Abstract
Acute lung injury (ALI) during hematopoietic stem cell transplant (HSCT) is associated with substantial morbidity; however, the frequency of ALI in HSCT patients is poorly characterized. Platelets are postulated to play a critical role in the pathogenesis of ALI. Using a transfusion trial of pathogen inactivated platelet components (PC-Test) compared with conventional PC (Reference) populated with HSCT patients, data were reviewed by an adjudication panel to determine the frequency of ALI overall, by treatment groups, and key outcomes: PC exposure, ventilator-free days, and mortality. The diagnosis of ALI was based on American European Consensus Criteria. Of 645 patients who received PC over 28 days, 100 (15.5%) had clinically serious pulmonary adverse events, and 35 (5.4%) met criteria for ALI. Days of platelet support and number of platelet transfusions for patients with ALI were not significantly different from patients without ALI (P > .05). Mortality was greater for patients with ALI (57%) than those without (17%, P < .001) but not significantly different between treatment groups. For patients with ALI, the distributions of time to onset of mechanical ventilation were significantly different (P = .04). Patients supported with Reference PC were more likely to be ventilated sooner than patients receiving Test PC.
Introduction
Acute lung injury (ALI) is a syndrome of respiratory failure characterized by high morbidity and mortality1-3 and is a significant cause of morbidity in recipients of hematopoetic stem cell transplants (HSCTs).4 In this patient population, ALI may arise from either infectious or noninfectious causes.4 In addition to ALI, patients undergoing HSCT who develop other forms of respiratory failure that require mechanical ventilation may experience mortality ranging from 85%-90%.5 The true prevalence of ALI may be underestimated in this population as many studies have not used specific criteria to diagnose ALI.6,7 Correct diagnosis of ALI is important for delivery of optimal respiratory therapy.8
Blood transfusion is recognized as a risk factor for ALI.1,2 Specifically, transfusion of platelet and plasma components to critically ill medical patients is associated with development of ALI.9 Importantly, transfusion of platelet and plasma components has been associated with increased mortality independent of the severity of hypoxemia (PaO2/FiO2) or other risk factors such as multisystem organ failure or disseminated intravascular coagulation.10 However, platelet transfusion remains a critical supportive therapy for HSCT patients, who may be at risk for ALI. Paradoxically, animal studies have suggested that P-selectin–mediated platelet-neutrophil-endothelial cell interactions may play a critical role in the pathology of ALI.11
Previously, we conducted a prospective, randomized, controlled, double-blinded clinical trial to evaluate the therapeutic efficacy and safety of platelet components (PC) prepared with pathogen inactivation treatment in a population predominantly composed of patients undergoing HSCT.12 During the course of this study 5 of 645 (0.8%) patients were diagnosed by the clinical investigators with acute respiratory distress syndrome (ARDS), a subset of ALI. The unexpectedly low incidence of ALI in this study population compared with the reported incidence of related syndromes, such as idiopathic pneumonia,4 suggested that ALI had been under-recognized during the course of the trial. After completion of this trial, we performed an independent retrospective blinded review of the primary medical records of patients reported to have clinically serious pulmonary adverse events (CSPAEs) during this trial to identify more precisely patients with ALI and the frequency by treatment group. For the post-study review, a prespecified protocol with defined criteria for diagnosis of ALI was used to (1) define the frequency and outcome of ALI in this population and (2) characterize the potential relationship of ALI to platelet transfusion in a population of HSCT patients intensively supported with PCs.
Methods
Study design
The SPRINT study (Protocol P3A99) was a multicenter, randomized, double-blinded controlled trial in which the hemostatic efficacy of PCs treated with photochemical pathogen inactivation (PCT-PCs) transfused to patients (Test, N = 318) was compared with that of conventional platelet components (C-PCs) transfused to patients (Reference, N = 327).12 The trial was approved by the institutional review boards of all participating institutions. During the clinical trial, thrombocytopenic patients, 78% of whom had received HSCT, were transfused with study platelet products (PCT-PC or C-PC) for a period of up to 28 days (active study period) with surveillance for adverse events (AEs). After completion of study platelet transfusions, each subject was followed to evaluate AEs for an additional 7 days to a maximum of 35 days.
In the SPRINT study, AEs were reported by investigators, blinded to treatment assignment, using the National Cancer Institute Common Toxicity Criteria with 4 severity grades. AE reports were based on daily review of primary medical records by study research nurses trained to the study protocol and blinded to treatment assignment. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) terminology (version 3.3) to Preferred Term (PT) and mapped to the primary and nonprimary respiratory, thoracic, and mediastinal disorders System Organ Class (SOC), and the frequencies of the respective AEs were calculated (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).13 No PT for ALI was available in version 3.3 of MedDRA. During the conduct of the SPRINT trial, investigators were not provided with specific per-protocol criteria for the diagnosis of ALI or ARDS.14
The frequencies of AEs at the PT level and summarized by SOC were determined as part of the analysis of safety.13 Fisher exact test was used to test for treatment differences for categorical data with 2-sided P values with a 5% significance level.12,13 No statistically significant differences were observed between Test and Reference groups for grade 3 and 4 AEs overall and in the MedDRA respiratory, thoracic, and mediastinal disorders SOC. However, statistically significant differences between groups were observed for 3 low-frequency AEs at the PT level: ARDS (5/318 [1.6%] Test vs 0/327 Reference, P = .029), pneumonia not otherwise specified (7/318 [2.2%] Test vs 0 Reference, P = .007), and pleuritic pain (12/318 [3.8%] Test vs 3/327 [0.9%] Reference, P = .018).13 As a result of the unexpectedly low frequency of ARDS, and the lack of a MedDRA coding term for ALI in version 3.3, a retrospective review and analysis of patients with potential CSPAEs using specific diagnostic criteria for ALI, including the subset of ARDS, was conducted to determine more precisely the frequency of ALI in the P3A99 trial.
Methods to determine the frequency of acute lung injury in the post-trial review study
A study protocol was developed to use an independent Expert Physician Panel (EPP) for assessment of the clinical course of all subjects who may have experienced potentially CSPAEs during the P3A99 study. The objective of this post-clinical trial review was to determine more precisely the frequency of treatment-emergent ALI using established diagnostic criteria (Table 1).14 Adverse events for all 645 subjects in the P3A99 trial were reviewed by the EPP and used to identify subjects with potential CSPAEs. Subjects identified by the EPP with a potential CSPAEs in the P3A99 study (either worsening of a pre-existing event or a treatment-emergent event) were selected for secondary review and on the basis of this review further categorized by the EPP using a hierarchy of pulmonary disease categories (supplemental Table 1) and assessed for ALI using American European Consensus Criteria (AECC) criteria. The hierarchy was based on the anatomy of the respiratory system and the disease categories representing pulmonary conditions or diagnoses associated with and/or relevant to the evaluation of pulmonary conditions that can precede ALI. For the post-trial review, the study period for AE collection was extended up to 49 days (28 days of study platelet transfusions, plus 7 days of additional AE surveillance after the final study transfusion, plus an extra 14 days for ongoing serious pulmonary AEs). The AE surveillance period was extended for the review analysis to specifically capture any delayed pulmonary AEs, including delayed onset of ALI. A detailed description of the methods used to manage the study is provided in the data supplement.
AECC diagnostic criteria for ALI and ARDS
| ALI |
|
| ARDS |
|
| ALI |
|
| ARDS |
|
PAOP indicates pulmonary artery occlusion pressure; and PCWP, pulmonary capillary wedge pressure.
Statistical analyses
All statistical analyses were performed after a prospectively developed statistical analysis plan. The primary analysis was a calculation of the frequency of clinically serious pulmonary AEs as determined by the EPP in Test and Reference subjects from study P3A99, with a comparison of group event rates. The denominator for the primary analysis was 645 (318 Test and 327 Reference subjects). Based on rigorous collection of all AEs, and specific collection of respiratory AEs after each study transfusion in the P3A99 trial, the EPP used pulmonary AEs with severity of grade 2 and greater as the threshold to identify patients with clinically serious pulmonary AEs. All subjects determined by the EPP to have a potential clinically serious pulmonary event were further categorized in 1 or more respiratory anatomical and disease categories, as well as infection-related or antineoplastic therapy-related event categories. The frequencies of these categories were also calculated and compared between groups.
The secondary analyses included a calculation of the frequency of diagnoses by AECC criteria of ALI, including the subset of ARDS, as determined by the EPP. The denominator for these analyses was 645. The inclusion of all study subjects in the analysis was supported by the use of comprehensive AE reporting by the initial study investigators, including specific per-protocol reporting of respiratory signs and symptoms after each study platelet transfusion. With use of these protocol assessments, all subjects with bilateral pulmonary infiltrates, a PaO2/FiO2 ratio ≤ 300, or evidence of pulmonary edema were captured in the AE listing used by the EPP to select patients for review. No study subjects with potential pulmonary events above grade 1 were excluded for review. In addition, pulmonary, infectious, and cardiovascular AEs in the subjects selected for review from the P3A99 study, as reported in subject medical records, were coded using MedDRA version 6.0. These AEs were listed, by verbatim term, MedDRA lower level term, PT, high level term, high level group term, and SOC, by primary axis classification (supplemental Figure 1). For each AE for which the primary or nonprimary SOC was the respiratory, thoracic, and mediastinal disorders SOC, the number of subjects who experienced at least 1 AE was counted and tabulated per treatment group and overall. For each PT, the number of subjects who experienced the AE at least once during the active study period was counted and tabulated per treatment group and overall. Frequencies were calculated by PT and by primary and nonprimary respiratory, thoracic, and mediastinal disorders SOC and were compared between Test and Reference groups. Frequencies were also calculated and compared between Test and Reference groups for all pulmonary events classified as pulmonary infiltrative disorders. The denominator for these analyses was based on all subjects selected by the EPP for review, because it could not be assumed that all nonreviewed subjects did not experience any primary or nonprimary AE in the respiratory, thoracic, and mediastinal disorders SOC.
The treatment differences for the occurrence of categorical outcomes of interest were statistically evaluated via Fisher exact test. The treatment differences for count variables, including duration of platelet support in days and total platelet transfusions, were statistically evaluated via Wilcoxon rank sum tests. Nonparametric statistical testing methods were chosen for count variables due to the right-skewed nature of the distributions. Statistical significance was defined as a P value less than .05.
Results
Patient demographics
Among 645 patients in the P3A99 study, 148 subjects (78 Test, 70 Reference) were selected for review by the EPP based on the criteria for potential CSPAEs using PT (supplemental Figure 1). Each of the 12 study sites contributed patients to this review. The proportion of P3A99 study subjects who were selected by the EPP for review was comparable site-to-site (approximately 22%) except for site (810), which contributed 78% of the site's P3A99 subjects to the review, and 2 sites (808 and 812), which each contributed 5% and 7%, respectively, of the site's P3A99 subjects for review. These differences were due to the high frequency of allogeneic HSCTs at site 810 and low frequency at sites 808 and 812. Among the 148 patients reviewed, the EPP determined that 100 (55 Test, 45 Reference) had confirmed CSPAEs.
The mean age of subjects (49.5 years) and sex of subjects (55% male) selected for review were comparable between treatment groups and similar to the entire population.12 The proportions of patients by primary diagnosis were similar between the treatment groups (Table 2). The pulmonary, hepatic, infectious, and cardiovascular medical histories for baseline clinical conditions of study subjects were similar between Test and Reference groups by MedDRA primary SOC. There was a high prevalence of prior respiratory events at study entry, with 65% of subjects having at least 1 event classified in the respiratory, thoracic, and mediastinal disorders SOC, but without significant differences between treatment groups (supplemental Table 2). Events involving the vascular disorders SOC (45.3%) and the infections and infestations SOC (41.2%) were also common at baseline. There were no significant differences between treatment groups for events in these SOC at baseline.
Primary diagnosis for patients with potential clinically serious pulmonary adverse events
| . | Treatment group . | Total, N (%) . | |
|---|---|---|---|
| Test, N (%) . | Reference, N (%) . | ||
| Total patients | 78 (100) | 70 (100) | 148 (100) |
| Hematologic malignancy | 74 (94.9) | 64 (91.4) | 138 (93.2) |
| Acute leukemia | 31 (39.7) | 25 (35.7) | 56 (37.8) |
| ALL | 6 (7.7) | 5 (7.1) | 11 (7.4) |
| ANLL/AML | 25 (32.1) | 20 (28.6) | 45 (30.4) |
| Chronic leukemia/HCL | 11 (14.1) | 11 (15.7) | 22 (14.9) |
| CML | 7 (9.0) | 7 (10.0) | 14 (9.5) |
| CLL | 3 (3.8) | 3 (4.3) | 6 (4.1) |
| CMML | 1 (1.3) | 1 (1.4) | 2 (1.4) |
| HCL | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 12 (15.4) | 20 (28.6) | 32 (21.6) |
| NonHodgkin | 12 (15.4) | 20 (28.6) | 32 (21.6) |
| Hodgkin | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myelodysplasia | 5 (6.4) | 1 (1.4) | 6 (4.1) |
| Plasma cell dyscrasia | 15 (19.2) | 7 (10.0) | 22 (14.9) |
| Myeloma | 15 (19.2) | 6 (8.6) | 21 (14.2) |
| Other | 0 (0.0) | 1 (1.4) | 1 (0.7) |
| Solid tumor | 2 (2.6) | 3 (4.3) | 5 (3.4) |
| Carcinoma | 2 (2.6) | 3 (4.3) | 5 (3.4) |
| Sarcoma | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other diseases* | 2 (2.6) | 3 (4.3) | 5 (3.4) |
| . | Treatment group . | Total, N (%) . | |
|---|---|---|---|
| Test, N (%) . | Reference, N (%) . | ||
| Total patients | 78 (100) | 70 (100) | 148 (100) |
| Hematologic malignancy | 74 (94.9) | 64 (91.4) | 138 (93.2) |
| Acute leukemia | 31 (39.7) | 25 (35.7) | 56 (37.8) |
| ALL | 6 (7.7) | 5 (7.1) | 11 (7.4) |
| ANLL/AML | 25 (32.1) | 20 (28.6) | 45 (30.4) |
| Chronic leukemia/HCL | 11 (14.1) | 11 (15.7) | 22 (14.9) |
| CML | 7 (9.0) | 7 (10.0) | 14 (9.5) |
| CLL | 3 (3.8) | 3 (4.3) | 6 (4.1) |
| CMML | 1 (1.3) | 1 (1.4) | 2 (1.4) |
| HCL | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 12 (15.4) | 20 (28.6) | 32 (21.6) |
| NonHodgkin | 12 (15.4) | 20 (28.6) | 32 (21.6) |
| Hodgkin | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myelodysplasia | 5 (6.4) | 1 (1.4) | 6 (4.1) |
| Plasma cell dyscrasia | 15 (19.2) | 7 (10.0) | 22 (14.9) |
| Myeloma | 15 (19.2) | 6 (8.6) | 21 (14.2) |
| Other | 0 (0.0) | 1 (1.4) | 1 (0.7) |
| Solid tumor | 2 (2.6) | 3 (4.3) | 5 (3.4) |
| Carcinoma | 2 (2.6) | 3 (4.3) | 5 (3.4) |
| Sarcoma | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other diseases* | 2 (2.6) | 3 (4.3) | 5 (3.4) |
ALL indicates acute lymphoblastic leukemia; ANLL, acute nonlymphocytic leukemia; AML, acute myeloid leukemia; HCL, hairy cell leukemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; and CMML, chronic myelomonocytic leukemia.
Other diseases included 5 patients with nonmalignant disorders undergoing stem cell transplantation.
Because of the potential pulmonary toxicity of chemotherapy and radiation therapy, particularly total body irradiation pre-HSCT, data were collected on chemotherapy and radiation therapy given up to 1 year before study entry. Test and Reference subjects with confirmed CSPAEs appeared balanced in terms of exposure to pulmonary toxic medications with the exception of cyclophosphamide (supplemental Table 3). This difference was not significant for patients with and without confirmed ALI (supplemental Table 4). The proportion of patients treated with total body irradiation immediately before or during the study was similar between treatment groups (supplemental Table 5).
The majority (77%) of study subjects selected for review received 1 or more HSCTs at some time during the active phase of the study. This was similar to the frequency of HSCTs in the entire population of the P3A99 study.12 The proportion of Test patients treated with allogeneic HSCT was lower than Reference (56.7% vs 68.5%). The mean duration of the active study period was slightly longer for the Test group: 24 days compared with 22 days for the Reference group.
Frequency of CSPAEs and ALI
Of 148 patients with potential CSPAEs selected for review by the EPP from the total study population (N = 645), 100 (15.5%) were determined to have had 1 or more treatment-emergent clinically serious pulmonary AE (Table 3). There was no statistically significant difference between Test and Reference groups overall in the frequency of these events (17.3% Test vs 13.8% Reference, P = .232). All of these subjects had at least 1 event involving the lung parenchyma. Clinically serious events involving the tracheal bronchial tree (0.9%) or pleural space (5.9%) were less common and occurred with similar frequency between treatment groups (Table 3). The EPP determinations of treatment-emergent pneumonitis from the hierarchy of pulmonary disease categories demonstrated no statistically significant differences for any diagnosis.
Confirmed treatment-emergent clinically serious pulmonary events, as determined by the EPP
| . | Treatment group . | Total, N = 645 (%) . | P* . | |
|---|---|---|---|---|
| Test, N = 318 (%) . | Reference, N = 327 (%) . | |||
| Any subject with a clinically serious pulmonary AE | 55 (17.3) | 45 (13.8) | 100 (15.5) | .232 |
| Tracheal bronchial tree | 4 (1.3) | 2 (0.6) | 6 (0.9) | .445 |
| Bronchospasm | 3 (0.9) | 2 (0.6) | 5 (0.8) | .682 |
| Hemorrhage | 1 (0.3) | 0 (0.0) | 1 (0.2) | .493 |
| Bronchitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Lung parenchyma | 55 (17.3) | 45 (13.8) | 100 (15.5) | .232 |
| Pneumonitis any | 39 (12.3) | 32 (9.8) | 71 (11.0) | .379 |
| Pneumonitis without ALI or ARDS | 20 (6.3) | 16 (4.9) | 36 (5.6) | .495 |
| Pneumonitis with ALI or ARDS | 19 (6.0) | 16 (4.9) | 35 (5.4) | .604 |
| Pneumonitis with ALI only | 7 (2.2) | 11 (3.4) | 18 (2.8) | .475 |
| Pneumonitis with ARDS only | 12 (3.8) | 5 (1.5) | 17 (2.6) | .088 |
| Atelectasis/other | 16 (5.0) | 13 (4.0) | 29 (4.5) | .572 |
| LAH with infiltrates | 16 (5.0) | 12 (3.7) | 28 (4.3) | .443 |
| Pleural space | 18 (5.7) | 20 (6.1) | 38 (5.9) | .868 |
| Pleuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Pleural effusion | 18 (5.7) | 20 (6.1) | 38 (5.9) | .868 |
| Pnemothorax | 0 (0.0) | 1 (0.3) | 1 (0.2) | 1.000 |
| . | Treatment group . | Total, N = 645 (%) . | P* . | |
|---|---|---|---|---|
| Test, N = 318 (%) . | Reference, N = 327 (%) . | |||
| Any subject with a clinically serious pulmonary AE | 55 (17.3) | 45 (13.8) | 100 (15.5) | .232 |
| Tracheal bronchial tree | 4 (1.3) | 2 (0.6) | 6 (0.9) | .445 |
| Bronchospasm | 3 (0.9) | 2 (0.6) | 5 (0.8) | .682 |
| Hemorrhage | 1 (0.3) | 0 (0.0) | 1 (0.2) | .493 |
| Bronchitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Lung parenchyma | 55 (17.3) | 45 (13.8) | 100 (15.5) | .232 |
| Pneumonitis any | 39 (12.3) | 32 (9.8) | 71 (11.0) | .379 |
| Pneumonitis without ALI or ARDS | 20 (6.3) | 16 (4.9) | 36 (5.6) | .495 |
| Pneumonitis with ALI or ARDS | 19 (6.0) | 16 (4.9) | 35 (5.4) | .604 |
| Pneumonitis with ALI only | 7 (2.2) | 11 (3.4) | 18 (2.8) | .475 |
| Pneumonitis with ARDS only | 12 (3.8) | 5 (1.5) | 17 (2.6) | .088 |
| Atelectasis/other | 16 (5.0) | 13 (4.0) | 29 (4.5) | .572 |
| LAH with infiltrates | 16 (5.0) | 12 (3.7) | 28 (4.3) | .443 |
| Pleural space | 18 (5.7) | 20 (6.1) | 38 (5.9) | .868 |
| Pleuritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Pleural effusion | 18 (5.7) | 20 (6.1) | 38 (5.9) | .868 |
| Pnemothorax | 0 (0.0) | 1 (0.3) | 1 (0.2) | 1.000 |
NA indicates not applicable; LAH, Left Atrial Hypertension.
Based on Fisher exact test.
The frequency of ALI, including the subset of ARDS, was 6.0% (19/318) for the Test group compared with 4.9% (16/327) for the Reference group (P = .60). The frequency of ARDS was 3.8% (12/318) for Test and 1.5% (5/327) for Reference (P = .09), and for the subset with ALI without criteria for ARDS, the frequency was 2.2% (7/318) for Test and 3.4% (11/327) for Reference (P = .48). The diagnosis of ARDS by the EPP was based upon documentation of PaO2/FiO2 ratios ≤ 200. Among the 17 subjects with ARDS, all 17 were on mechanical ventilation, and the PaO2/FiO2 ratio was available for all 17 subjects. Among the 18 subjects with ALI, without criteria for ARDS determined by the EPP, PaO2/FiO2 ratios were available for only 4 subjects. For the remaining 14 subjects, the EPP relied on oxygen saturation values for 11 subjects, and 3 subjects had concurrent oxygen saturation and PaO2 measurements. However, the PaO2/FiO2 ratio could not be accurately calculated because only face mask FiO2 was available for these subjects. In the absence of actual PaO2/FiO2 ratios, the EPP categorized these subjects as meeting the criteria for ALI, though they may have met the criteria for ARDS if the information for calculation of PaO2/FiO2 ratios had been in the medical record. No patient had documented transfusion-related acute lung injury (TRALI) with confirmed cognate antigen-antibody mismatch.
Exposure to blood components in patients with CSPAEs and ALI
The exposure to PCs was greater in patients with CSPAEs (N = 100) than in patients without CSPAEs (N = 545) for each treatment group (Table 4). Among patients without CSPAEs, Test patients received more PCs (Table 4). This was also the case for exposure to red blood cell (RBC) components. Among the 99 of 100 patients with CSPAEs who received RBC, there was no significant difference in the distribution of RBC units transfused between the treatment groups. Transfusion of plasma components was infrequent, and only 23 of 100 patients with CSPAEs received plasma. Comparison of the extent of platelet or RBC transfusion exposure between patients with criteria for the diagnosis of ALI (N = 35) and those without criteria for the diagnosis of ALI (N = 65) did not demonstrate a significant difference. Moreover, the extent of platelet and RBC transfusion among patients with or without treatment-emergent ALI was not significantly different between treatment groups (Table 4). The mean duration of platelet support and exposure to platelet transfusion was not different between treatment groups for patients with ALI (Table 5). Test patients with the diagnostic criteria for ARDS had a longer period of platelet support and more platelet transfusions compared with Reference patients (Table 5), but this difference was not significant due to the small number of patients with ARDS. The mean age of transfused PC was similar across all levels of confirmed pulmonary injury, and for those patients without CSPAEs, ranging from 3.3 to 3.7 days.
Blood product usage in patients without and with clinically serious pulmonary adverse events (CSPAE) and without and with acute lung injury
| Platelet components* . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 7.3 (7.59) | 5.0 | 1.0, 46.0 | .0002 |
| Reference | 282 | 5.3 (6.26) | 3.0 | 1.0, 58.0 | ||
| CSPAE | Test | 55 | 17.8 (13.35) | 16.0 | 1.0, 55.0 | .3492 |
| Reference | 45 | 15.2 (12.44) | 11.0 | 1.0, 64.0 | ||
| No ALI | Test | 36 | 18.7 (13.92) | 16.0 | 1.0, 55.0 | .1595 |
| Reference | 29 | 14.6 (13.94) | 9.0 | 1.0, 64.0 | ||
| ALI | Test | 19 | 16.1 (12.39) | 12.0 | 2.0, 39.0 | .6073 |
| Reference | 16 | 16.3 (9.45) | 14.0 | 4.0, 36.0 | ||
| Platelet components* . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 7.3 (7.59) | 5.0 | 1.0, 46.0 | .0002 |
| Reference | 282 | 5.3 (6.26) | 3.0 | 1.0, 58.0 | ||
| CSPAE | Test | 55 | 17.8 (13.35) | 16.0 | 1.0, 55.0 | .3492 |
| Reference | 45 | 15.2 (12.44) | 11.0 | 1.0, 64.0 | ||
| No ALI | Test | 36 | 18.7 (13.92) | 16.0 | 1.0, 55.0 | .1595 |
| Reference | 29 | 14.6 (13.94) | 9.0 | 1.0, 64.0 | ||
| ALI | Test | 19 | 16.1 (12.39) | 12.0 | 2.0, 39.0 | .6073 |
| Reference | 16 | 16.3 (9.45) | 14.0 | 4.0, 36.0 | ||
| Red cell components . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 4.7 (4.13) | 4.0 | 0.0, 27.0 | .0140 |
| Reference | 282 | 4.0 (3.64) | 4.0 | 0.0, 34.0 | ||
| CSPAE | Test | 55 | 9.0 (5.07) | 8.0 | 0.0, 20.0 | .5848 |
| Reference | 45 | 10.4 (6.89) | 8.0 | 2.0, 31.0 | ||
| No ALI | Test | 36 | 8.8 (4.76) | 8.0 | 0.0, 20.0 | .9100 |
| Reference | 29 | 10.2 (7.59) | 8.0 | 2.0, 31.0 | ||
| ALI | Test | 19 | 9.4 (5.74) | 8.0 | 2.0, 20.0 | .5501 |
| Reference | 16 | 10.7 (5.61) | 9.5 | 3.0, 21.0 | ||
| Red cell components . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 4.7 (4.13) | 4.0 | 0.0, 27.0 | .0140 |
| Reference | 282 | 4.0 (3.64) | 4.0 | 0.0, 34.0 | ||
| CSPAE | Test | 55 | 9.0 (5.07) | 8.0 | 0.0, 20.0 | .5848 |
| Reference | 45 | 10.4 (6.89) | 8.0 | 2.0, 31.0 | ||
| No ALI | Test | 36 | 8.8 (4.76) | 8.0 | 0.0, 20.0 | .9100 |
| Reference | 29 | 10.2 (7.59) | 8.0 | 2.0, 31.0 | ||
| ALI | Test | 19 | 9.4 (5.74) | 8.0 | 2.0, 20.0 | .5501 |
| Reference | 16 | 10.7 (5.61) | 9.5 | 3.0, 21.0 | ||
| Plasma components . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 0.1 (0.57) | 0.0 | 0.0, 6.0 | .4851 |
| Reference | 282 | 0.1 (0.66) | 0.0 | 0.0, 8.0 | ||
| CSPAE | Test | 55 | 1.2 (3.15) | 0.0 | 0.0, 16.0 | .0871 |
| Reference | 45 | 4.8 (14.64) | 0.0 | 0.0, 72.0 | ||
| No ALI | Test | 36 | 0.3 (1.37) | 0.0 | 0.0, 8.0 | .0321 |
| Reference | 29 | 1.5 (3.49) | 0.0 | 0.0, 13.0 | ||
| ALI | Test | 19 | 2.9 (4.63) | 0.0 | 0.0, 16.0 | .6669 |
| Reference | 16 | 10.8 (23.39) | 0.0 | 0.0, 72.0 | ||
| Plasma components . | ||||||
|---|---|---|---|---|---|---|
| Group . | Treatment . | N . | Mean (SD) . | Median . | Min, Max . | P† . |
| No CSPAE | Test | 263 | 0.1 (0.57) | 0.0 | 0.0, 6.0 | .4851 |
| Reference | 282 | 0.1 (0.66) | 0.0 | 0.0, 8.0 | ||
| CSPAE | Test | 55 | 1.2 (3.15) | 0.0 | 0.0, 16.0 | .0871 |
| Reference | 45 | 4.8 (14.64) | 0.0 | 0.0, 72.0 | ||
| No ALI | Test | 36 | 0.3 (1.37) | 0.0 | 0.0, 8.0 | .0321 |
| Reference | 29 | 1.5 (3.49) | 0.0 | 0.0, 13.0 | ||
| ALI | Test | 19 | 2.9 (4.63) | 0.0 | 0.0, 16.0 | .6669 |
| Reference | 16 | 10.8 (23.39) | 0.0 | 0.0, 72.0 | ||
Includes all platelet components transfused during the active study period, both on and off protocol.
Wilcoxon rank sum test.
Platelet transfusion exposure in patients with criteria for treatment-emergent ALI and ARDS
| . | Treatment group . | Total . | |
|---|---|---|---|
| Test . | Reference . | ||
| Patients With ALI (N)* | 19 | 16 | 35 |
| Mean duration of platelet support in days (SD) | 15.6 (9.2) | 17.4 (8.5) | 16.5 (8.8) |
| Mean total platelet transfusions (SD) | 16.1 (12.4) | 16.3 (9.5) | 16.2 (11.0) |
| Patients With ARDS (N)† | 12 | 5 | 17 |
| Mean duration of platelet support in days (SD) | 18.3 (9.6) | 10.6 (5.6) | 16.1 (10.9) |
| Mean total platelet transfusions (SD) | 19.4 (13.4) | 11.4 (7.8) | 17.1 (12.4) |
| . | Treatment group . | Total . | |
|---|---|---|---|
| Test . | Reference . | ||
| Patients With ALI (N)* | 19 | 16 | 35 |
| Mean duration of platelet support in days (SD) | 15.6 (9.2) | 17.4 (8.5) | 16.5 (8.8) |
| Mean total platelet transfusions (SD) | 16.1 (12.4) | 16.3 (9.5) | 16.2 (11.0) |
| Patients With ARDS (N)† | 12 | 5 | 17 |
| Mean duration of platelet support in days (SD) | 18.3 (9.6) | 10.6 (5.6) | 16.1 (10.9) |
| Mean total platelet transfusions (SD) | 19.4 (13.4) | 11.4 (7.8) | 17.1 (12.4) |
Patients with AECC criteria and a PaO2/FiO2 ratio < 300.
Patients with AECC criteria and a PaO2/FiO2 ratio < 200.
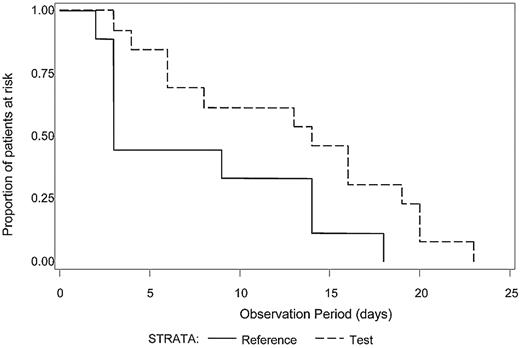
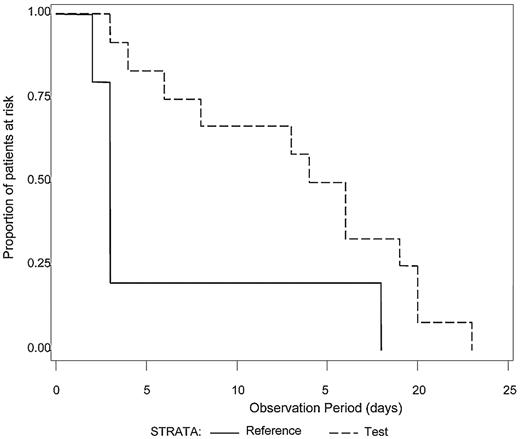
The median number of platelet transfusions before the first diagnosis of ALI was not significantly different between Test and Reference patients (7.0 and 7.5, respectively). Because intubation with mechanical ventilation is a major medical intervention indicative of severe respiratory failure, we examined the time to initiation of mechanical ventilation after the first study platelet transfusion (Test or Reference) for all patients with ALI (N = 21) who required mechanical ventilation (Figure 1). A log-rank test was used to determine whether a difference in the distributions of time to mechanical ventilation after the first platelet transfusion existed between the treatment groups. Patients in the Test group diagnosed with ALI exhibited a longer time after the first study platelet transfusion before initiation of mechanical ventilation compared with Reference group patients (P = .04). For patients with criteria for diagnosis of ARDS (N = 17), all of whom required mechanical ventilation, Test patients demonstrated a significantly longer ventilator-free interval (P = .03) after the first study platelet transfusion (Figure 2). A similar analysis for RBC transfusions was conducted. Among patients with confirmed CSPAE, RBC transfusion was initiated after platelet transfusion and preceded mechanical ventilation in 30.9% of Test patients and 35.6% of Reference patients. For patients with ALI, the distributions of time to onset of mechanical ventilation after the first RBC transfusion were similar to those observed for the initial platelet transfusion although not significantly different between Test and Reference patients (P = .25). Reference patients were more likely to be ventilated sooner after transfusion with RBC compared with Test patients (median time to onset of mechanical ventilation was 4 days for Reference patients and 11 days for Test patients). Too few patients received fresh frozen plasma to conduct a similar analysis.
Time from first study transfusion to onset of mechanical ventilation for patients with ALI. The time (days) to the onset of mechanical ventilation after the first study platelet transfusion was determined for patients with a diagnosis of ALI who required mechanical ventilation (N = 21). The distributions of time to onset of mechanical ventilation were significantly different between the Test group (dashed line) in comparison to the Reference group (solid line), P = .04. Reference patients were more likely to be mechanically ventilated sooner than Test patients.
Time from first study transfusion to onset of mechanical ventilation for patients with ALI. The time (days) to the onset of mechanical ventilation after the first study platelet transfusion was determined for patients with a diagnosis of ALI who required mechanical ventilation (N = 21). The distributions of time to onset of mechanical ventilation were significantly different between the Test group (dashed line) in comparison to the Reference group (solid line), P = .04. Reference patients were more likely to be mechanically ventilated sooner than Test patients.
Time from first study transfusion to onset of mechanical ventilation for patients with diagnostic criteria for ARDS. The time (days) to the onset of mechanical ventilation after the first study platelet transfusion was determined for patients with a diagnosis of ALI meeting criteria for ARDS who required mechanical ventilation (N = 17). The distributions of time to onset of mechanical ventilation were significantly different between the Test group (dashed line) in comparison to the Reference group (solid line), P = .03. Reference patients were more likely to be mechanically ventilated sooner than Test patients.
Time from first study transfusion to onset of mechanical ventilation for patients with diagnostic criteria for ARDS. The time (days) to the onset of mechanical ventilation after the first study platelet transfusion was determined for patients with a diagnosis of ALI meeting criteria for ARDS who required mechanical ventilation (N = 17). The distributions of time to onset of mechanical ventilation were significantly different between the Test group (dashed line) in comparison to the Reference group (solid line), P = .03. Reference patients were more likely to be mechanically ventilated sooner than Test patients.
Because ALI is associated with high mortality, we examined mortality during the active study and surveillance periods up to 49 days after the first study platelet transfusion (Table 6). Patients with CSPAEs (N = 100) had a high rate of mortality that was further increased for patients diagnosed with treatment-emergent ALI. Of note, mortality among patients with clinically serious pulmonary AE without criteria for ALI (N = 65) was lower (17% vs 57%, P < .001) compared with patients with ALI (N = 35). There were no significant differences in mortality rates between treatment groups with the exception of subjects meeting the criteria for ALI only (PaO2/FiO2 ratio < 300 but > 200) in which mortality was marginally lower among Test patients (Table 6).
Mortality among subjects with confirmed clinically serious pulmonary adverse events
| Mortality . | Treatment Group . | Total, N (%) . | P* . | |
|---|---|---|---|---|
| Test, N (%) . | Reference, N (%) . | |||
| Subjects with CSPAE | 14/55 (25.4) | 17/45 (37.8) | 31/100 (31) | .20 |
| Among subjects with ALI† | 9/19 (47.4) | 11/16 (68.8) | 20/35 (57.1) | .31 |
| Among subjects with ALI only‡ | 0/7 (0) | 6/11 (54.5) | 6/18 (33.3) | .04 |
| Among subjects with ARDS§ | 9/12 (75.0) | 5/5 (100.0) | 14/17 (82.4) | .52 |
| Mortality . | Treatment Group . | Total, N (%) . | P* . | |
|---|---|---|---|---|
| Test, N (%) . | Reference, N (%) . | |||
| Subjects with CSPAE | 14/55 (25.4) | 17/45 (37.8) | 31/100 (31) | .20 |
| Among subjects with ALI† | 9/19 (47.4) | 11/16 (68.8) | 20/35 (57.1) | .31 |
| Among subjects with ALI only‡ | 0/7 (0) | 6/11 (54.5) | 6/18 (33.3) | .04 |
| Among subjects with ARDS§ | 9/12 (75.0) | 5/5 (100.0) | 14/17 (82.4) | .52 |
Based upon Fisher exact test.
Patients with AECC criteria and a PaO2/FiO2 ratio < 300.
Patients with AECC criteria and a PaO2/FiO2 ratio < 300 but > 200.
Patients with AECC criteria and a PaO2/FiO2 ratio < 200.
Discussion
ALI, defined by AECC criteria, is a syndrome with significant morbidity and mortality that can be ameliorated by appropriate medical management.8 TRALI comprises a small subset of patients with ALI.1 As opposed to TRALI, which is defined by temporal proximity (6 hours) to transfusion and is commonly defined by a cognate antigen-antibody mismatch, the current study examined the global incidence of treatment-emergent ALI arising from all potential causes during a period of repeated platelet transfusion exposure. The incidence of ALI among HCST patients is not well characterized due to inconsistent application of diagnostic criteria, even by critical care clinicians.2 To optimize identification of patients with ALI, the EPP used prespecified per-protocol assessments for review of all patients with potential pulmonary AEs with severity of grade 2 and higher reported during the active phase of the initial study. Of necessity, this review used retrospectively collected data, but all data were obtained from prospectively recorded medical records during the randomized, controlled, blinded clinical trial.12 While the use of retrospective analysis is a potential limitation due to incomplete data collection, a similar approach has been used in other studies to elucidate the impact of clinical factors on the incidence of pulmonary injury in HSCT recipients.15,16 Our review protocol used an expert adjudication panel with re-extraction of data from primary medical records by trained research personnel blinded to treatment to use all available data reported in the medical record and to minimize bias.
The P3A99 study population is of specific interest with respect to the impact of platelet transfusion on the frequency and outcome of ALI. Firstly, patients with malignant hematology-oncology disorders, especially those treated with allogeneic HSCT, require intensive platelet transfusion support.17 Secondly, this population has a substantial incidence of pulmonary injury, including ALI, due to various comorbid clinical factors resulting in high mortality4 that may be potentiated by platelet transfusion.10 Thirdly, the study focused on an intervention, pathogen inactivation treatment that could impact pulmonary injury. There are no studies that have specifically examined the impact of platelet transfusion on the incidence of ALI in this population. Thus, it was germane to determine, as accurately as feasible, the frequency of ALI in this population and to assess if there was a relationship between the intensity of platelet transfusion and the incidence of ALI compared with the intensity of platelet transfusion exposure in patients with less severe pulmonary AEs.
In contrast to the low incidence of ALI (5/645, 0.8%) reported by the clinical investigators during conduct of the clinical trial,13 the EPP review using AECC criteria observed a higher frequency of ALI (35/645, 5.4%). We believe this difference was due to the use of specific criteria for the diagnosis of ALI, including ARDS, by the expert panel and the lack of a specific code for ALI in the MedDRA coding dictionary used in the initial clinical trial. As part of the review study, we analyzed the diagnoses reported in the initial clinical trial for the patients subsequently identified by the EPP with ALI. The EPP review confirmed that 32 of 35 patients with ALI were diagnosed with grade 3 or grade 4 pulmonary infiltrative disorders by the P3A99 investigators, and as noted, 5 patients also were given a code of ARDS. Two patients were diagnosed with grade 2 pulmonary AEs. A single patient diagnosed with grade 1 pneumocystis pneumonia and pulmonary alveolar hemorrhage was classified with grade 3 hypoxemia. Thus, while the majority of patients subsequently diagnosed with ALI by the EPP were not diagnosed with ARDS by the P3A99 investigators, all of these patients were identified with severe pulmonary disorders by the original investigators, and there were no significant differences between treatment groups.13 Based on the review study, it is clear that prespecified per-protocol assessments and use of AECC criteria for ALI, including the ARDS subset, are required to diagnose ALI. This experience is consistent with other reports indicating that ALI is underdiagnosed, even in intensive care facilities.2,18 In addition, the review indicated that the incidence of ALI varied with primary therapeutic modality. Among the total patient population (N = 645), the incidence of ALI for recipients of autologous HSCT was 0.9%, 13.9% for recipients of allogeneic HSCT, and 5.4% for patients treated with chemotherapy without HSCT. This observation is consistent with other studies showing that death attributed to respiratory failure is lower among autologous HSCT patients compared with allogeneic HSCT patients.6
Based on experimental animal models of ALI, it has been postulated that platelets play a critical role in the pathogenesis of ALI through expression of P-selectin with attendant neutrophil and endothelial interactions potentiating pulmonary injury.11,19-21 None of these models have examined the contribution of platelet transfusion in the setting of transfusion-dependent thrombocytopenia. The P3A99 study provided an opportunity to examine the relationship of the intensity of platelet transfusion to the incidence and outcome of clinically serious pulmonary disorders, including ALI, in a patient cohort receiving intense platelet transfusion support with concurrent comorbid factors associated with ALI. Patients who developed CAPAEs had more platelet and RBC transfusions than patients who did not develop CSPAEs (Table 4). This difference may be largely determined by severity of the primary disease and therapy with attendant complications resulting in delayed recovery of endogenous platelet production. Of interest, patients with ALI, the pulmonary AEs with the most severe lung injury and highest mortality, experienced a similar intensity of platelet support to patients with less severe lung injury and lower mortality. A similar trend was observed for RBC transfusion support. Thus, we were unable to detect evidence that more exposure to platelet transfusion was associated with more severe lung injury.
In addition, we observed that for patients with pulmonary injury who required mechanical ventilation, an objective indicator of more severe lung injury, the time from the first platelet transfusion to the initiation of mechanical ventilation was significantly longer for patients supported with PCs prepared with pathogen inactivation (Test) compared with conventional components (Reference). This observation in conjunction with comparable mortality rates between treatment groups suggests that pathogen inactivation treatment did not potentiate the morbidity of lung injury. Although there were no differences in the preparation of RBC components between treatment groups, a similar trend between treatment groups was observed for RBC transfusions but did not reach statistical significance. It is of interest that the population enrolled in this study had profound neutropenia and thrombocytopenia, as a result of disease and therapy induced hematopoietic suppression, during the period of transfusion support and lung injury. Thus, the postulated interaction between platelets, neutrophils, and endothelium may not be analogous to conditions in the experimental animal models of ALI. Looney and coworkers20 have observed that modest reductions in neutrophil levels in animals maintained in clean rooms as opposed to open environments reduced the sensitivity to induction of experimental ALI in a 2-event model.
In light of the present review, we conclude that AECC criteria for the diagnosis of ALI are required to insure consistent identification of patients with ALI, especially in clinical platelet transfusion trials. Importantly, this retrospective review using specific criteria for diagnosis of ALI did not demonstrate an association of PCs treated with amotosalen and ultraviolet A light with an increased incidence of ALI. In contrast, the data analyses suggest, but do not prove, that pathogen inactivation treatment of PCs may reduce the morbidity of ALI, as measured by the objective parameter of ventilator-free days. The P3A99 study was designed with sufficient power to evaluate platelet transfusion efficacy but did not have sufficient power to analyze conclusively various cofactors that could impact infrequent AEs, such as ALI. However, we did not detect any trends to indicate that pathogen inactivation of PC affected the frequency of ALI and did detect a significant increase in ventilator-free days among recipients of PC treated with pathogen inactivation during the period of transfusion support. This finding requires confirmation via subsequent prospective clinical trials or large-scale active hemovigilance studies22,23 before concluding pathogen inactivation of PCs can reduce the severity of concomitant ALI in this patient population.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contributions of the clinical investigators who participated in the P3A99 clinical trial: Jeffrey McCullough, University of Minnesota; David H. Vesole, Medical College of Wisconsin; Richard J. Benjamin, American Red Cross; Sherrill J. Slichter, Puget Sound Blood Center; Alvaro Pineda, Mayo Clinic; Edward Snyder, Yale-New Haven Hospital; Edward A. Stadtmauer, University of Pennsylvania Medical Center; Ileana Lopez-Plaza, University of Pittsburgh; Steven Coutre, Stanford Medical Center; Ronald G. Strauss, University of Iowa; Lawrence T. Goodnough, Washington University School of Medicine; Joy L. Fridey, Blood Bank of San Bernardino County; Thomas Raife, Blood Center of Southeastern Wisconsin; Ritchard Cable, American Red Cross; Scott Murphy, American Red Cross; and Frank Howard IV, Loma Linda University Cancer Institute. The authors also acknowledge the contribution of the Expert Physician Panel for their diligent efforts in conducting the pulmonary adverse event review: Robert A. Balk, Rush Medical Center, Chicago, IL; R. Phillip Dellinger, Cooper Health System, Camden, NJ; and Scott Rowley, Hackensack University Medical Center, Hackensack, NJ. R. Phillip Dellinger served as Chairman of the Expert Physician Panel. The authors thank Cornelius H. Wortel, Clinquest Inc, and Susan D. Ross, MetaWorks Inc, for administration of the review study protocol. The authors are indebted to Michael Matthay for critical advice on design of the review study and the manuscript.
Authorship
Contribution: L.C. designed the P3A99 clinical trial and contributed to the design of the review study of pulmonary adverse events; J.S.L. and C.D.S. supervised data analysis for the review study and performed supplemental analyses for the manuscript; and J.E., L.C., and C.D.S. wrote the manuscript.
Conflict-of-interest disclosure: L.C. and C.D.S. are employees of Cerus Corporation and own stock and or stock options in Cerus Corporation. J.S.L. and J.E. were formerly employees of Cerus Corporation and held stock or stock options during the time of their employment.
Correspondence: Laurence Corash, MD, Clinical Research and Medical Affairs, Cerus Corporation, 2550 Stanwell Dr, Concord, CA 94520; e-mail: lcorash@cerus.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal