Abstract
Treatment outcome of acute myeloid leukemia (AML) in elderly patients remains unsatisfactory. It has been shown that the infusion of granulocyte colony-stimulating factor–mobilized donor peripheral blood stem cells (G-PBSCs) can enhance graft-versus-leukemia effects and speed hematopoietic recovery. Fifty-eight AML patients aged 60-88 years were randomly assigned to receive induction chemotherapy with cytarabine and mitoxantrone (control group; n = 28) or it plus human leukocyte antigen–mismatched G-PBSCs (G-PBSC group; n = 30). Patients who achieved complete remission received another 2 cycles of postremission therapy with intermediate-dose cytarabine or it plus G-PBSCs. The complete remission rate was significantly higher in the G-PBSC group than in the control group (80.0% vs 42.8%; P = .006). The median recovery times of neutrophils and platelets were 11 days and 14.5 days, respectively, in the G-PBSC group and 16 days and 20 days, respectively, in the control group after chemotherapy. The 2-year probability of disease-free survival was significantly higher in the G-PBSC group than in the control group (38.9% vs 10.0%; P = .01). No graft-versus-host disease was observed in any patient. Persistent donor microchimerism was successfully detected in all of the 4 female patients. These results indicate that G-PBSCs in combination with conventional chemotherapy may provide a promising treatment method for AML in elderly patients.
Introduction
The outcome of acute myeloid leukemia (AML) in elderly patients older than the age of 60 years still remains unsatisfactory, with a low complete remission (CR) rate and poor overall survival (OS) due to prolonged pancytopenia, elevated chemotherapy-related mortality, and intrinsic resistance of leukemic blasts to therapeutic agents.1-3 The CR rate of standard induction chemotherapy with cytarabine plus anthracycline for such patients is low (40%), and the remission is usually transient and rarely lasts for > 12 months.4,5 Recently, Löwenberg et al6 reported that giving a dose of daunorubicin that was 2-fold higher than the conventional dose resulted in a higher response rate than the conventional dose, but it did not prolong OS time. The response of AML to the attenuated doses used in standard regimens or to granulocyte colony-stimulating factor (G-CSF) used after standard induction chemotherapy for reducing intensive chemotherapy-related toxicity or shortening the duration of neutropenia is still low because of inadequate antileukemic cytotoxicity or other reasons.7-9
Allogeneic stem cell transplantation (alloSCT) after nonmyeloablative or reduced intensity conditioning (RIC) shows some curative effects for AML in elderly patients. However, it is associated with severe graft-versus-host disease (GVHD), and the lack of available human leukocyte antigen (HLA)–matched donors for RIC remains a major obstacle.10-12 RIC-haploidentical transplantation may provide available donors, but it is limited by significant morbidity and mortality (eg, GVHD and infections).13-15 To date, a number of studies have shown that donor lymphocyte infusion (DLI) can mediate stronger graft-versus-leukemia (GVL) effects but is associated with severe GVHD and delayed neutrophil recovery on the basis of alloSCT.12,16 Furthermore, it has been reported that G-CSF–mobilized donor peripheral blood stem cell (G-PBSC) infusion can also mediate GVL effects and hasten hematologic recovery without amplifying GVHD.17 Irradiated DLI in conjunction with low-dose total body irradiation (TBI) has been used in rescue treatment of advanced leukemia in elderly patients, but it also shows disappointing outcomes due to poor response to treatment and severe GVHD.18 Moreover, our experimental studies showed that mice infused with a high dose of G-CSF–mobilized allogeneic spleen cells (3-12 × 107) after cytarabine chemotherapy without immunosuppressive pretreatment exhibited rapid hematopoietic recovery and persistent microchimerism without GVHD. On the basis of these observations, we designed a clinical control study to investigate the effects of G-PBSC infusion combined with conventional induction or intensive postremission chemotherapy on outcomes of AML in elderly patients.
Methods
Patients and donors
Patients with AML > 60 years in age and lacking a HLA-matched sibling from 2 hospitals were enrolled in this study from May 2004 to December 2009. Their diagnoses were defined by the French-American-British and World Health Organization criteria.2,4,19 Chromosomal and immunophenotyping analyses were performed with pretreatment bone marrow obtained from all patients at diagnosis, but the alterations of FLT3 and NPM1 genes were not analyzed because of insufficient patient data. Patients with acute promyelocytic leukemia and patients with a blast crisis of chronic myeloid leukemia were excluded, but patients with secondary AML (with a history of myelodysplasia syndrome) were also included. High risk was defined by the presence of complex cytogenetic abnormalities (with ≥ 3 cytogenetic abnormalities), del (7q, −5, −7), t (9,22), q(34,11.2), secondary AML, or a white blood cell count of ≥ 50 × 109/L (≥ 50 000/μL). Other patients were considered to be standard risk.2,4
Before transplantation, donor and recipient HLA-A, -B, -C, -DRB1, and -DQB1 alleles were typed by a polymerase chain reaction (PCR) with sequence-specific priming–based molecular method (Invitrogen). All patients had related donors who were HLA mismatched, including a HLA-C mismatch in the G-PBSC group. Donors who had more matched HLA loci and matched red blood cell (RBC) type were first chosen in this study. However, donor sex, age, and other characteristics were not considered with priority. Of the 30 patient/donor pairs, 21 were mismatched at 5/10 of HLA loci and 9 at 4/10. Median donor age was 35 years (range, 30-53 years), including 18 sons, 10 daughters, and 2 younger brothers.
The final protocols were approved by the Affiliated Hospital of Academy of Military Medical Sciences (Beijing, China) and the Second Artillery General Hospital (Beijing, China). In accordance with the Helsinki Declaration, written informed consent for enrollment in this study was obtained from all of the patients or their legal guardians and the donors. An independent physician explained the risks associated with peripheral mononuclear cell apheresis.
Treatment design
Induction therapy.
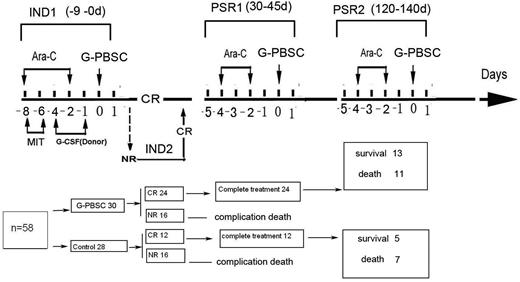
Eligible patients were randomly assigned to receive induction chemotherapy with mitoxantrone (8-10 mg/m2 intravenously for 3 days) and cytarabine (150 mg/m2 intravenously for 7 days) (control group; n = 28) or a combination of that treatment with HLA-mismatched G-PBSCs (G-PBSC group; n = 30). Three patients older than 80 years in the G-PBSC group received induction chemotherapy with a reduced dose of mitoxantrone (3 mg/m2) and cytarabine (50 mg/m2) for 3 and 5 days, respectively. HLA-mismatched G-PBSCs were administered 36 hours after the administration of cytarabine. Patients who experienced no remission or partial remission received a second course of the identical induction chemotherapy (Figure 1).
Protocol and results of HLA-mismatched G-PBSC infusion in combination with conventional chemotherapy for AML in elderly patients. Ara-C indicates cytarabine; MIT, mitoxantrone; CR, complete remission; IND1, first induction chemotherapy; IND2, second induction chemotherapy; PSR1, first postremission treatment; PSR2, second postremission treatment.
Protocol and results of HLA-mismatched G-PBSC infusion in combination with conventional chemotherapy for AML in elderly patients. Ara-C indicates cytarabine; MIT, mitoxantrone; CR, complete remission; IND1, first induction chemotherapy; IND2, second induction chemotherapy; PSR1, first postremission treatment; PSR2, second postremission treatment.
Postremission treatment.
Patients who achieved CR received 2 further courses of cytarabine postremission chemotherapy (0.5-1.0 g/m2 for 6 dosages in the control group) or the combination of that with G-PBSC therapy (G-PBSC group) at intervals of 3-3.5 months. Patients who relapsed during the treatment were considered therapy failure and received another identical reinduction chemotherapy on the basis of their group membership or stopped treatment. None of the patients received any GVHD prophylactic treatment or further maintenance therapy; however, G-CSF (Filgrastim; Kirin Corp) was given when absolute neutrophil count (ANC) was < 5 × 108/L.
Mobilization and apheresis of donor peripheral mononuclear cells
Apheresis of HLA-mismatched donor peripheral mononuclear cells was carried out with a CS-3000S cell separator (Baxter) after the donor was subcutaneously injected with 5 μg/kg G-CSF (Kirin Corp) twice a day for 5 days. Donor cells were divided into aliquots and were cryopreserved in liquid nitrogen, but freshly collected cells were used in the first course. Each infusion of the cells was performed at 36 hours after each chemotherapy regimen on day 0. The median numbers of mononuclear, CD34+, CD3+, CD3−CD16+CD56+ and CD3+CD16+CD56+ cells infused per course were 2.9 × 108/kg (range, 1.2-5.2 × 108/kg), 1.7 × 106/kg (range, 1.1-4.6)× 106/kg), 0.9 × 108/kg (range, 0.5-2.6 × 108/kg), 0.19 × 108/kg (range, 0.075-0.25 × 108/kg), and 0.13 × 108/kg (range, 0.05-0.45 × 108/kg), respectively.
Detection of donor chimerism and microchimerism
Hematopoietic donor chimerism was assessed in all patients in the G-PBSC group with the use of standard cytogenetic and semiquantitative PCR-based analysis of short tandem repeats with 1% sensitivity as previously described.12
Hematopoietic donor microchimerism (donor cells < 1%) was continuously monitored in all of the 4 female patients with available male donors by detection of the sex-determining region of the Y chromosome with a real-time quantitative PCR protocol with a sensitivity of 10−7 as previously described.20
Criteria for responses and evaluation of outcomes
Complete remission was defined as recovery of hematopoiesis, with ANC > 1.5 × 109/L, platelet count > 100 × 109/L, and normalization of marrow blasts (5%). Early death was defined as mortality within the first 4 weeks after induction chemotherapy. Relapse was defined as marrow infiltration of > 5% leukemia cells in previously normal bone marrow or evidence of extramedullary leukemia. Neutrophil recovery time was defined as the first of 3 consecutive days in which the ANC was > 0.5 × 109/L, whereas that of platelets was defined as the first of 3 consecutive days in which the platelet count was > 30 × 109/L as previously described.2,4,5,19
GVHD was defined according to published criteria.21 Disease-free survival (DFS) time was the time from CR to relapse or death. The OS time refers to the time from the diagnosis to either death or the last day of follow-up until March 2010.
Statistical analysis
SAS 9.0 software (SAS Institute) was used in all statistical analysis. Survival data were analyzed by the log-rank test, and survival curves were plotted with the Kaplan-Meier method. A t test or Wilcoxon test was used to assess the probability of significant differences in patient survival time. P < .05 was considered statistically significant.
Results
Demographics and characteristics of patients
The characteristics of the patients in the 2 groups are summarized in Table 1. Patient age, sex and subtype, stage and risk category of disease were compared between the 2 groups. The percentages of unfavorable karyotypes, secondary AML, and patients older than 70 years were 16.7%, 26.7% and 46.7%, respectively, in the G-PBSC group; these values were slightly higher than values in control group (14.3%, 21.4%, and 28.6%, respectively).
Patient characteristics
| . | Control group . | G-PBSCs group . | P . |
|---|---|---|---|
| Total no. of patients | 28 | 30 | |
| 60-70 y | 20 | 16 | |
| ≥ 71 y | 8 | 14 | .07 |
| Age, y, range (median) | 60-77 (65) | 60-88 (68) | |
| Sex | |||
| Female, n | 11 | 16 | |
| Male, n | 17 | 14 | .3 |
| WHO classification | |||
| AML with recurrent cytogenetic translocations, n | 1 | 0 | |
| AML with t(8;21)(q22;q22) AML1/CBFα/ETO, n | 1 | 0 | |
| AML with multilineage dysplasia, n | 6 | 8 | |
| With prior MDS, n | 2 | 3 | |
| Without prior MDS, n | 4 | 5 | .999 |
| AML with myelodysplastic syndrome, therapy related, n | 0 | 1 | |
| Other types, n | 0 | 1 | |
| AML not otherwise categorized, n | 21 | 21 | |
| AML minimally differentiated, n | 4 | 0 | |
| AML without maturation, n | 1 | 3 | |
| AML with maturation, n | 0 | 6 | |
| Acute myelomonocytic leukemia, n | 10 | 11 | |
| Acute monocytic leukemia, n | 5 | 1 | |
| Acute erythroid leukemia, n | 1 | 0 | |
| WBC count | |||
| < 50 000/L, n | 25 | 27 | |
| ≥ 50 000/L, n | 3 | 3 | .999 |
| High-risk category, n* | 13† | 12‡ | |
| Standard-risk category, n | 15 | 18 | .999 |
| . | Control group . | G-PBSCs group . | P . |
|---|---|---|---|
| Total no. of patients | 28 | 30 | |
| 60-70 y | 20 | 16 | |
| ≥ 71 y | 8 | 14 | .07 |
| Age, y, range (median) | 60-77 (65) | 60-88 (68) | |
| Sex | |||
| Female, n | 11 | 16 | |
| Male, n | 17 | 14 | .3 |
| WHO classification | |||
| AML with recurrent cytogenetic translocations, n | 1 | 0 | |
| AML with t(8;21)(q22;q22) AML1/CBFα/ETO, n | 1 | 0 | |
| AML with multilineage dysplasia, n | 6 | 8 | |
| With prior MDS, n | 2 | 3 | |
| Without prior MDS, n | 4 | 5 | .999 |
| AML with myelodysplastic syndrome, therapy related, n | 0 | 1 | |
| Other types, n | 0 | 1 | |
| AML not otherwise categorized, n | 21 | 21 | |
| AML minimally differentiated, n | 4 | 0 | |
| AML without maturation, n | 1 | 3 | |
| AML with maturation, n | 0 | 6 | |
| Acute myelomonocytic leukemia, n | 10 | 11 | |
| Acute monocytic leukemia, n | 5 | 1 | |
| Acute erythroid leukemia, n | 1 | 0 | |
| WBC count | |||
| < 50 000/L, n | 25 | 27 | |
| ≥ 50 000/L, n | 3 | 3 | .999 |
| High-risk category, n* | 13† | 12‡ | |
| Standard-risk category, n | 15 | 18 | .999 |
GDMI indicates [insert expansion]; WHO, World Health Organization; MDS, myelodysplastic syndrome; and WBC, white blood cell.
ETO gene expression.
Four patients exhibited complex cytogenetic abnormalities, including with ≥ 3 cytogenetic abnormalities (eg, del (7q, −5, −7), t (9,22), q(34,11.2)).
Five patients exhibited complex cytogenetic abnormalities.
Response to induction chemotherapy
The CR rate in the G-PBSC group was significantly higher than that in the control group (80.0% vs 42.8%; P = .006). The CR rate in the G-PBSC group was also higher than that in the control group after the first cycle of induction chemotherapy (63.3% vs 28.6%; P = .006). The CR rate of patients older than 70 years in the G-PBSC group was much higher than that in the control group (92.8% vs 12.5%; P = .0003), whereas the disease resistance rate in the G-PBSC group was significantly lower than that in the control group (10.0% vs 39.2%; P = .01; Table 1). The early death rates were 6.7% and 14.3% (P = .69) in the G-PBSC group and in the control group, respectively.
Hematopoietic recovery
The median recovery times for neutrophils and platelets were 11 days and 14.5 days, respectively, in the G-PBSC group and 16 days and 20 days, respectively, in the control group after the first cycle of induction chemotherapy (P = .02). A slight difference was also observed in the median recovery time for neutrophils and platelets between the 2 groups after postremission therapy (10 days and 14 days vs 12.5 days and 17 days, respectively; P = .06; Table 2). There were no differences in median neutrophil and platelet recovery times among patients of different ages or risk categories.
Outcome of therapy in elderly patients with AML
| . | Control group . | G-PBSC group . | P . |
|---|---|---|---|
| Complete remission rates | |||
| After the first induction, n/N | 8/28 | 19/30 | .006 |
| After the second induction, n/N | 12/28 | 24/30 | .006 |
| Patients >70 y, n/N | 1/8 | 13/14 | .0003 |
| Patients <70 y, n/N | 11/20 | 11/16 | .4 |
| In high-risk category, n/N | 4/13 | 7/12 | .23 |
| In standard-risk category, n/N | 8/15 | 17/18 | .01 |
| Disease resistance, n/N | 11/28 | 3/30 | .01 |
| Early death rate, n/N | 4/28 | 2/30 | .69 |
| Median time of ANC > 0.5 × 109/L, d | |||
| After the first induction | 16 | 11 | .02 |
| After the post-remission | 12.5 | 10 | .06 |
| Median time of platelet count > 30 × 109/L, d | |||
| After the first induction | 20 | 14.5 | .02 |
| After the post-remission | 17 | 14 | .06 |
| Severe infection | |||
| After the first induction, n/N | 16/28 | 8/30 | .02 |
| After the post-remission, n/N | 4/12 | 5/24 | .44 |
| . | Control group . | G-PBSC group . | P . |
|---|---|---|---|
| Complete remission rates | |||
| After the first induction, n/N | 8/28 | 19/30 | .006 |
| After the second induction, n/N | 12/28 | 24/30 | .006 |
| Patients >70 y, n/N | 1/8 | 13/14 | .0003 |
| Patients <70 y, n/N | 11/20 | 11/16 | .4 |
| In high-risk category, n/N | 4/13 | 7/12 | .23 |
| In standard-risk category, n/N | 8/15 | 17/18 | .01 |
| Disease resistance, n/N | 11/28 | 3/30 | .01 |
| Early death rate, n/N | 4/28 | 2/30 | .69 |
| Median time of ANC > 0.5 × 109/L, d | |||
| After the first induction | 16 | 11 | .02 |
| After the post-remission | 12.5 | 10 | .06 |
| Median time of platelet count > 30 × 109/L, d | |||
| After the first induction | 20 | 14.5 | .02 |
| After the post-remission | 17 | 14 | .06 |
| Severe infection | |||
| After the first induction, n/N | 16/28 | 8/30 | .02 |
| After the post-remission, n/N | 4/12 | 5/24 | .44 |
DFS and OS
Of the patients experiencing CR, 12 in the control group received treatment with cytarabine and 24 in the G-PBSC group received cytarabine with G-PBSCs as a postremission chemotherapy. Three patients older than 80 years received another 2 cycles of G-PBSC therapy without chemotherapy. The range of follow-up time was 7-62 months, and no patient was lost during the follow-up period. Of the 56 patients, 23 patients relapsed, of whom 11 gave up treatment and 12 received reinduction chemotherapy. Seven of the 12 patients died during therapy, and 5 (including 2 patients in the G-PBSC group and 3 in the control group) had a second CR. The probabilities of 2-year DFS and OS were 38.9% and 39.3%, respectively, in the G-PBSC group (Figure 1A). These probabilities were significantly higher (10.0% and 10.3%, respectively, P = .01 and P = .0006) than those in the control group (Figure 2). Exploratory analyses of subgroups showed that the patients who had donors with HLA-CLys80 (C2; n = 13) showed a much higher OS rate than those without C2 (n = 17) in the G-PBSC group (57.1% vs 12.5%; P = .01; Figure 1B); however, there was no significant difference in DFS and OS in the group with C1/C1(C1, HLA-CAsn 80) ligands (C1 epitope present on both HLA-C alleles; n = 15) and those without C1/C1 ligands (n = 15). In further analysis of DFS and OS between other subgroups (age older than 70 years and younger than 70 years, high-risk category and standard, HLA mismatched at 4/10 loci and at 5/10, respectively), no significant difference was observed (Figure 2).
G-PBSC infusion improves the probability of DFS and OS for elderly patients with AML. (A) The probabilities of 2-year DFS and OS were 38.9% and 39.3%, respectively, in the G-PBSC group. These rates are significantly higher than those in the control group (10.0% and 10.3%, respectively; P = .01 and P = .0006). (B) The patients whose donor associated with HLA-C2 ligands (n = 13) had significantly higher OS compared with donor having no C2 ligands (n = 17) in the G-PBSC group (57.1% vs 12.5%; P = .01).
G-PBSC infusion improves the probability of DFS and OS for elderly patients with AML. (A) The probabilities of 2-year DFS and OS were 38.9% and 39.3%, respectively, in the G-PBSC group. These rates are significantly higher than those in the control group (10.0% and 10.3%, respectively; P = .01 and P = .0006). (B) The patients whose donor associated with HLA-C2 ligands (n = 13) had significantly higher OS compared with donor having no C2 ligands (n = 17) in the G-PBSC group (57.1% vs 12.5%; P = .01).
GVHD and severe infection
No signs of acute or chronic GVHD, such as unexplained skin rashes or diarrhea, were observed in any of the patients during treatment. One patient developed a slight skin rash on the second day after the first G-PBSC therapy session, but this rash disappeared within 12 hours after an antiallergic therapy.
Severe infection rate was lower in the G-PBSC group than in the control group (26.7% [(8 of 30] vs 57.1% [16 of 28]) after the first cycle of induction chemotherapy (P = .03). No significant difference in severe infection rates was observed between the 2 groups (20.8% [5 of 24] vs 33.3% [4 of 12]) after postremission therapy (P = .44).
Donor chimerism and microchimerism
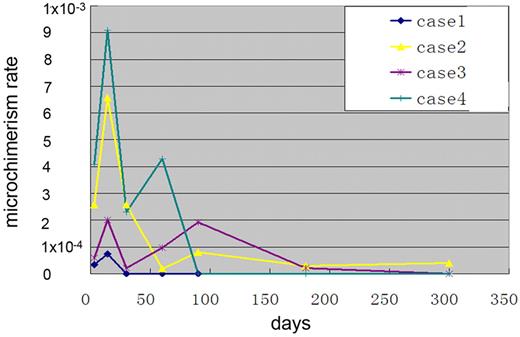
No full or mixed donor chimerism was found in the G-PBSC group, but donor microchimerism was successfully detected in all of the 4 female patients (range, 0.000033-0.0009 gene expression copies) compared with the internal control gene β-globin (1.0 copy). The kinetics of donor microchimerism showed that it emerged on day 2 and reached its first peak on days 7-14 after the first G-PBSC treatment. A second peak was observed after the second or third course of G-PBSC therapy, lasting 2 weeks to 10 months (Figure 3).
The kinetics of donor microchimerism was detected in all 4 female patients (range, 0.000033-0.0009 gene expression copies) compared with the internal control gene β-globin (1.0 copy). The kinetics of donor microchimerism showed that microchimerism emerged on day 2 and reached its first peak on days 7-14 after the first G-PBSC treatment and its second peak after the second or third course of G-PBSC therapy, lasting 2 weeks to 10 months.
The kinetics of donor microchimerism was detected in all 4 female patients (range, 0.000033-0.0009 gene expression copies) compared with the internal control gene β-globin (1.0 copy). The kinetics of donor microchimerism showed that microchimerism emerged on day 2 and reached its first peak on days 7-14 after the first G-PBSC treatment and its second peak after the second or third course of G-PBSC therapy, lasting 2 weeks to 10 months.
Discussion
In the present study, HLA-mismatched G-PBSC infusion in combination with conventional chemotherapy achieved a higher CR rate (80.0%) and lower disease-resistant rate (10%) than conventional chemotherapy alone (42.8% and 39.2%) for AML in elderly patients (P = .006 and P = .01, respectively). Furthermore, the CR rate after the first cycle of induction chemotherapy was also higher, which combined with a short duration of pancytopenia and lower severe infection rate. The increased CR rate was also independent of age, which was particularly apparent in patients older than 70 years. More importantly, the 2-year DFS and OS in the G-PBSC group were longer than those in the control group (38.9% and 39.3% vs 10.0% and 10.3%, respectively; P = .01 and P = .006), suggesting that G-PBSC therapy improved the outcome of AML in elderly patients.
A first plausible explanation for these findings is that G-PBSC therapy can significantly speed hematopoietic recovery after chemotherapy, thereby reducing the induction chemotherapy-related rates morbidity and mortality. In this study, the median recovery times for neutrophils and platelets were only 11 days and 14.5 days after the induction chemotherapy in the G-PBSC group. The duration of neutropenia and thrombopenia were shorter by 5 and 5.5 days, respectively, than those in the control group. As a result, severe infections during the hypoplastic phase were also reduced. This finding is inconsistent with the reported data,8,9 indicating that G-CSF therapy can shorten the duration of neutropenia. As a result, it can also prevent infectious complications after chemotherapy, which, as an adjuvant standard or intensive chemotherapy, is particularly important for elderly patients. We speculate that, in contrast to unstimulated DLI, the larger number of lymphocytes, CD34+ cells, natural killer (NK) cells, as well as cytokines, contained in G-PBSCs22 may contribute roles in promoting hematopoietic recovery, although only donor microchimerism was observed after the G-PBSC treatment. Other potential mechanism may be related to the joint action of G-PBSCs and G-CSF on hematopoietic recovery.
Another explanation for these positive findings is the enhanced antileukemia effects mediated by the alloreactive G-PBSCs infused. Antileukemic alloactivities in DLI and donor stems infusion (DSI) has been well demonstrated.23-27 Differently, in this study, many more NK cells (0.075-0.25 × 108/kg) were infused, and all of patient-donor pairs were HLA-C mismatched. We observed that the patients with C2 donors had a significantly higher OS than patients without (P = .01). This result suggests that NK cells contributed to the antileukemic effects and improved clinical outcome, although the killer cell immunoglobulin-like receptors of NK cells in the patients and donors were not examined. However, a large number of HLA haploidentical donor T cells (≤ 8.4 × 108/kg) and other kinds of immune cells infused may strengthen the antileukemic effects through interaction with the recipient immune system.25,26,28
It is well known that even the GVL effects induced by DLI generally depend on full or mixed donor engraftment. In the absence of engraftment, the infused donor cells will be immediately rejected before a graft-versus-tumor (GVT) response is observed.25,26 In this study, however, no full or mixed donor chimerism was observed in any patient. In contrast, persistent donor microchimerism was observed in all of the 4 available female patients. This finding implies that at least a small portion of the donor cells infused remains as a microchimerism in the recipients; this microchimerism may play a role in inducing antileukemia effects. Another important fact in this study was that no acute or chronic GVHD was observed in any of the patients during the entire treatment period, even though a large amount of nonirradiated donor CD3+ cells (≤ 8.4 × 108/kg) was infused and no GVHD prophylaxis was used. Previous reports of RIC haploidentical transplantation have used immunosuppressive and partially myelosuppressive conditioning to achieve full or mixed donor chimerism, but these procedures have induced GVL effects and were associated with significant GVHD.14,15,29 Other reports have used DLI after alloSCT, which induces stronger GVL effects, but is also associated with severe GVHD.16-18 In contrast, our results showed that combining programmed G-PBSC infusion with cytarabine or mitoxantrone or both chemotherapy without immunosuppressive treatment formed donor microchimerism or microtransplantation, mediated specific antileukemic effects, prevented GVHD, and improved patient outcomes. Although the exact mechanism of the prevention of GVHD in this study is still unknown, our findings of donor microchimerism and the condition without immunosuppression before G-PBSC therapy can explain some of it.30,31 Importantly, the lack of prior immunosuppressive treatment reserved immune function in the recipients which may be critical for preventing GVHD. Acute GVHD has been reported to occur in only patients undergoing autologous stem cell transplantation or TBI alone before DLI.25 This finding indicates that other regimens, including drugs such as fludarabine and TBI that possesses stronger immunosuppressive effects, should be used with caution in such an approach. 32,33
In conclusion, the combination of HLA-mismatched G-PBSC infusion with conventional chemotherapy can result in a higher CR rate, a longer survival time, and a shorter duration of pancytopenia than conventional chemotherapy alone. This method also offers a much safer and much more effective anti-AML regimen for elderly patients. Further investigation will focus on the modification of G-PBSC therapy to enhance its antileukemic effects and the mechanism responsible for the avoidance of GVHD.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Bob Löwenberg (Erasmus University Medical Center, Netherlands) and Dr Mingyao Liu (Faculty of Medicine, University of Toronto, ON) for critically reviewing the manuscript.
This work was supported by grants from the “863 Projects” of Ministry of Science and Technology of PR China (no. 2006AA02A109), National Basic Research Program of China (2011CB964803 and 2010CB529404), and National Natural Science Foundation of China (30672387 and 30800277).
The funding agencies/sponsors had no role in data collection, analysis, manuscript preparation, or authorization for publication.
Authorship
Contribution: H.-S.A. provide the study concept and design; M.G., K.-X.H., C.-L.Y., Q.-Y.S., J.-H.Q., D.-H.W., W.-J.S., L.W., X.-D.S., Y.-J.H., J.-X.Q., and Z.-D. acquired data; H.-S.A., K.-X.H., G.-X.L., L.W., X.-D.S., and Z.-D. analyzed and interpreted data; H.-S.A., K.-X.H., and G.-X.L. drafted the manuscript; H.-S.A., K.-X.H., and G.-X.L. critically revised the manucript; K.-X.H. provided statistical expertise; H.-S.A. obtained funding, and as principal investigator, had full access to all the data in this study and takes responsibility for integrity of the data and accuracy of data analysis; and L.W. and X.-D.S. provided administrative, technical, or material support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hui-Sheng Ai, Department of Hematology and Transplantation, Affiliated Hospital of the Academy of Military Medical Sciences, 8 Dongdajie, Beijing 100071, China; e-mail: huishengai@163.com


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal