Abstract
We have recently reported that CD8+ T-cell memory maintenance after immunization with recombinant human adenovirus type 5 (rHuAd5) is dependent upon persistent transgene expression beyond the peak of the response. In this report, we have further investigated the location and nature of the cell populations responsible for this sustained response. The draining lymph nodes were found to be important for primary expansion but not for memory maintenance, suggesting that antigen presentation through a nonlymphoid source was required. Using bone marrow chimeric mice, we determined that antigen presentation by nonhematopoietic antigen-presenting cells (APCs) was sufficient for maintenance of CD8+ T-cell numbers. However, antigen presentation by this mechanism alone yielded a memory population that displayed alterations in phenotype, cytokine production and protective capacity, indicating that antigen presentation through both hematopoietic and nonhematopoietic APCs ultimately defines the memory CD8+ T-cell response produced by rHuAd5. These results shed new light on the immunobiology of rHuAd5 vectors and provide evidence for a mechanism of CD8+ T-cell expansion and memory maintenance that relies upon both hematopoietic and nonhematopoietic APCs.
Introduction
Recombinant adenovirus vectors have proven to be robust immunogens for eliciting T-cell immunity.1-3 Vaccines derived from recombinant human adenovirus serotype 5 (rHuAd5) have displayed remarkable potency in various models prompting further investigation. To better understand the immunobiology of rHuAd5, we have been studying both the nature of the CD8+ T cells elicited by these vaccines and the mechanisms of CD8+ T-cell priming and memory maintenance. Immunization with rHuAd5 typically produces a sustained memory population with a protracted contraction phase,4-8 although these kinetics may be influenced by vector configuration and route of administration.9 The memory CD8+ T-cell population is composed primarily of effector T cells (TEFF) and effector memory T cells (TEM),5,6 which is indicative of a persistent viral infection. Indeed, we have recently determined that sustained, low-level antigen expression from the rHuAd5 vector plays a key role in maintaining the CD8+ T-cell memory population.10 Premature extinction of transgene expression causes pronounced CD8+ T-cell contraction, but only modestly affects phenotype, suggesting that memory maintenance and phenotype may be regulated by distinct mechanisms.
The relationship between memory CD8+ T-cell phenotype and protective immunity remains to be fully established. TEM provide optimal immune protection against certain agents11-13 ; therefore, understanding the nature of the antigen-presenting cells (APCs) involved in the generation and maintenance of CD8+ TEM will provide important information for vaccine design. Dendritic cells (DCs) are thought to be critical for the induction of antiviral CD8+ T-cell responses. At least 7 distinct DC populations have been identified14 ; however, the blood-derived CD8α+ DC may be specialized for antigen presentation.15-19 A central role for CD8α+ DCs in priming CD8+ T cells has been established after acute and chronic viral infection15-20 and intracellular bacterial infection.19 The requirement of DCs for priming CD8+ T-cell immunity was confirmed with a model that allowed inducible in vivo depletion of CD11c+ DCs.21,22 Because conventional CD11c+ DCs are derived from hematopoietic precursors, it is not surprising that most studies identified an essential role for bone marrow (BM)–derived hematopoietic APCs in the generation of CD8+ T-cell immunity after infection.23-26 Despite the evidence supporting a primary role for DCs in triggering CD8+ T-cell immunity, several reports have demonstrated a role for nonhematopoietic APCs (nhAPCs),24,25,27,28 indicating that the relative contribution of conventional APCs and nhAPCs may be pathogen-dependent. How priming by the respective APCs influences the CD8+ T-cell response remains to be determined.
Antigen is available in the draining lymph nodes (DLNs) for months after rHuAd5 immunization.5,6 Coupled with our recent report demonstrating that continuous transgene expression is required for maintaining the CD8+ T-cell response over the first 2 months after rHuAd5 immunization,10 we suspected that extended transgene expression within the DLNs leads to prolonged antigen presentation and maintenance of the early CD8+ T-cell memory population. However, the nature of the APC involved remains unknown. Prolonged antigen presentation has been noted after infection with herpes simplex virus type 1,29 influenza,30-32 and γ-herpesvirus,20 which appears to be mediated by DCs in the lymphoid tissue.20,29,32 Because the rHuAd5 vector does not replicate, the infected cell population must be reasonably long-lived to sustain continuous transgene expression. It is questionable whether conventional DCs could support long-term transgene expression because they typically have a short half-life in vivo, although recent reports have suggested that they can divide and pass antigen on to daughter cells.33 It is equally possible that the prolonged antigen presentation is not mediated through conventional BM-derived APCs. We have reported that nhAPCs are capable of priming and expanding rHuAd5-induced CD8+ T cells under conditions of extreme leukopenia.34 Whether these cells play a significant role in the generation or maintenance of CD8+ T-cell immunity in mice replete with leukocytes is unknown. In the current study, we have investigated the importance of the DLNs in maintenance of the CD8+ T-cell response and the relative contribution of nhAPCs to CD8+ T-cell memory.
Methods
Mice
Female C57BL/6 (B6) mice 6 to 8 weeks of age were purchased from Charles River Breeding Laboratories or Taconic Farms. Kb-deficient Db knockout mice (Kb−/−Db−/− mice) were also from Taconic Farms. Thy1.1+ (B6.PL-Thy1a/CyJ) mice were purchased from The Jackson Laboratory. OT-1 (Thy1.1+) and Kb−/− mice were bred in the Central Animal Facility at McMaster University. All procedures were approved by the McMaster Animal Research Ethics Board.
Adenoviruses, injections, and doxycycline treatment
The following vectors have been described elsewhere: rHuAd5-SIINFEKL-Luc (expressing SIINFEKL linked to luciferase [Luc]),4 doxycycline (DOX)–regulated rHuAd5-tTA-SIINFEKL-Luc (expressing SIINFEKL-Luc under the control of the tetracycline transactivator [tTA] SIINFEKL),10 rHuAd5-NP (expressing PR8 influenza virus nucleoprotein [NP] 35 and rHuAd5-β-galactosidase ([β-gal], expressing β-gal).36 The rHuAd5-GP33-ER virus encodes an immunodominant CD8+ T-cell epitope from lymphocytic choriomeningitis virus (LCMV) glycoprotein (GP) included (KAVYNFATM; C-terminal residue modified to improve binding to Db) linked to the adenovirus E3gp19K endoplasmic reticulum (ER)–targeting sequence. Viruses were propagated using 293 cells and purified using CsCl gradient centrifugation.5 For intramuscular immunizations, virus was diluted in phosphate-buffered saline and injected in both rear thighs using a 0.5-cc insulin syringe. DOX was administered initially as an intraperitoneal injection of 500 μg and maintained by addition of DOX in the drinking water as described.10
Challenge viruses
Recombinant vaccinia virus (rVV) expressing the SIINFEKL epitope linked to an ER-insertion sequence (rVV-ESOVA)37 was injected intraperitoneally at 107 plaque-forming units (pfu). Virus replication was measured in the ovaries 7 days later as published.5 Mouse-adapted influenza A/FM/1/47 (H1N1) was delivered intranasally at 105 pfu, and virus titers were measured in the lung 5 days later as described.38 LCMV strain WE, kindly provided by Dr Alain Lamarre (INRS-Institut Armand-Frappier, Quebec, QC), was injected intravenously at 200 pfu, and virus titers were measured in the spleen 3 days later as described.39
Tumor challenge
Challenge with ovalbumin (OVA)–expressing B16M05 melanoma cells was performed as previously described,34 except tumor cells were injected intradermally.
Flow cytometric antibodies and analytic instruments
All antibodies used for flow cytometry were purchased from BD Biosciences with the exception of: anti–killer cell lectin-like receptor subfamily G1 member (KLRG1), anti-CD62L, and anti-CD127 (eBioscience); anti-CD43 (Biolegend); anti–granzyme B (Caltag Laboratories). Kb/SIINFEKL tetramers were obtained from the Molecular Biology Core at the Trudeau Institute. Results were acquired using a FACSCanto (BD Biosciences) and analyzed using FlowJo Version 8.7.3 (TreeStar).
Flow cytometry and staining
Antigen presentation assay
A total of 5 × 105 carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled OT-I CD8+ T cells were infused into immunized mice. Tissues were retrieved 24 hours later, and cells were seeded into 96-well plates at 106 cells/well. Cells were stained for CD8 and Thy1.1 after 72 hours of culture, and CFSE proliferation was analyzed by flow cytometry.
Lymphadenectomies
Mice were anaesthetized by isoflurane. An incision was made on each hind leg and the popliteal lymph nodes (LNs) were excised. A vertical incision was made on the abdomen to expose the peritoneum and the inguinal LNs were removed. The peritoneum was then cut to allow for removal of the iliac LNs. The peritoneum and skin incisions were closed with adsorbable and nonadsorbable sutures (Ethicon), respectively.
Lethal irradiation and BM transplantation
Mice were lethally irradiated as described34 and rescued by intravenous infusion of 5 × 106 BM cells. BM was depleted of T cells using a cocktail of anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), and anti–Thy-1.2 (clone 30-H12) followed by treatment with low-toxicity guinea pig complement (Cedarlane Laboratories). All B6 mice transplanted with Kb−/− bone marrow (Kb−/−→B6 mice) received 200 μg each of anti-CD4 and anti-CD8 antibodies 4 and 6 days after BM transplantation to deplete residual radioresistant T cells. All antibodies were produced and purified from hybridoma supernatants at McMaster University.
Antigen presentation and cytotoxicity assays
Statistical analysis
Student t tests were conducted with Microsoft Excel Version 11.6.2. The 1-way ANOVAs were conducted with STATISTICA Version 8 (StatSoft) or Prism Version 4 (GraphPad). Differences were considered significant at P < .05. Data are presented as mean (± SEM) for each experiment shown.
Results
Antigen presentation occurs in the DLNs after intramuscular rHuAd5 immunization
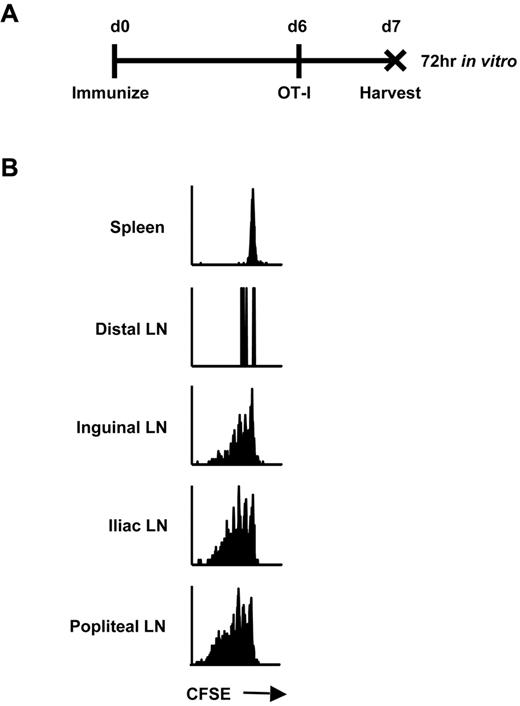
We hypothesized that continual presentation of vector-derived antigen within the DLNs was responsible for maintenance of early memory CD8+ T cells after immunization with rHuAd5. We previously determined that the antigen-specific CTL response was initiated in the popliteal, inguinal, and iliac LNs, suggesting that these were the sites of antigen priming.4 Consistent with those findings, we also observed that transgene expression was limited to these secondary lymphoid organs using Luc4 and green fluorescence protein reporters (data not shown). To confirm that these nodes were actually sites of antigen presentation, CFSE-labeled OT-I CD8+ T cells were adoptively transferred 6 days after rHuAd5-SIINFEKL-Luc immunization. This time point was selected because live virus is no longer measurable in the mouse (data not shown), and the CTL response is established4 but dependent upon transgene expression.10 So, there is no concern about vector spreading to additional sites and the observed antigen presentation is relevant to the CD8+ T-cell response. Lymphoid tissues were removed 24 hours after OT-I transfer, providing the T cells sufficient time to engage SIINFEKL-loaded APCs, but not enough time to proliferate and migrate to other sites. The OT-I cells were incubated in vitro for 3 days to allow time for proliferation (Figure 1A). OT-I cells retrieved from the popliteal, inguinal, and iliac LNs displayed evidence of proliferation in vitro, whereas cells isolated from distal LNs and spleen did not, confirming the DLNs as sites of antigen presentation (Figure 1B).
Antigen presentation occurs only in the DLNs after intramuscular rHuAd5 immunization. (A) Experimental design where mice were injected intravenously with 5 × 105 CFSE-labeled OT-I CD8+ T cells 6 days after rHuAd5-SIINFEKL-Luc immunization. Tissues were harvested at day 7, and cells were cultured separately in vitro for 72 hours. (B) CFSE profile of OT-I cells isolated from the spleen, distal LNs (axillary, brachial) or DLNs (inguinal, iliac, popliteal) at 7 days after immunization.
Antigen presentation occurs only in the DLNs after intramuscular rHuAd5 immunization. (A) Experimental design where mice were injected intravenously with 5 × 105 CFSE-labeled OT-I CD8+ T cells 6 days after rHuAd5-SIINFEKL-Luc immunization. Tissues were harvested at day 7, and cells were cultured separately in vitro for 72 hours. (B) CFSE profile of OT-I cells isolated from the spleen, distal LNs (axillary, brachial) or DLNs (inguinal, iliac, popliteal) at 7 days after immunization.
Immunofluorescent analysis revealed that antigen expressed from rHuAd5 in the DLNs was largely restricted to regions surrounding the B-cell follicles and localized primarily to CD11b+ cells and some CD11c+ cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), similar to recent results using vaccinia virus and vesicular stomatitis virus.40 Furthermore, CD11c+ cells isolated from the DLNs were the primary sources of antigen for at least 12 days after immunization (supplemental Figure 2), consistent with the concept that DCs in the DLNs were responsible for CD8+ T-cell activation and maintenance.
The DLNs are required for maximal CD8+ T-cell expansion but not for memory maintenance
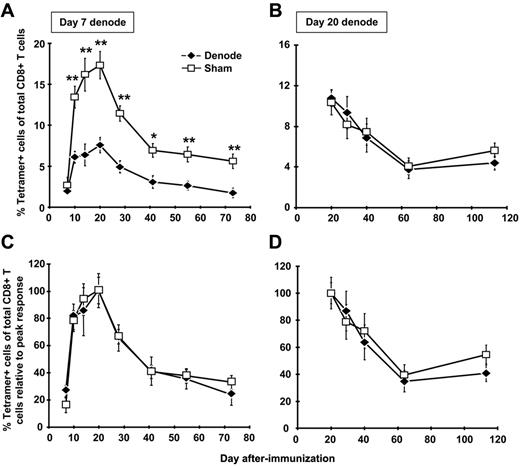
To directly address the role of antigen presentation within the DLNs in expansion and maintenance of the CD8+ T-cell memory population, we removed the DLNs after rHuAd5 immunization. day 7 after immunization was chosen as the first time point for lymphadenectomy because antigen-specific CD8+ T cells are already measurable in the periphery,4 yet continued expansion of the response is critically dependent upon transgene expression (and presumably antigen presentation) beyond this time point.10 These experiments were conducted with a high dose (108 pfu) and low dose (107 pfu) of virus. Similar results were obtained with both doses, so only the results for 107 pfu are shown. Removal of the DLNs had a major impact on the magnitude of primary expansion, consistent with a role for these nodes in initial expansion (Figure 2A); the magnitude of the CD8+ T-cell response was reduced by approximately 60% in the peripheral blood (Figure 2A), and other tissues, including lung, spleen, peritoneal cavity, and BM (data not shown).
The DLNs are required for maximal CD8+ T-cell expansion but not for memory maintenance. B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. The DLNs (popliteal, inguinal, iliac) were surgically removed 7 or 20 days later. Sham mice received the same incisions as denoded mice, but their LNs were not removed. The frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood after lymphadenectomy at day 7 (A) or day 20 (B). Each data point represents the average (± SEM) of 5 mice in each experiment. (C-D) Data in panels A and B were normalized to the peak response of each group (ie, day 20 for both denode and sham mice). *P < .05, **P < .01.
The DLNs are required for maximal CD8+ T-cell expansion but not for memory maintenance. B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. The DLNs (popliteal, inguinal, iliac) were surgically removed 7 or 20 days later. Sham mice received the same incisions as denoded mice, but their LNs were not removed. The frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood after lymphadenectomy at day 7 (A) or day 20 (B). Each data point represents the average (± SEM) of 5 mice in each experiment. (C-D) Data in panels A and B were normalized to the peak response of each group (ie, day 20 for both denode and sham mice). *P < .05, **P < .01.
LN removal at day 7 did not affect memory maintenance as the memory population in mice that underwent lymphadenectomy displayed a contraction rate that was identical to the rate observed in sham controls (Figure 2C). To directly address the role of antigen within the DLNs in maintenance of the memory population, lymphadenectomy was performed at the peak of the primary response (day 20). Extinction of transgene expression at this time point causes a marked contraction of the memory pool,10 confirming the need for antigen beyond the peak of the response to maintain CD8+ T-cell numbers. At this time point, lymphadenectomy had no significant effect on maintenance of the CD8+ T-cell response (Figure 2B,D). Thus, although antigen presentation within the DLNs was required for primary expansion, these sites were not responsible for the long-term antigen presentation required for memory maintenance.
Memory maintenance requires a source of antigen outside the lymphoid tissue
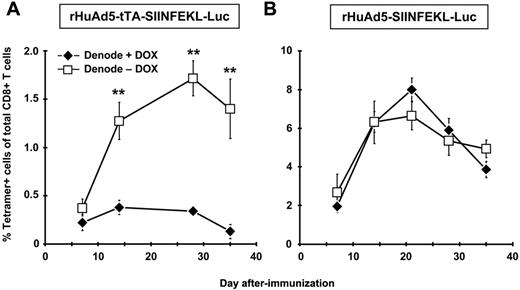
The results thus far suggest that although the DLNs play a key role in primary expansion of the CD8+ T-cell population, they are likely not the sites of the persistent transgene expression required for maintenance of the early memory population. To confirm this result, we used the DOX-regulated rHuAd5-tTA-SIINFEKL-Luc vector,10 which permits controlled extinction of transgene expression. Mice were immunized with rHuAd5-tTA-SIINFEKL-Luc and the DLNs were removed 7 days later. Treatment with DOX at the time of LN removal completely abrogated the CD8+ T-cell response (Figure 3A). In contrast, the CD8+ T-cell response continued to expand in mice that were not treated with DOX, confirming a role for APC outside the DLNs in both primary expansion and maintenance of the CD8+ T-cell population. As a control, mice were immunized with rHuAd5-SIINFEKL-Luc, which uses a constitutive promoter, subjected to lymphadenectomy, and treated with DOX. In this case, DOX treatment had no effect on the CD8+ T-cell response (Figure 3B), confirming that the effect of DOX in Figure 3A was a result of extinction of transgene expression.
CD8+ T-cell maintenance is dependent upon antigen in the periphery and not the DLNs. B6 mice were immunized with 109 pfu of rHuAd5-tTA-SIINFEKL-Luc (A) or 107 pfu of rHuAd5-SIINFEKL-Luc (B) intramuscularly. The DLNs (popliteal, inguinal, iliac) were removed 7 days later. On day 7, one group of denoded mice received DOX in their drinking water (Denode + DOX) and the other group received no DOX (Denode − DOX). Frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood at various time points. Data points represent a total (± SEM) of 5 mice. **P < .01.
CD8+ T-cell maintenance is dependent upon antigen in the periphery and not the DLNs. B6 mice were immunized with 109 pfu of rHuAd5-tTA-SIINFEKL-Luc (A) or 107 pfu of rHuAd5-SIINFEKL-Luc (B) intramuscularly. The DLNs (popliteal, inguinal, iliac) were removed 7 days later. On day 7, one group of denoded mice received DOX in their drinking water (Denode + DOX) and the other group received no DOX (Denode − DOX). Frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood at various time points. Data points represent a total (± SEM) of 5 mice. **P < .01.
Early removal of the DLNs alters memory CD8+ T-cell phenotype but not secondary responses
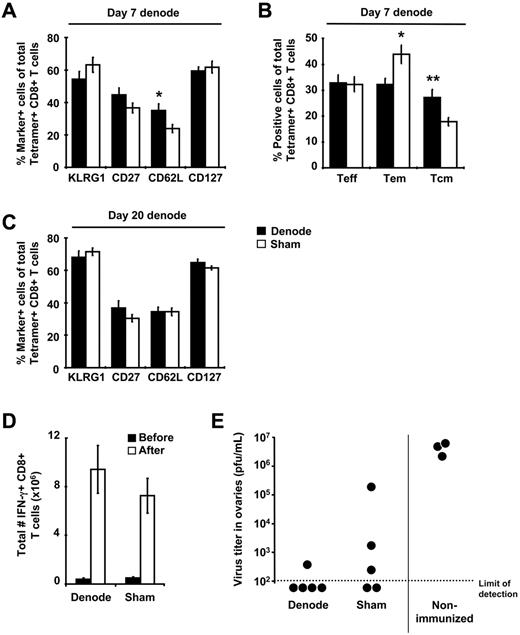
Immunization with rHuAd5 elicits a memory CD8+ T-cell population characterized by a predominance of TEM (CD127+CD62L−), high levels of KLRG1, and low levels of CD27.10 Removal of the DLNs at day 7 after immunization led to an increase in the frequency of CD62L+ cells and a corresponding increase in the proportion of central memory T cells (TCM, CD127+CD62L+) at 4 months after immunization, but no change in any other memory markers (Figure 4A-B). Removal of the DLNs at day 20 after immunization had no effect on the expression of phenotypic markers (Figure 4C).
Early removal of the DLNs alters memory CD8+ T cell phenotype but not secondary responses. (A,C) B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and their DLNs were surgically removed 7 or 20 days later. Spleens were harvested from these mice approximately 100 days after surgery and SIINFEKL-specific CD8+ T cells were identified using tetramer staining. Expression of various phenotypic markers on tetramer-positive CD8+ T cells was examined after lymphadenectomy shown at day 7 (A-B) or day 20 (C). (B) Frequency of TEFF (CD127−CD62L−), TEM (CD127+CD62L−), and TCM (CD127+CD62L+) within the tetramer-positive population after LN removal at day 7. Data points represent a total (± SEM) of 8 to12 mice. (D-E) B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and their DLNs were surgically removed 7 days later. Mice were challenged intraperitoneally with 107 pfu of rVV-ESOVA approximately 100 days after surgery. (D) Total number of SIINFEKL-specific IFN-γ+ CD8+ T cells in the spleen before and 7 days after rVV challenge. Data points represent a total (± SEM) of 5 mice. (E) Virus titers in the ovaries 7 days after rVV challenge. Each point represents a single mouse. *P < .05, **P < .01.
Early removal of the DLNs alters memory CD8+ T cell phenotype but not secondary responses. (A,C) B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and their DLNs were surgically removed 7 or 20 days later. Spleens were harvested from these mice approximately 100 days after surgery and SIINFEKL-specific CD8+ T cells were identified using tetramer staining. Expression of various phenotypic markers on tetramer-positive CD8+ T cells was examined after lymphadenectomy shown at day 7 (A-B) or day 20 (C). (B) Frequency of TEFF (CD127−CD62L−), TEM (CD127+CD62L−), and TCM (CD127+CD62L+) within the tetramer-positive population after LN removal at day 7. Data points represent a total (± SEM) of 8 to12 mice. (D-E) B6 mice were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and their DLNs were surgically removed 7 days later. Mice were challenged intraperitoneally with 107 pfu of rVV-ESOVA approximately 100 days after surgery. (D) Total number of SIINFEKL-specific IFN-γ+ CD8+ T cells in the spleen before and 7 days after rVV challenge. Data points represent a total (± SEM) of 5 mice. (E) Virus titers in the ovaries 7 days after rVV challenge. Each point represents a single mouse. *P < .05, **P < .01.
The production of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) by the memory CD8+ T cells was not influenced by lymphadenectomy (supplemental Figure 3). To examine secondary responses, mice were challenged with rVV-ESOVA, which shares only the SIINFEKL epitope with rHuAd5-SIINFEKL-Luc. The magnitude of the secondary response was identical in both sham controls and mice that had undergone lymphadenectomy at day 7 (Figure 4D), and protection from rVV infection was also similar (Figure 4E). These data indicate that antigen presentation in the DLNs plays an important role in primary CD8+ T-cell expansion but only modestly influences maintenance and functionality of the memory CD8+ T cells.
Preparation of BM chimeric mice to examine the contribution of nhAPCs
Based on our previous report34 that radioresistant cells can prime CD8+ T cells after rHuAd5 immunization in leukopenic hosts, we hypothesized that persistent transgene expression within nonhematopoietic cells residing outside the DLNs was responsible for CD8+ T-cell expansion and maintenance as suggested by Figure 3A. To address this possibility, we prepared BM chimeric mice where wild-type mice were reconstituted with Kb−/− BM to limit presentation of the Kb-restricted SIINFEKL epitope to nhAPCs. CD8+ and CD4+ T cells, B cells, and CD11b+ cells were all derived from the donor BM in these chimeras (supplemental Figure 4 right panels and data not shown). To carefully examine the origin of the DCs in the chimeric mice, we purified CD11c+ cells before analysis. CD8α+ DCs and plasmacytoid DCs were of donor origin, whereas approximately 50% of the Langerin-positive DCs were found to come from the host, consistent with previous reports41 (supplemental Figure 4 left panels). Using Langerin-diphtheria toxin receptor-enhanced green fluorescence protein mice (DTREGFP mice), which allows for inducible depletion of Langerin-positive DCs after treatment with diphtheria toxin,42 we confirmed that Langerin-positive DCs were not involved in the CD8+ T-cell response produced by rHuAd5 (supplemental Figure 5). Therefore, we reasoned that the Kb−/−→B6 chimeras would be suitable tools for investigating the role of antigen presentation by nhAPCs after rHuAd5 immunization.
Antigen presentation is dramatically reduced when nonhematopoietic cells are the only functional APCs
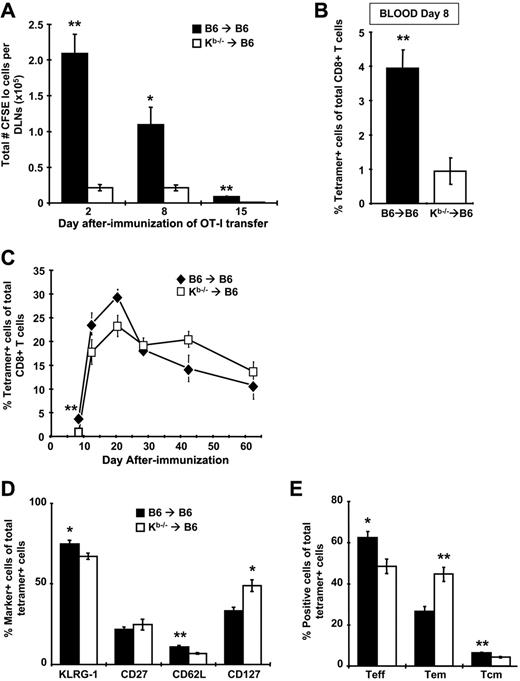
To determine the relative contribution of nhAPCs to CD8+ T-cell priming after rHuAd5 immunization, BM chimeras were immunized with rHuAd5-SIINFEKL-Luc and CFSE-labeled OT-I cells were adoptively transferred at various days after immunization. The OT-I cells were recovered from the DLNs after 96 hours and assessed for proliferation. At all time points, CFSElo OT-I cells were measurable in Kb−/−→B6 chimeras, indicating that naive T-cell priming was indeed occurring in these mice. However, the number of OT-I cells that had proliferated was 5- to 10-fold lower in Kb−/−→B6 mice compared with B6→B6 controls, suggesting that the OT-I cells in Kb−/−→B6 chimeras were exposed to significantly less antigen (Figure 5A). Consistent with diminished antigen presentation, we observed that initial expansion of endogenous SIINFEKL-specific CD8+ T-cells was reduced by 4-fold in Kb−/−→B6 chimeras (Figure 5B).
Antigen presentation is dramatically reduced and memory CD8+ T-cell phenotype is altered when nonhematopoietic cells are the only functional APCs, but kinetics and magnitude of the CD8+ T-cell response are similar. B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. (A) At various days after immunization, mice were injected intravenously with 5 × 105 CFSE-labeled cells harvested from the LNs of Thy1.1+ OT-I mice. The DLNs were harvested 4 days after transfer and CFSE dilution was measured in the Thy1.1+ population. Total number of proliferated (CFSElo) cells in the DLNs is shown. Each data point represents the mean (± SEM) from a total of 4 mice. (B-C) Frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood at day 8 (B) or at various time points (C) after immunization. Data points represent a total of 5 to 12 mice (± SEM). (D) Expression of various phenotypic markers on tetramer-positive CD8+ T cells in the spleen at 42 days after immunization. (E) The frequency of TEFF (CD127−CD62L−), TEM (CD127+CD62L−), and TCM (CD127+CD62L+) within the tetramer-positive population at day 42. Data in panels D and E represent the mean (± SEM) for 4 mice and are reflective of 2 experiments with similar results (n = 4 for each experiment). *P < .05, **P < .01.
Antigen presentation is dramatically reduced and memory CD8+ T-cell phenotype is altered when nonhematopoietic cells are the only functional APCs, but kinetics and magnitude of the CD8+ T-cell response are similar. B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. (A) At various days after immunization, mice were injected intravenously with 5 × 105 CFSE-labeled cells harvested from the LNs of Thy1.1+ OT-I mice. The DLNs were harvested 4 days after transfer and CFSE dilution was measured in the Thy1.1+ population. Total number of proliferated (CFSElo) cells in the DLNs is shown. Each data point represents the mean (± SEM) from a total of 4 mice. (B-C) Frequency of SIINFEKL-specific CD8+ T cells was measured by tetramer staining of peripheral blood at day 8 (B) or at various time points (C) after immunization. Data points represent a total of 5 to 12 mice (± SEM). (D) Expression of various phenotypic markers on tetramer-positive CD8+ T cells in the spleen at 42 days after immunization. (E) The frequency of TEFF (CD127−CD62L−), TEM (CD127+CD62L−), and TCM (CD127+CD62L+) within the tetramer-positive population at day 42. Data in panels D and E represent the mean (± SEM) for 4 mice and are reflective of 2 experiments with similar results (n = 4 for each experiment). *P < .05, **P < .01.
Nonhematopoietic APCs are capable of expanding and maintaining a CD8+ T-cell response
As shown in Figure 3A, primary expansion of the CD8+ T-cell response requires a source of antigen outside the DLNs that sustains transgene expression beyond day 7. Interestingly, despite the 4-fold difference in the frequency of SIINFEKL-specific CD8+ T cells at day 8 in the Kb−/−→B6 mice, we observed no significant differences in frequency at later time points (Figure 5C). These results demonstrate that although the lack of conventional hematopoietic APCs results in attenuation of CD8+ T-cell expansion, the memory population achieves similar levels. Furthermore, the rate of contraction in Kb−/−→B6 mice was blunted in comparison to B6→B6 controls (Figure 5C), supporting the concept that the memory population produced by rHuAd5 is maintained by nhAPCs.
Priming by nhAPCs alone leads to increased accumulation of effector memory cells
We observed a redistribution of the memory CD8+ T-cell phenotype in Kb−/−→B6 mice with an almost 2-fold increase in the proportion of TEM (Figure 5D-E). Expression of KLRG1 and CD27 was similar in both groups (Figure 5D). We have previously observed increased levels of CD43 on CD8+ T cells produced by rHuAd5 in the absence of CD4+ T-cell help36 and we reasoned that the environment of the nhAPCs might not be conducive to adequate CD4+ T-cell engagement. Similarly, CD8+ T cells generated in the absence of CD4+ T-cell help displayed reduced levels of CD44.43 However, levels of both CD43 and CD44 were comparable on CD8+ T cells generated in both groups of mice (data not shown).
The CD8+ T-cell memory population produced by nhAPCs alone exhibits defects in protective immunity and cytokine production
The in vivo lytic activity of the SIINFEKL-specific T cells in Kb−/−→B6 mice was equal to control mice (Figure 6A). Secondary expansion of SIINFEKL-specific CD8+ T cells was measured after challenge with rVV-ESOVA. Kb−/−→B6 and B6→B6 mice that had been immunized with rHuAd5-SIINFEKL-Luc 42 days previously were challenged intraperitoneally with rVV-ESOVA, and the frequency of SIINFEKL-specific cells was measured 7 days later. Equivalent numbers of antigen-specific CD8+ T cells were measured in both groups, indicating that the secondary response was intact (Figure 6B). The ovaries of the irradiated mice had atrophied, so it was not possible to measure rVV titers after challenge.
Antigen presentation by nhAPCs alone alters CD8+ T-cell protective capacity but not lytic activity or secondary responses. B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. (A) In vivo lytic activity of SIINFEKL-specific CD8+ T cells at 42 days after immunization. SIINFEKL- and KAVYNFATM-pulsed Thy1.1+ splenocytes were labeled with a low concentration (0.5μM) and high concentration (5μM) of CFSE, mixed at a 1:1 ratio and adoptively transferred into immunized mice. Spleens and DLNs were removed 4 hours later. Data shown are gated on Thy1.1+ cells. The specific killing in each histogram represents the mean percentage (± SEM) for 5 mice. (B) Secondary expansion of SIINFEKL-specific CD8+ T cells after rVV challenge. Chimeras that were immunized 42 days previously with rHuAd5-SIINFEKL-Luc were challenged intraperitoneally with 107 pfu of rVV-ESOVA. SIINFEKL-specific CD8+ T cells were measured using tetramer staining 7 days after challenge. Data represent the mean (± SEM) for 10 mice per group. Cells measured from blood, spleen, and peritoneal cavity lavage (PCL) are shown. (C) Tumor growth after B16M05 challenge. Chimeras that were immunized 20 days previously with 107 pfu of rHuAd5-SIINFEKL-Luc or a control virus (rHuAd5-βgal) were challenged intradermally with 106 B16M05 tumor cells, and tumor size was monitored daily. Data represent the mean tumor volume (± SEM) for 5 mice per group. (D) Virus titers in the spleen after LCMV-WE challenge. Chimeras that were immunized intramuscularly 24 days previously with 108 pfu of rHuAd5-GP33-ER (Ad-GP33) or rHuAd5-βgal (Ad-βgal) were challenged intravenously with 200 pfu of LCMV-WE. Spleens were harvested 3 days after challenge. (E) Virus titers in the lung after influenza virus challenge. Chimeras that were immunized intranasally 30 days previously with 5 × 107 pfu of rHuAd5-NP (Ad-NP) or rHuAd5-βgal (Ad-βgal) were challenged intranasally with 105 pfu of mouse-adapted influenza A virus. Lungs were harvested 5 days after challenge. For data in panels D and E, each point represents a single mouse. *P < .05, ***P < .001.
Antigen presentation by nhAPCs alone alters CD8+ T-cell protective capacity but not lytic activity or secondary responses. B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. (A) In vivo lytic activity of SIINFEKL-specific CD8+ T cells at 42 days after immunization. SIINFEKL- and KAVYNFATM-pulsed Thy1.1+ splenocytes were labeled with a low concentration (0.5μM) and high concentration (5μM) of CFSE, mixed at a 1:1 ratio and adoptively transferred into immunized mice. Spleens and DLNs were removed 4 hours later. Data shown are gated on Thy1.1+ cells. The specific killing in each histogram represents the mean percentage (± SEM) for 5 mice. (B) Secondary expansion of SIINFEKL-specific CD8+ T cells after rVV challenge. Chimeras that were immunized 42 days previously with rHuAd5-SIINFEKL-Luc were challenged intraperitoneally with 107 pfu of rVV-ESOVA. SIINFEKL-specific CD8+ T cells were measured using tetramer staining 7 days after challenge. Data represent the mean (± SEM) for 10 mice per group. Cells measured from blood, spleen, and peritoneal cavity lavage (PCL) are shown. (C) Tumor growth after B16M05 challenge. Chimeras that were immunized 20 days previously with 107 pfu of rHuAd5-SIINFEKL-Luc or a control virus (rHuAd5-βgal) were challenged intradermally with 106 B16M05 tumor cells, and tumor size was monitored daily. Data represent the mean tumor volume (± SEM) for 5 mice per group. (D) Virus titers in the spleen after LCMV-WE challenge. Chimeras that were immunized intramuscularly 24 days previously with 108 pfu of rHuAd5-GP33-ER (Ad-GP33) or rHuAd5-βgal (Ad-βgal) were challenged intravenously with 200 pfu of LCMV-WE. Spleens were harvested 3 days after challenge. (E) Virus titers in the lung after influenza virus challenge. Chimeras that were immunized intranasally 30 days previously with 5 × 107 pfu of rHuAd5-NP (Ad-NP) or rHuAd5-βgal (Ad-βgal) were challenged intranasally with 105 pfu of mouse-adapted influenza A virus. Lungs were harvested 5 days after challenge. For data in panels D and E, each point represents a single mouse. *P < .05, ***P < .001.
To measure protective immunity, rHuAd5-SIINFEKL-Luc–immunized chimeras were challenged with an OVA-expressing melanoma cell line. Although both Kb−/−→B6 and B6→B6 mice were protected against tumor growth, the T cells elicited by nhAPCs alone were less effective (Figure 6C). At day 28 after tumor challenge, B6→B6 controls were still completely protected, whereas only 20% of Kb−/−→B6 chimeras remained tumor-free. To assess protection against a virus, mice were immunized with rHuAd5-GP33-ER, which expresses only the GP33-41 CD8+ T-cell epitope from LCMV, and challenged with LCMV-WE. Again, both groups of chimeras were protected against LCMV-WE compared with mice immunized with rHuAd5-βgal, although the B6→B6 mice displayed greater protection (Figure 6D). Finally, we examined protective immunity after intranasal delivery of rHuAd5-NP. Because NP is an intracellular antigen, protection against influenza will be CD8+ T-cell–mediated. Both Kb−/−Db−/−→B6 and B6→B6 mice immunized with rHuAd5-NP displayed similar protection against influenza infection compared with mice immunized with rHuAd5-βgal (Figure 6E). These data indicate that although the CD8+ T cells generated by nhAPCs possess normal lytic activity and proliferative capacity, depending on the nature of the challenge, they may be less protective than those activated in a wild-type environment.
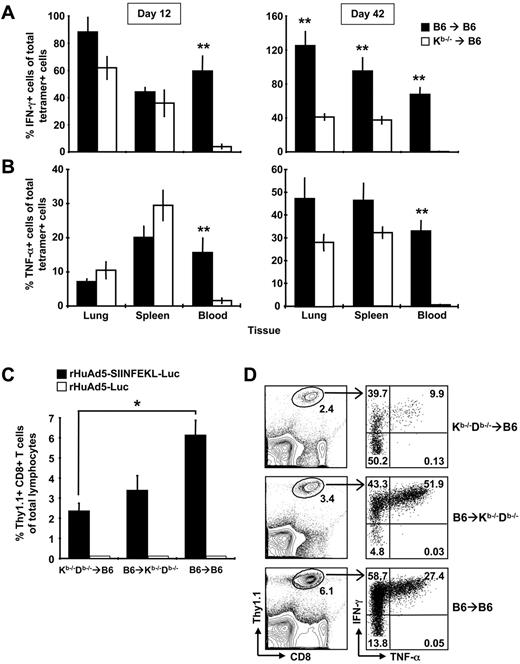
Further examination revealed that the CD8+ T cells in the Kb−/−→B6 mice were defective in cytokine production. Although we could readily measure tetramer-positive cells in the blood of Kb−/−→B6 chimeras at early and late time points, we detected few IFN-γ–secreting cells (Figure 7A). In the spleen and lung, IFN-γ production by antigen-specific CD8+ T cells was similar in both groups at early time points (Figure 7A left panel), but was impaired in Kb−/−→B6 mice at later time points (Figure 7A right panel). Antigen-specific cells from Kb−/−→B6 mice also produced significantly less IL-2 (data not shown). Similar, but not identical, results were observed for TNF-α production. Again, tetramer-positive cells in the blood produced very little cytokine. However, SIINFEKL-specific cells in the spleen and lung produced comparable levels of TNF-α in Kb−/−→B6 and B6→B6 mice at early and late time-points (Figure 7B). Thus, nonhematopoietic cells can expand and maintain a functional CD8+ T-cell population, but memory cells generated without help from the hematopoietic compartment display an altered pattern of cytokine production characterized by a striking deficiency in IFN-γ expression. We suspected that the CD8+ T cells in Kb−/−→B6 mice might be exhausted; however, PD-1 was not measurable on SIINFEKL-specific CD8+ T cells from either group of mice (data not shown). We interpret these data to suggest that expansion on nhAPCs alone results in differential programming of the CD8+ T cells rather than development of an exhausted phenotype.
Both conventional APCs and nhAPCs are required for optimal CD8+ T-cell expansion and cytokine production. (A-B) B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and spleens, lungs and blood were harvested 12 or 42 days later. SIINFEKL-specific CD8+ T cells were identified using tetramer staining or intracellular cytokine staining. The percentage of tetramer-positive cells that produce IFN-γ (A) or TNF-α (B) was calculated by dividing the frequency of IFN-γ+ cells or TNF-α+ cells by the frequency of tetramer-positive cells and multiplying by 100. Each histogram represents the mean percentage (± SEM) for 8 mice. (C-D) Kb−/−Db−/−→B6, Kb−/−Db−/−→B6, and B6→B6 mice chimeras received 5 × 104 Thy1.1+ OT-I CD8+ T cells intravenously. The next day, mice were immunized with 108 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. The frequency of SIINFEKL-specific CD8+ T cells were measured in the peripheral blood at day 8 by staining for Thy1.1 (C) or by intracellular cytokine staining for IFN-γ (D). Data points represent a total of 3 to 6 mice (± SEM) in each experiment. (D) Right panels show coexpression of IFN-γ and TNF-α by Thy1.1+ CD8+ T cells in each type of chimera. Value in each quadrant represents the mean frequency of Thy1.1+ cells in that quadrant. Frequency in the left panels represents the mean of Thy1.1+ cells of total lymphocytes. Frequency was calculated from a total of 3 to 6 mice per group. *P < .05, **P < .01.
Both conventional APCs and nhAPCs are required for optimal CD8+ T-cell expansion and cytokine production. (A-B) B6→B6 and Kb−/−→B6 chimeras were immunized with 107 pfu of rHuAd5-SIINFEKL-Luc intramuscularly and spleens, lungs and blood were harvested 12 or 42 days later. SIINFEKL-specific CD8+ T cells were identified using tetramer staining or intracellular cytokine staining. The percentage of tetramer-positive cells that produce IFN-γ (A) or TNF-α (B) was calculated by dividing the frequency of IFN-γ+ cells or TNF-α+ cells by the frequency of tetramer-positive cells and multiplying by 100. Each histogram represents the mean percentage (± SEM) for 8 mice. (C-D) Kb−/−Db−/−→B6, Kb−/−Db−/−→B6, and B6→B6 mice chimeras received 5 × 104 Thy1.1+ OT-I CD8+ T cells intravenously. The next day, mice were immunized with 108 pfu of rHuAd5-SIINFEKL-Luc intramuscularly. The frequency of SIINFEKL-specific CD8+ T cells were measured in the peripheral blood at day 8 by staining for Thy1.1 (C) or by intracellular cytokine staining for IFN-γ (D). Data points represent a total of 3 to 6 mice (± SEM) in each experiment. (D) Right panels show coexpression of IFN-γ and TNF-α by Thy1.1+ CD8+ T cells in each type of chimera. Value in each quadrant represents the mean frequency of Thy1.1+ cells in that quadrant. Frequency in the left panels represents the mean of Thy1.1+ cells of total lymphocytes. Frequency was calculated from a total of 3 to 6 mice per group. *P < .05, **P < .01.
Conventional APCs and nhAPCs cooperate to promote CD8+ T-cell expansion and programming of cytokine production
Our results suggested that conventional hematopoietic APCs play a role in the initial expansion and programming of rHuAd5-induced CD8+ T cells. To formally investigate the contribution of hematopoietic APCs, we prepared reciprocal chimeras (B6→Kb−/−Db−/− and Kb−/−Db−/−→B6), in which antigen presentation was localized to either conventional APCs or nhAPCs. For this model, we transferred Thy1.1+ OT-I cells 1 day before rHuAd5-SIINFEKL-Luc immunization because the major histocompatibility complex–deficient hosts do not possess SIINFEKL-specific CD8+ T-cell precursors. Although both conventional hematopoietic APCs and nhAPCs were capable of priming OT-I cells (Figure 7C), maximal expansion required both cell populations (B6→B6, Figure 7C). Cytokine analysis revealed that most of the OT-I cells produced IFN-γ in both Kb−/−Db−/−→B6 and B6→B6 mice, whereas only half of the OT-I cells activated in Kb−/−Db−/−→B6 mice could produce this cytokine (Figure 7D). TNF-α levels were also reduced in the CD8+ T cells expanded exclusively by nhAPCs. The frequency of IFN-γ/TNF-α double-positive OT-I cells was highest in Kb−/−Db−/−→B6 mice, followed by B6→B6, and lowest in Kb−/−Db−/−→B6 mice (P < .01, Figure 7D). Similar results were observed at day 12 after immunization, although as with the endogenous population (Figure 7A-B), the cytokine defect was more pronounced in the blood than in the spleen and lung (data not shown). These data argue that conventional hematopoietic APCs and nhAPCs cooperate to promote maximal CD8+ T-cell expansion and that the cytokine profile of the overall population is a composite of cells stimulated by both APCs.
Discussion
Overall, these data indicate that antigen presentation by both conventional hematopoietic APCs and nhAPCs ultimately defines the rate of expansion, magnitude, and functionality of the antigen-specific CD8+ T-cell population produced by rHuAd5. We propose the following model to explain the CD8+ T-cell response produced by rHuAd5 vectors (supplemental Figure 6): priming of the CD8+ T-cell population is driven primarily by conventional hematopoietic APCs within the DLNs (Figures 1–2 and 5), recently activated T cells interact with nhAPCs resulting in further expansion of the population (Figure 3) and maintenance of the early memory population is reliant upon transgene expression in nhAPCs outside the LNs (Figure 5).10 A recent report suggested that after intravenous immunization with rHuAd5, marginal metallophilic macrophages capture antigen and transfer it to CD8α+ DCs.44 Because we observed that most of the antigen within DLNs was located in CD11b+ cells (supplemental Figure 1), macrophages in the subcapsular sinus of the LNs may play a similar role after intramuscular rHuAd5 injection. However, our data suggest that this process would only be important for initial priming and not long-term maintenance.
Previous studies designed to test the requirements for nhAPCs in eliciting primary CD8+ T-cell responses to pathogens focused on initial expansion rather than memory maintenance.23-25,27,28 The broad conclusion from early studies23-25 was that nhAPCs contributed little to primary CD8+ T-cell expansion and hematopoietic cells were essential for priming CTL responses to multiple pathogens with the exception of LCMV. It was suggested that LCMV might replicate in the lymphoid tissues and infect the few host professional APCs that survived irradiation to induce a CTL response.24 Probst et al22 argued that conventional CD11c+ DCs were crucial for the CD8+ T-cell response to LCMV; however, that model did not allow nhAPCs to participate in antigen priming. Subsequently, Thomas et al27 showed that not only was it possible for nhAPCs to elicit CD8+ T cells after intraperitoneal LCMV infection, but these APCs were actually essential for maximal expansion. Thomas et al27 also noted that primary expansion was delayed when only nhAPCs were available for CD8+ T-cell priming but the magnitude of the primary response ultimately reached levels comparable to fully reconstituted mice, similar to the results we report. These data suggest that conventional CD11c+ DCs cooperate with nhAPCs to promote maximal expansion after both rHuAd5 and LCMV infection. A role for nhAPCs has also been noted in primary26 and memory26,45 CD8+ T-cell expansion in the lung after intranasal influenza virus infection, consistent with our observation that immunization with rHuAd5-NP provides equivalent protection in Kb−/−Db−/−→B6 and B6→B6 mice. Thus, nhAPCs are relevant to multiple pathogens and infection routes; however, their involvement in primary CD8+ T-cell expansion is pathogen-dependent, as the CD8+ T-cell responses produced by vaccina virus, vesicular stomatitis virus, or Listeria monocytogenes were only marginally impaired when nhAPCs were unable to present antigen.27
Our report offers several important and novel insights. Notably, we examined CD8+ T cells not only during the priming phase, but also during the memory phase. We showed that CD8+ T cells primed and maintained exclusively by nhAPCs exhibit a cytokine defect in the memory phase and a shift toward a TEM phenotype. Thus, conventional hematopoeitic APCs are required for appropriate programming of the memory response. Furthermore, we interrogated the endogenous T-cell population, so our results would not be unduly influenced by the unusual properties manifested in the memory phase by T-cell receptor transgenic cells.46 Finally, we used a moderate dose (107 pfu) of a replication-defective vector for immunization. Unlike LCMV, which produces high levels of viremia that could lead to infection of residual host APCs, the rHuAd5 vector used in this study is replication-defective, thereby avoiding possible mitigating effects of in vivo virus amplification.
Although prolonged antigen presentation was originally thought to be a characteristic of chronic pathogens,47 it is now recognized that antigen presentation persists for a prolonged period after “acute” infections like rHuAd55,10 and influenza.30-32 In the case of rHuAd5, transgene expression was required for at least 30 days after immunization to sustain the memory CD8+ T-cell population.10 Likewise, maintenance of the influenza-specific CD8+ T-cell memory pool was dependent upon stimulation of memory cells by antigen that persisted for up to 2 months in the lung DLNs.30 At later time points, antigen was not required to maintain an activated population of flu-specific CD8+ T cells in the lung,48 similar to our results with rHuAd5.10 Although we expected the DLNs were the site of continued antigen presentation in our rHuAd5 model, this proved not to be the case, indicating that the antigen required for sustaining the CD8+ TEM was located in nonlymphoid tissue. It has been suggested that memory CD8+ T cells may provide a long-lived source of adenovirus antigen6 ; however, our report argues against a role for hematopoietic cells in this process. During herpes simplex virus type 1 latency, ongoing antigen presentation at the trigeminal ganglia by nhAPCs has been implicated in maintaining a local population of activated CD8+ T cells.49 Gene expression after immunization with rHuAd5 can persist at low levels for months5,6,36 and we suspect that the nhAPCs may exist within the injection site. Given the potent immune responses produced by rHuAd5 vectors in murine and nonhuman primate models, further investigation of the nature of these nhAPCs is merited as this cell population may reflect a useful target for vaccine development.
Although we cannot explain the mechanisms underlying the cytokine defect in the antigen-specific CD8+ T cells generated by nhAPCs alone, our data suggest that hematopoietic APCs are more effective at programming cytokine production. OT-I cells activated exclusively by hematopoietic APCs expressed higher cytokine levels than those activated in control mice, whereas OT-I cells induced by nhAPCs alone expressed less IFN-γ and TNF-α than controls (Figure 7D). One factor that may be provided solely by professional APCs is appropriate costimulation. Interestingly, the CD8+ T-cell population elicited in CD80/CD86−/− mice after murine gammaherpesvirus 68 infection exhibited strong similarities to the CD8+ T cells elicited by rHuAd5 in Kb−/−→B6 chimeras.50 Regardless of the mechanism, our data demonstrate that although nhAPCs can promote a robust CD8+ T-cell response, conventional APCs are also required to ensure optimal development of memory cells.
This report adds to the growing body of literature demonstrating an important role for nonhematopoietic cells in peripheral CD8+ T-cell homeostasis. We propose a model where both conventional hematopoietic DCs in the LNs and nhAPCs in the periphery cooperate to promote maximal CD8+ T-cell expansion and progression to memory. The relative contribution of each population is dependent upon the pathogen and the importance of each APC population remains to be determined. Proper understanding of the contribution of both hematopoietic and nhAPCs will be required for optimal development of CD8+ T-cell vaccines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research grant MOP-42433 (J.L.B.). J.D.B. was supported by a studentship from the Canadian Institutes for Health Research.
Authorship
Contribution: J.D.B. and J.L.B. wrote the paper; J.D.B., T.C.Y. and J.L.B. designed research and analyzed data; Z.X. and Y.W. provided intellectual input and helped design research; and J.D.B., T.C.Y., D.B., J.B.M., S.L.S., A.J.R.M., H.V., J.E.B., J.D.F., R.P., C.E., D.D., N.G., M.D., and L.Z. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan L. Bramson, Department of Pathology and Molecular Medicine, McMaster University, Rm MDCL-4016, 1200 Main St West, Hamilton, ON, L8N 3Z5 Canada; e-mail: bramsonj@mcmaster.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal