In this issue of Blood, Guo and colleagues from Beijing report that infusion of human leukocyte antigen (HLA)–mismatched peripheral blood stem cells (PBSC) improves survival in elderly patients with acute myeloid leukemia (AML) when used in combination with chemotherapy.1
Despite rapid advances in our understanding of the biology of AML, most patients with this disease continue to have poor outcomes with chemotherapy. This is particularly so in individuals older than 60 years of age, who constitute the majority of patients and where overall survival remains stubbornly low (reviewed in Estey2 ). Higher rates of poor-risk disease are linked to more chemotherapy resistance. Additional comorbidities, which are common in this age group, also lead to more treatment-related deaths. Thus, there continues to be an urgent need to improve treatment for elderly patients with AML.
One important advance has been the use of reduced-intensity conditioned allogeneic stem cell transplantation with the intent to exploit the graft-versus-leukemia (GVL) effect mediated by donor immune cells contained within the graft. Indeed, evidence from recent series suggests that this approach can overcome some of the therapeutic resistance of AML in older patients.3 However, individuals enrolled into such studies represent only a select group who are probably not representative of most elderly patients with AML. Furthermore, only patients with a suitably HLA-matched donor are eligible for this approach. The intention of Guo and colleagues was to devise a means of delivering immune antitumor effects without requiring that a patient undergo allogeneic stem cell transplantation.1 In their approach, patients would get standard chemotherapy followed by mobilized peripheral blood stem cells from a haploidentical-related donor. Unpublished preclinical data had suggested that this led to rapid hematopoietic recovery without durable donor engraftment and no graft-versus-host disease (GVHD).
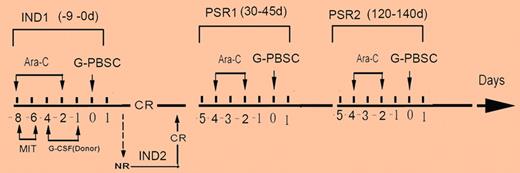
In their study, 58 patients over 60 years of age with AML and without an HLA-identical sibling donor were randomized to receive either standard AML chemotherapy (mitoxantrone and cytarabine according to a “3 + 7” schedule) or the same treatment followed by infusion of granulocyte-colony stimulating factor (G-CSF)–mobilized PBSC from an HLA-mismatched relative.1 Patients achieving complete remission went on to receive consolidation with 2 further cycles of cytarabine with or without PBSC infusion (see figure). A single mobilized PBSC product was split into aliquots to be given during induction and consolidation. Over a third of the study population was over 70 years of age and as might be expected, there were a high proportion of patients with high-risk features including multilineage dysplasia (26%) and/or poor-risk cytogenetics (43%). Both the PBSC and control group were well balanced in terms of their risk profile. The key findings were a significantly higher complete remission rate in the PBSC group (80% vs 43%) and importantly, a higher 2-year probability of overall survival (39% vs 10%). Although hematopoietic recovery was more rapid in the PBSC group, this could not be explained by engraftment of donor hematopoietic stem cells. Associated with the lack of significant donor cell engraftment, GVHD did not occur. Although the study was small and selected for patients who were chemotherapy candidates, the randomized design was likely to have reduced the potential for bias that affects other single-arm studies of new treatments in AML. This study is therefore a potentially important advance in a clinical arena where improvements in outcome have been exceptionally difficult to achieve.
Trial design for Guo et al.1 Ara-C indicates cytarabine; MIT, mitoxantrone; IND, induction; and PSR, postremission treatment.
Trial design for Guo et al.1 Ara-C indicates cytarabine; MIT, mitoxantrone; IND, induction; and PSR, postremission treatment.
Over the past decade, several other groups have also performed early-phase studies involving transfer of donor leukocytes to induce graft-versus-tumor responses in patients with solid or hematologic malignancies without prior allogeneic stem cell transplantation.4-6 The protocols involved have varied with the cells transferred derived from steady-state or mobilized aphereses, irradiated or nonirradiated, from HLA-identical or mismatched donors and administered with no or some level of immunosuppressive treatment. In several of these studies, objective tumor responses were observed, often in association with engraftment of transferred cells and the development of GVHD or, unfortunately, in some cases, aplasia as is seen in transfusion-associated GVHD.5 Engraftment of donor cells and GVHD is more likely in patients who have had extensive prior treatment, particularly prior autologous transplantation.5 A striking difference between these studies and the Beijing trial is that in the latter case, engraftment of donor cells was minimal and no GVHD was observed.1 This is likely because patients undergoing anthracycline-based chemotherapy for AML still retain substantial host cellular immunity sufficient to rapidly reject the majority of infused cells.7
This of course then leaves the question of what mechanisms underlie the accelerated hematopoietic recovery and improved leukemia responsiveness even though the infused cells fail to survive. Are mobilized PBSC required for the effect or would cells derived from steady-state aphereses be just as effective? What are the cellular constituents within the infused product that mediate the positive effects? Dissection of potential mechanisms will require detailed preclinical experiments, but the failure to observe T-cell engraftment makes it unlikely that the increased responses observed in this study relate to a “classical” GVL response. It is of interest that in other contexts, rejection of donor hematopoietic cells has been linked to reduced rates of relapse.8,9 For example, after dual cord transplantation, where only 1 cord unit engrafts while the other is rejected, rates of acute leukemia relapse are significantly lower than after single-cord transplantation.9 Durable responses in patients with previously refractory disease have also been reported after allogeneic transplantation despite early rejection of the donor graft.8 Together, these findings suggest the possibility that the rejection response itself is important in mediating antileukemia effects. Indeed, animal experiments deliberately simulating the process of rejection of donor hematopoietic cells have demonstrated enhancement of specific antitumor responses that involve interferon-γ and both host (eg, CD4 and invariant natural killer T cells) and donor (eg, CD8 T cells) immune cells.10,11
It will be important for future studies to evaluate this novel approach in larger numbers of patients, including in other age groups. Caution, however, should be exercised in patients at risk of transfusion-associated GVHD, for example, those treated with purine analogues or those who have received prior autologous stem cell transplantation, where the risks of GVHD might be greater. For the same reason, this approach would also be inadvisable where the donor is homozygous for an HLA haplotype that is shared by the recipient. These concerns aside, the study of the Beijing group could ultimately help to open another front in the battle to cure AML.
Conflict-of-interest disclosure: S.M. has received an unrestricted educational grant from Bayer-Schering. R.C. declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal