2Jα33 expression in sorted subsets was performed with a duplex PCR with sequence-specific hVα7.2Jα33 and hCα Taqman probes.20 Quantitative RT-PCR for hRORC expression in sorted subsets was performed with a duplex PCR with sequence-specific hRORC and hActin Taqman probes (Applied Biosystems). Total RNA was extracted with the RNeasy kit (QIAGEN), in accordance with the manufacturer's instructions, and cDNA was generated with standard methods. Reactions were run on an ABI 7900-HT real-time PCR System (Applied Biosystems).
Rh123 efflux assays
Fluorescent drug efflux assay was performed as previously described.18 Shortly, fresh peripheral blood mononuclear cells from healthy donors were loaded in efflux buffer containing RPMI 1640 (Invitrogen) and 1% bovine serum albumin (Sigma-Aldrich) with 10 μg/mL Rh123 (Sigma-Aldrich) for 30 minutes on ice. They were then washed and cultured for 30 minutes at 37°C in the presence or absence of cyclosporine A (Sigma-Aldrich), as indicated. Rh123 was detected at 530/30 nm.
Statistical analysis
All quantitative data were analyzed by Prism Version 5 (GraphPad) with paired or unpaired t tests as appropriate.
Results
MAIT cells express tissue-specific chemokine receptors and are abundant in the liver
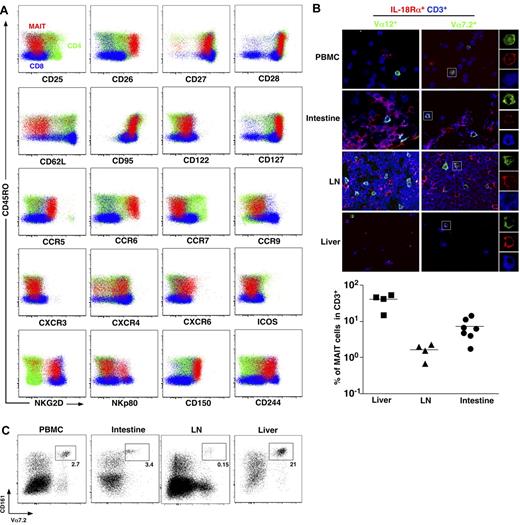
To better understand MAIT cell physiology, we first analyzed thoroughly the phenotype of MAIT cells. MAIT cells are defined as CD161hiVα7.2+CD4−CD3+ lymphocytes.3 In addition to their already known memory phenotype (CD45RA−CD45RO+), compared with mainstream T cells, MAIT cells stood out as CD62LloCD122intCD127hiCD95hi, indicating an effector memory phenotype (Figure 1A). MAIT cells expressed very high level of CD26, a dipeptidase able to process several chemokine and peptidic mediators.21-24 Like most memory CD8 T cells, MAIT cells expressed heterogeneous levels of NKp80, high levels of NKG2D, and 2 SLAM family members, CD150 and CD244, although they do not depend on these pathways for their development contrary to NKT cells.3,25 The pattern of chemokine receptor expression was, however, very specific, as MAIT cells exhibited high levels of CCR6 and CXCR6, heterogeneous levels of CXCR4, and intermediate expression of CCR9. These chemokine receptors (CXCR6 and CCR9) are involved in trafficking to peripheral tissues, especially the intestine and the liver.26-30 MAIT cells did not express CCR7, a chemokine receptor involved in migration into lymph nodes.31,32 Altogether, these results clearly indicate that MAIT cells are circulating lymphocytes with tissue tropism, unlike other conventional T cells.
MAIT cells express tissue-specific chemokine receptors and are abundant in the liver. (A) Representative staining for indicated activation markers, chemokine receptors, and NK receptors on CD4+ (green) or CD8β+ (blue) T cells (CD3+γδ−) and MAIT cells (CD3+γδ−CD4−CD8β+/−CD161+Vα7.2+) (red) from blood. Representative of at least 3 donors. (B) Peripheral blood mononuclear cells, intestine, lymph node (LN), and liver triple immunostaining with the indicated antibodies. MAIT cells are triple-stained cells in the right panel. Examples of mono-color layers on a zoomed triple-stained cell are shown on the right. (Bottom panel) Proportion of MAIT (Vα7.2+IL-18Rα+CD3+) in liver, LN, and intestine. Each symbol represents an individual donor. (C) Representative fluorescence-activated cell sorter staining on CD4−CD3+γδ− lymphocytes from the indicated organs (n > 100, 3, 4, and 2 for peripheral blood lymphocytes (PBLs), intestine, LN, and liver, respectively).
MAIT cells express tissue-specific chemokine receptors and are abundant in the liver. (A) Representative staining for indicated activation markers, chemokine receptors, and NK receptors on CD4+ (green) or CD8β+ (blue) T cells (CD3+γδ−) and MAIT cells (CD3+γδ−CD4−CD8β+/−CD161+Vα7.2+) (red) from blood. Representative of at least 3 donors. (B) Peripheral blood mononuclear cells, intestine, lymph node (LN), and liver triple immunostaining with the indicated antibodies. MAIT cells are triple-stained cells in the right panel. Examples of mono-color layers on a zoomed triple-stained cell are shown on the right. (Bottom panel) Proportion of MAIT (Vα7.2+IL-18Rα+CD3+) in liver, LN, and intestine. Each symbol represents an individual donor. (C) Representative fluorescence-activated cell sorter staining on CD4−CD3+γδ− lymphocytes from the indicated organs (n > 100, 3, 4, and 2 for peripheral blood lymphocytes (PBLs), intestine, LN, and liver, respectively).
To further document this specific ability of human MAIT cells to localize into peripheral tissues, we studied MAIT cell distribution in healthy intestine, peripheral lymph node, and liver using immunofluorescence microscopy of frozen sections stained with anti-CD3, anti-Vα7.2, and anti–IL-18Rα antibodies as this combination enables MAIT cell identification.3,4 Triple-positive MAIT cells were detected in intestinal tissues, with a frequency comparable with that observed in blood (Figure 1B). By contrast, their frequency was remarkably higher within the liver and low in the peripheral lymph nodes. These observations were confirmed by fluorescence-activated cell sorter analysis of lymphocytes isolated from the same tissues (Figure 1C). Therefore, human MAIT cells are present in the intestine but more specifically accumulate in the liver under physiologic conditions.
MAIT cells express IL-18Rα in cord blood before rapidly acquiring a memory phenotype after birth
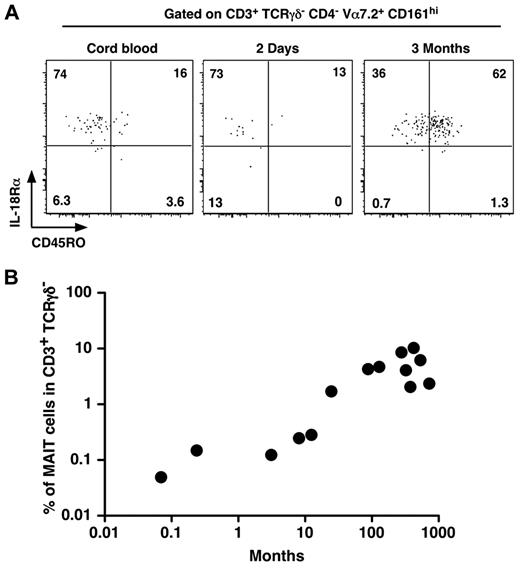
As MAIT cells are in low numbers and display a naive phenotype in cord blood whereas they are memory and numerous in adult blood, we set out to determine when MAIT cells would expand and acquire their memory phenotype. Interestingly, MAIT cells were already CD161hi and IL-18Rα+ in cord blood (Figure 2A). They gradually acquired a memory phenotype, as few MAIT cells from a 2-day-old infant expressed CD45RO, although most of them were CD45RO+ at 3 months of age (Figure 2A). Although we studied a limited number of patients, after birth, the frequency of MAIT cells increased steadily with age to reach adult levels after 1 to 2 years (Figure 2B). These data confirm that MAIT cells acquire their specific phenotypic features (eg, CD161 and IL-18Rα expression) during development, whereas the commensal flora is involved in the postbirth expansion and acquisition of the memory features.
MAIT cells express IL-18Rα at birth and rapidly acquire a memory phenotype. (A) Representative CD45RO and IL-18Rα staining of MAIT cells (gated as in Figure 1A) from human cord blood (left panel) or 2-day-old (middle panel) and 3-month-old (right panel) infant blood. (B) Proportion of MAIT cells in blood T lymphocytes of healthy donors from 0.1 to 1000 months of age. Each symbol represents an individual donor.
MAIT cells express IL-18Rα at birth and rapidly acquire a memory phenotype. (A) Representative CD45RO and IL-18Rα staining of MAIT cells (gated as in Figure 1A) from human cord blood (left panel) or 2-day-old (middle panel) and 3-month-old (right panel) infant blood. (B) Proportion of MAIT cells in blood T lymphocytes of healthy donors from 0.1 to 1000 months of age. Each symbol represents an individual donor.
MAIT cells express RORC and a mixed Th1/Th17-associated pattern of cytokines on in vitro stimulation
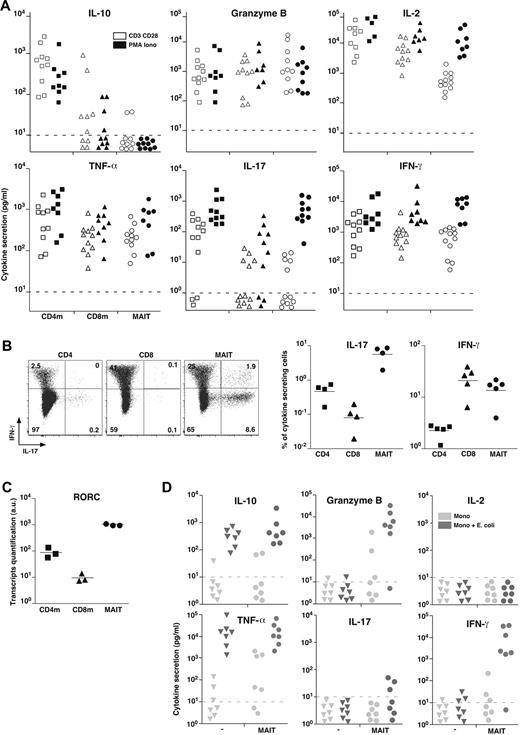
To better analyze their effector functions, we purified MAIT cells from blood and analyzed their secretion pattern after in vitro stimulation with PMA and ionomycin or anti-CD3 and anti-CD28 (CD3 + CD28) antibodies compared with mainstream memory CD4 and CD8 T cells. In the absence of stimulation, no cytokine was produced (not shown). After stimulation, MAIT cells did not produce Th2 (IL-4, IL-5, IL-13) or Tr1-associated cytokines, in particular no IL-10 nor transforming growth factor-β (Figure 3A; and data not shown). MAIT cells secreted high levels of Granzyme B and tumor necrosis factor-α (TNF-α), in similar amounts than memory CD8 T cells. They also produced IFN-γ and some IL-2 but in low amounts compare with the memory mainstream CD4 or CD8 T cells after CD3 + CD28 stimulation. Interestingly, this difference was absent with PMA and ionomycin stimulation, showing that MAIT cells are able to produce high levels of these cytokines when they are fully stimulated. The much lower amount of IL-2 was confirmed at the transcriptional level (not shown). Like CD8 T cells, MAIT cells secreted barely detectable IL-17 after CD3 + CD28 stimulation. Surprisingly, after PMA and ionomycin stimulation, they secreted as much IL-17 as CD4 T cells. Taken together, these results show that MAIT cell cytokine secretion pattern differs from both CD4 and CD8 mainstream memory T cells.
MAIT cells express RORC and display a mixed Th1/Th17 cytokine secretion pattern. (A) IL-10, Granzyme B, IL-2, TNF-α, IL-17, and IFN-γ secretion by sorted CD4 or CD8 memory T cells and MAIT cells after 48 hours of stimulation by anti-CD3 + CD28 or PMA and ionomycin. Dotted line represents detection limit. (B) Representative IL-17 and IFN-γ intracellular staining of CD4, CD8 T, and MAIT cells after 12 hours of PMA and ionomycin stimulation (left panel). Proportion of CD4, CD8 T, and MAIT cells producing IL-17 or IFN-γ (right panel). (C) Quantitative PCR analysis of RORC expression normalized to actin on sorted CD4 or CD8 memory T and MAIT cells. Each symbol represents an individual donor. (D) IL-10, Granzyme B, IL-2, TNF-α, IL-17, and IFN-γ in the 48-hour culture supernatant of monocytes fed or not with bacteria for 3 hours followed or not by addition of sorted autologous MAIT cells.
MAIT cells express RORC and display a mixed Th1/Th17 cytokine secretion pattern. (A) IL-10, Granzyme B, IL-2, TNF-α, IL-17, and IFN-γ secretion by sorted CD4 or CD8 memory T cells and MAIT cells after 48 hours of stimulation by anti-CD3 + CD28 or PMA and ionomycin. Dotted line represents detection limit. (B) Representative IL-17 and IFN-γ intracellular staining of CD4, CD8 T, and MAIT cells after 12 hours of PMA and ionomycin stimulation (left panel). Proportion of CD4, CD8 T, and MAIT cells producing IL-17 or IFN-γ (right panel). (C) Quantitative PCR analysis of RORC expression normalized to actin on sorted CD4 or CD8 memory T and MAIT cells. Each symbol represents an individual donor. (D) IL-10, Granzyme B, IL-2, TNF-α, IL-17, and IFN-γ in the 48-hour culture supernatant of monocytes fed or not with bacteria for 3 hours followed or not by addition of sorted autologous MAIT cells.
To define what proportion of MAIT cells produce IFN-γ and/or IL-17, we carried out intracytoplasmic staining after PMA and ionomycin stimulation: 20% plus or minus 7% of MAIT cells produced IFN-γ compared with 3.2% plus or minus 0.26% and 21% plus or minus 9.7% for conventional memory CD4 and CD8 T cells, respectively (Figure 3B). Confirming the secretion assay, a high percentage of MAIT cells produced IL-17, representing a higher proportion than in conventional CD4 T cells. The low proportion of IL-17–secreting CD4 T cells found in Figure 3B is in part related to the presence of numerous naive cells here, whereas memory CD4 T cells were studied in Figure 3A. However, taking into account the relative proportion of naive and memory CD4 cells, this apparent discrepancy may also indicate that MAIT cells express IL-17 more rapidly than CD4 T cells considering the timing of the stimulation (16 vs 48 hours) or that MAIT cells produce less IL-17 on a per-cell basis.
Very few mainstream CD8 T cells secreted IL-17 indicating that, in the CD8 subset, the ability to secreted IL-17 is restricted to MAIT cells. Consistent with these data, unstimulated MAIT cells expressed high levels of the Th17-associated transcription factor RORC (RORγt; Figure 3C). Thus, blood MAIT cells display a mixed Th1/Th17 pattern of cytokine secretion after in vitro stimulation. IFN-γ and IL-17 secretions were essentially mutually exclusive, although a small proportion of MAIT cells were a double producer, which might reflect the existence of functionally different subsets of MAIT cells.
The absence of IL-17 secretion after CD3 + CD28 stimulation contrasts with the high level of IL-17 seen after PMA and ionomycin stimulation. This suggests that, in addition to TCR and costimulation, triggering another transduction pathway is necessary for IL-17 production. As MAIT cells express high levels of IL-23R and IL-18R, we tested the addition of IL-23 and/or IL-18 to anti-CD3 + CD28 stimulation without getting IL-17 secretion (not shown). Because MAIT cells are activated by monocytes fed with bacteria,4 we measured the lymphokine production after this stimulation and compared it with MAIT cells cocultured with uninfected monocytes (Figure 3D). After infection, monocytes produced high amount of TNF-α and IL-10, precluding the study of their production by MAIT cells. High levels of Granzyme B and IFN-γ were produced by MAIT cells stimulated by bacteria fed monocytes, whereas no IL-2 was found, similar to what is observed after CD3 + CD28 stimulation. Barely detectable IL-17 was found in 3 of 7 donors, suggesting that monocytes are missing an IL-17 triggering lymphokine or membrane ligand. Alternatively infected monocytes could express or secrete an IL-17 inhibitory molecule, such as IL-12.
Blood MAIT cells are nonproliferating cells
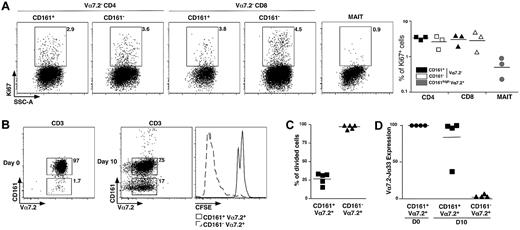
CD161 expression is a feature ascribed to both IL-17–secreting and stem cell–like memory CD8 T cells.18,19 The noncycling G0 status can be estimated by expression of Ki67. We observed very low frequency of Ki67+ events in the MAIT cell population compared with CD161+ or CD161− conventional memory CD4 and CD8 cells (Figure 4A), showing that MAIT cells are essentially in G0 in vivo.
MAIT cells are nonproliferating cells. (A) Representative Ki67 intracellular staining of CD4, CD8 T, or MAIT cells (left panel) and proportion from 3 different donors (right panel). (B) Representative Vα7.2-CD161 staining of freshly sorted MAIT cells (left panel) and after 10 days of culture in the presence of phytohemmaglutinin + IL-2, Il-7, and IL-15 (middle panel). CFSE pattern gated on MAIT (CD161+Vα7.2+) and CD161−Vα7.2+ cells after the 10-day culture period (right panel). (C) Proportion of CFSElow cells in MAIT cells and CD161−Vα7.2+ subset at day 10 in 5 independent experiments. (D) Vα7.2Jα33 transcript expression on sorted CD161+Vα7.2+ and CD161−Vα7.2+ at day10. Expression is normalized to that of freshly sorted MAIT cells.
MAIT cells are nonproliferating cells. (A) Representative Ki67 intracellular staining of CD4, CD8 T, or MAIT cells (left panel) and proportion from 3 different donors (right panel). (B) Representative Vα7.2-CD161 staining of freshly sorted MAIT cells (left panel) and after 10 days of culture in the presence of phytohemmaglutinin + IL-2, Il-7, and IL-15 (middle panel). CFSE pattern gated on MAIT (CD161+Vα7.2+) and CD161−Vα7.2+ cells after the 10-day culture period (right panel). (C) Proportion of CFSElow cells in MAIT cells and CD161−Vα7.2+ subset at day 10 in 5 independent experiments. (D) Vα7.2Jα33 transcript expression on sorted CD161+Vα7.2+ and CD161−Vα7.2+ at day10. Expression is normalized to that of freshly sorted MAIT cells.
Then, we analyzed their proliferation in vitro, after stimulation with phytohemaglutinin-L and a combination of IL-2, IL-7, and IL-15. CD3+ Vα7.2+CD161+ MAIT cells were electronically sorted to 96.6% plus or minus 1.7% of purity, stained with CFSE before stimulation with the aforementioned stimuli for 10 days, before fluorescence-activated cell sorter analysis. Surprisingly, we observed the appearance of numerous Vα7.2+CD161− T cells making 5% to 91% of the cultured cells (Figure 4B). Dilution of CFSE was only seen in the CD161− population, whereas the Vα7.2+CD161+ cells remained CFSEhi (Figure 4B-C). We sorted Vα7.2+C161− and Vα7.2+CD161+ T cells from the 10-day cell culture, and quantified the Vα7.2-Jα33 mRNA. Only the CD161+ fraction contained high levels of Vα7.2-Jα33 mRNA (Figure 4D), whereas it was undetectable within the appearing Vα7.2+CD161− population, showing that the few conventional T cells that were sorted together with MAIT cells strongly proliferated and finally outnumbered the noncycling MAIT cells. These experiments demonstrate the quiescent and hypoproliferative nature of MAIT cells compared with other T-cell subsets.
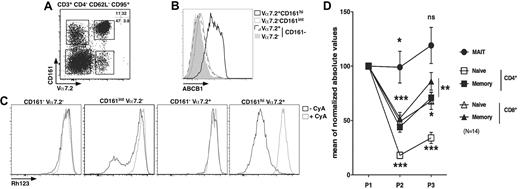
MAIT cells express ABCB1 and persist through chemotherapy
Long-lived cells are usually noncycling and often express the multidrug efflux protein ABCB1, which has been hypothesized to efflux toxic compounds from the cytoplasm.33-35 We analyzed the presence of ABCB1 at the surface of MAIT cells and other populations sorted according to their expression of several membrane markers. The effector-memory cell subset (CD4−CD95+CD62L−CD3+) was further divided according to Vα7.2 and CD161 expression. As shown in Figure 5A, this strategy enables the identification of 4 major populations: Vα7.2+CD161hi, Vα7.2+CD161−, Vα7.2−CD161int, and Vα7.2−CD161− cells. MAIT cells segregate in the Vα7.2+CD161hi gate, whereas other gates define subsets of conventional effector-memory T cells. Strikingly, the expression of ABCB1 within the CD4− effector-memory compartment was restricted to MAIT cells with all the cells expressing high levels of this receptor, whereas the other subsets expressed very low levels (Figure 5B). The ABCB1 transporter was functional as all MAIT cells rapidly lost Rh123 (a fluorescent substrate of the transporter), in a process that could be inhibited by cyclosporine A, a known inhibitor of ABCB1 (Figure 5C). By contrast, the other subsets did not contain any cells able to efflux Rh123 except for the Vα7.2−CD161int subset where a small proportion of cells did. This proportion was, however, very variable from one donor to another (Figure 5C second panel; and data not shown). Therefore, MAIT cells are noncycling G0 cells expressing high levels of a functional ABCB1 transporter, which enables them to rapidly efflux drugs.
MAIT cells express ABCB1 and persist through chemotherapy. (A) Representative Vα7.2-CD161 staining of CD3+CD4−CD62L−CD95+ PBLs. (B-C) Representative ABCB1 (B) or Rh123 (C) staining according to Vα7.2-CD161 expression as gated in panel A. (A-C) n = 4. (D) Blood numbers of naive (CD45RA+CD27+) or memory (CD45RA−CD27+) CD4 or CD8 T (CD3+γδ−) and MAIT cells of breast cancer patients before (P1), during (P2), and after (P3) chemotherapy (6 cycles of anthracycline-containing regimen). All values were normalized to prechemotherapy.
MAIT cells express ABCB1 and persist through chemotherapy. (A) Representative Vα7.2-CD161 staining of CD3+CD4−CD62L−CD95+ PBLs. (B-C) Representative ABCB1 (B) or Rh123 (C) staining according to Vα7.2-CD161 expression as gated in panel A. (A-C) n = 4. (D) Blood numbers of naive (CD45RA+CD27+) or memory (CD45RA−CD27+) CD4 or CD8 T (CD3+γδ−) and MAIT cells of breast cancer patients before (P1), during (P2), and after (P3) chemotherapy (6 cycles of anthracycline-containing regimen). All values were normalized to prechemotherapy.
To determine whether ABCB1 expression by MAIT cells would be correlated with cytotoxic drug resistance in vivo, we measured the number of MAIT cells in the blood of patients before, during, and after cancer treatment. MAIT cells were identified as CD4−Vα7.2+CD161hiCD3+ γδ− T lymphocytes, whereas naive and memory CD4 or CD8 cells were defined as CD45RA+CD27+ and CD45RA−CD27+ T cells, respectively.36 We followed the absolute number variations of these various subsets in patients undergoing 6 cycles of anthracycline (a drug that is effluxed by ABCB1) containing chemotherapy for breast cancer. To take into account interpatient variability, all the values are normalized to prechemotherapy. As shown in Figure 5D, the number of MAIT cells barely decreased after the third (P2) cycle of treatment and remain stable 1 month after the sixth cycle (P3). By comparison, conventional memory CD8 T cells significantly decreased in number during chemotherapy before slightly recovering after chemotherapy, but their number at P3 remained significantly different from the value obtained before treatment. Naive CD4 T cell numbers were the most affected by chemotherapy. However, all conventional T cells, naive or memory, CD4 or CD8 remained decreased after treatment. Thus, MAIT cells display a peculiar ability to persist during chemotherapy compared with other T cells.
Viral specific memory CD8 T cells are not contained in the CD161hiABCB1+ subset
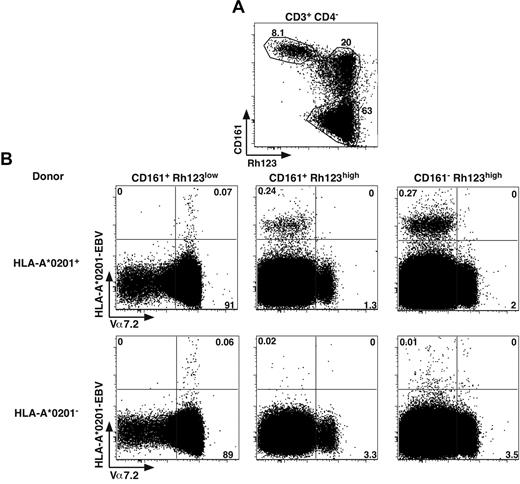
The G0 status, the expression of ABCB1 and expression of CD161 and IL-18Rα have been recently proposed as characteristics of antiviral CD8 memory stem T cells.18,19 However, the results seen in Figure 5 indicate that these features seemed to be restricted to MAIT cells within the effector memory CD8 compartment. Altogether, these data lead to an apparent contradiction as MAIT cells have a highly restricted TCR repertoire unlikely able to recognize classic MHC molecules loaded with viral peptides. We also recently demonstrated the inability of virus infected APCs to activate MAIT cells.
To tackle this apparent discrepancy, we analyzed ex vivo the distribution of cells binding HLA-A2 tetramers loaded with an EBV viral peptide, as previously used.18 Within the CD4− T cells, in the different CD161+ subsets expressing or not a functional ABCB1 transporter (Rh123lo or hi), we measured the proportion of Tet+ cells with respect to Vα7.2 expression (Figure 6). In HLA-A2+ donors, tetramer+ CD8 cells were essentially found in the Rh123hi subsets and expressed moderate to low levels of CD161 and were negative for Vα7.2, thereby representing conventional memory cells. We also observed some tetramer staining within the Vα7.2+CD161hiRh123lo population; however, the numbers were much smaller, and the staining appeared to be mostly nonspecific. Indeed, the same staining appears when analyzing HLA-A2− donors (Figure 6B bottom panels). Altogether, these data indicate that MAIT cells, which essentially constitute the CD161hiRh123lo subset, are unable to recognize viral peptides in the context of classic MHC.
MAIT cells do not recognize viral peptide presented by classic MHC. (A) Representative Rh123-CD161 staining of CD3+CD4− PBLs. Percentages are boxed. (B) Representative Vα7.2 and HLA-A*0201-EBV tetramer staining of PBLs from HLA-A*0201+ or HLA-A*0201− healthy donors, gated according to Rh123-CD161 expression as in panel A. Representative of 4 HLA-A*0201+ donors studied in independent experiments.
MAIT cells do not recognize viral peptide presented by classic MHC. (A) Representative Rh123-CD161 staining of CD3+CD4− PBLs. Percentages are boxed. (B) Representative Vα7.2 and HLA-A*0201-EBV tetramer staining of PBLs from HLA-A*0201+ or HLA-A*0201− healthy donors, gated according to Rh123-CD161 expression as in panel A. Representative of 4 HLA-A*0201+ donors studied in independent experiments.
Discussion
MAIT cells are very abundant in humans and display an effector-memory (CD45RO+CD27+) phenotype with chemokine receptor expression (CCR7−CCR9intCCR5hiCCR6hiCXCR6hi), indicating preferential homing to tissues, especially the liver and intestine where they are in high numbers. MAIT cells are noncycling cells that express high levels of ABCB1, rendering them resistant to chemotherapy and xenobiotics. They secrete high levels of IL-17 after stimulation with PMA and ionomycin. These characteristics indicate original properties and functions, which are fully compatible with their location and their ability to recognize cells infected with microbes.4
MAIT and NKT cells share many similarities.1 Interestingly, we found a high number of MAIT cells in human liver, which is reminiscent of the high number of CD1d-restricted NKT cells found in this organ in mouse. However, NKT cells are in low numbers in human liver and blood compared with MAIT cells.1 These intriguing facts could suggest that MAIT and NKT cell effector functions overlap, leading to the usage of one particular cell population in each of these 2 species. Alternatively, the functions of the 2 populations would be different, and the selection pressure imparted by the pathogens would lead to the requirement of NKT cells in mice and MAIT cells in humans.
Because MAIT cells are extremely abundant in human and express tissue homing integrins and chemokine receptors, to find MAIT cells in inflammatory tissues is not unexpected. Indeed, MAIT cells were present in several inflammatory tissues, such as multiple sclerosis neurologic lesions37,38 or solid cancers.15 The significance of these findings is difficult to assess in the absence of comparative analysis of blood and tissue MAIT cell numbers in several pathologic groups with similar inflammatory lesions, or without a functional analysis. On similar grounds, the significance of the high numbers of MAIT cells found in hepatitis C–infected livers becomes complex to assess because we show that they have a specific tropism for this organ. Indeed, the specific hepatitis C tetramer+ CD8 T cells studied previously10 are CD161int, suggesting that they are not MAIT cells.
We show that MAIT cells produce IL-17 after PMA and ionomycin stimulation. The ability to secrete this cytokine has been previously correlated to the expression of RORC as well as CCR6 and CD161.17 All these features are shared by the CD161hi CD8 T-cell subset first implicated in viral hepatitis10 before recently being further characterized by Billerbeck et al.11 These CD161hi CD8 T cells share many characteristics with MAIT cells. They express an intermediate level of CD8αβ and many other markers that we found using phenotypic and transcriptional analysis, such as IL-23R and CCR2. However, we found heterogeneous CXCR4 expression in both CD8 and DN MAIT cells, which contrasts with the complete positivity previously found.11 In addition, we were unable to detect IL-22 expression after CD3 + CD28 or PMA and ionomycin stimulations (data not shown). These differences could result from the different cell isolation procedures as cross-linking CD161 or TCR or using anti–IL-18Rα antibody could have positive or negative effects.7 Importantly, very few other CD8 T cells than MAIT cells were able to secrete IL-17.
Recently, another CD161hiIL-18Rα+ CD8 subset has been put forward as self-renewing stem cell–like memory lymphocytes able to restore antiviral immunity after chemotherapy, as they seem to survive to this treatment.18,19 Again, many common features suggested that this subset and MAIT cells were identical. However, we have shown that MAIT cells do not seem to be antiviral.4 This discrepancy is probably related to the absence of MAIT cell proliferation in vitro in the presence of mitogens and the IL-2, IL-7, IL-15 lymphokine cocktail, which in contrast induce proliferation of mainstream T cells (Figure 4B). Together with the absence of ex vivo specific viral tetramer staining by MAIT cells (Figure 6), these data indicate that the antiviral T cells previously found in the CD8+CD161hi population were probably CD161intVα7.2− mainstream T cells that proliferated during the 8- to 10-day culture period done by these authors before carrying out the antigen specificity analysis. Indeed, in the absence of anti-Vα7.2 antibody, cell sorting based on CD161+ expression of CD62LloCD95hi CD8 T cells leads to a mixed cell population containing a large majority of MAIT cells but also 5% to 25% of mainstream T cells (Figure 5A; and data not shown). Interestingly, some (15% in the donor displayed in Figure 5A-C) of these mainstream T cells efflux Rh123 (Figure 5C, CD161intVα7.2− panel). These cells might be the antiviral memory cells previously described.18 The exact phenotypic characterization of these antiviral cells remains to be done as the ex vivo analysis carried out to date was, indeed, on the much more abundant MAIT cells.
In any case, functionally and phenotypically, the IL-17–secreting and ABCB1+ CD161hi CD8 T cells previously described11,18 and the CD8+ MAIT cells seem to be the same and identical T-cell subset. It should be noted that MAIT cells also include CD4−CD8β− (DN) T cells. However, we have not yet found any functional or phenotypic difference between DN and CD8β+ MAIT cells.
The finding that MAIT cells include the 2 previously described CD161hi CD8 T-cell subsets sheds new light on these subsets. Although high CD161 expression on some human CD8 T cells initiated the definition of these 2 subsets,11,18,19 the effector-memory phenotype, CD161 and IL-18Rα expression, and IL-17 secretion are original features of MAIT cells, among many others. The restriction by MR1, the original development pathway, the specific location, and interactions with B cells and the commensal flora are probably the determining characteristics of MAIT cells leading to the specific phenotype and properties of these cells.
MAIT cells leave the thymus as naive cells before a peripheral expansion. The measurable number of CD161hi CD8 T cells in cord blood found by Billerbeck et al11 is in agreement with our previous data.3 These 2 sets of data are also in agreement with those of Turtle et al18 where no CD161hi CD8 T cells were apparent in cord blood as the focus was on memory cells. We show that MAIT cell express not only CD161 but also IL-18Rα as naive cells in cord blood, indicating the acquisition of particular features by MAIT cells during intrathymic selection. After birth, MAIT cells rapidly become memory with a progressive increase in numbers with age to reach adult levels at 1 to 2 years, confirming their intimate relationship with the commensal flora. We also show that they are nonproliferating cells when obtained from blood; however, it remains to be determined whether MAIT cells proliferate when stimulated inside the tissues. The high level of ABCB1 expression by MAIT cells is consistent with this G0 state. The role of ABCB1 expression is not clear in the literature. In view of the antibacterial activity of MAIT cells, we favor the idea that this high level of ABCB1 allows MAIT cells to resist the xenobiotics secreted by invasive bacteria either in the gut lamina propria or the liver.
The ability to secrete IL-17 by MAIT cells is consistent with their antibacterial and antifungal activity. To what extent MAIT cell properties may vary according to the context of MR1-dependent activation remains to be determined. Indeed, MAIT cell effector functions may be modulated by their location (blood or tissue) and the inflammatory context (presence of IL-18 secreted by epithelial cells after bacterial invasion or triggering of NKR [eg, CD161, NKp80, or NKG2D]). In any case, the G0 status, the high level of ABCB1 expression, and the ability to secrete IL-17 together with their abundance in the gut and liver make MAIT cells the ideal sentinels able to resist the harsh gut environment and fight pathogenic bacteria.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Nikon Imaging Center at Institut Curie-Centre National de la Recherche Scientifique, the anatomo-pathology department of the Curie Institute for biopsies, Zofia Maciorowski for cell sorting, and Dr Pierre Coulie for tetramer.
This work was supported by Inserm and Agence Nationale de la Recherche.
Authorship
Contribution: M.D., E.M., N.S., I.P., V.P., and D.L. performed the experiments; M.D., E.M., N.S., I.P., D.L., M.M., and E.T. processed the data; M.D., E.M., L.L.B., C.S., E.T., and O.L. wrote the manuscript; and O.L. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Lantz, Laboratoire d'Immunologie and Inserm U932, Institut Curie, Paris, France, 26 rue d'Ulm, 75005 Paris, France; e-mail: olivier.lantz@curie.net.
References
Author notes
M.D. and E.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal