Abstract
Two major pathways contribute to Ras-proximate-1–mediated integrin activation in stimulated platelets. Calcium and diacyglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI, RasGRP2) mediates the rapid but reversible activation of integrin αIIbβ3, while the adenosine diphosphate receptor P2Y12, the target for antiplatelet drugs like clopidogrel, facilitates delayed but sustained integrin activation. To establish CalDAG-GEFI as a target for antiplatelet therapy, we compared how each pathway contributes to thrombosis and hemostasis in mice. Ex vivo, thrombus formation at arterial or venous shear rates was markedly reduced in CalDAG-GEFI−/− blood, even in the presence of exogenous adenosine diphosphate and thromboxane A2. In vivo, thrombosis was virtually abolished in arterioles and arteries of CalDAG-GEFI−/− mice, while small, hemostatically active thrombi formed in venules. Specific deletion of the C1-like domain of CalDAG-GEFI in circulating platelets also led to protection from thrombus formation at arterial flow conditions, while it only marginally increased blood loss in mice. In comparison, thrombi in the micro- and macrovasculature of clopidogrel-treated wild-type mice grew rapidly and frequently embolized but were hemostatically inactive. Together, these data suggest that inhibition of the catalytic or the C1 regulatory domain in CalDAG-GEFI will provide strong protection from athero-thrombotic complications while maintaining a better safety profile than P2Y12 inhibitors like clopidogrel.
Introduction
Arterial thrombosis in the coronary or cerebrovascular circulation is the principal pathological process underlying acute coronary syndrome and ischemic stroke, which together represent the leading cause of morbidity and mortality in industrialized countries.1 Platelet activation is a central event in the pathogenesis of arterial thrombosis. Currently, the most powerful antiplatelet agents used in the clinic are inhibitors of cyclooxygenase-1 (acetylsalicylic acid, aspirin), the platelet adenosine diphosphate (ADP) receptor P2Y12 (eg, clopiodgrel or Plavix), and integrin αIIbβ3 (eg, abciximab or Reopro).2,3 These agents have all been shown to improve clinical outcomes in large-scale randomized controlled trials. However, all therapies have limitations that include uncertainty about optimal dosing, questions about resistance, and issues regarding the lack of reversibility in situations where bleeding risks are high.
αIIbβ3, the platelet fibrinogen receptor, is the best-studied member of the integrin family.4,5 Like most integrins, especially those regulating adhesion and trafficking of blood cells, it is expressed in a low-affinity state on resting platelets. Engagement of agonist receptors on the platelet surface triggers intracellular signaling events, which lead to inside-out activation of αIIbβ3. Deficiency in αIIbβ3 completely inhibits the ability of platelets to aggregate and adhere to sites of injury.6,7 Consequently, inhibitors to integrin αIIbβ3 show the strongest protection from thrombotic complications, but they also markedly increase the risk of fatal bleeding complications. In contrast, inhibitors to P2Y12 reduce but do not abolish platelet function. P2Y12 signaling is evoked when ADP is released from dense granules of activated platelets. In a growing thrombus, ADP is important for the amplification of the initial activation of adherent platelets as well as the recruitment of circulating platelets to the thrombus surface.8 Consequently, genetic knockout9 or inhibition10 of P2Y12 in mice led to the formation of unstable thrombi and continuous bleeding in the mouse tail bleed assay.
We recently established a simplified model for platelet activation in which 2 signaling pathways control the activation of the small GTPase Ras-proximate-1 (Rap1).11,12 Activation of phospholipase C, an event common to the signaling induced by all physiological agonists, leads to the generation of the second messengers calcium (Ca2+) and diacylglycerol (DAG). An increase in the intracellular Ca2+ concentration triggers the activation of calcium and diacyglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI, RasGRP2), a GEF that directly activates Rap1.13-15 In addition to the GEF domain, CalDAG-GEFI contains a pair of Ca2+ binding EF hand domains and a C1-like domain with hitherto unknown function.16 DAG is a well-established activator of protein kinase C (PKC) and therefore granule release in platelets.17,18 PKC-dependent integrin activation depends on positive feedback by the Gi-coupled P2Y12 receptor,19 which again leads to the activation of Rap1.11,12,20 Importantly, CalDAG-GEFI–mediated Rap1 activation is rapid but reversible, while P2Y12-mediated Rap1 activation occurs with a delay but is more stable. Thus, 2 kinetically distinct pathways converge at the level of Rap1, a molecular switch that drives platelet activation by triggering integrin activation, thromboxane A2 (TxA2) generation, and granule release.12,21
Our recent work suggested CalDAG-GEFI as a potential new target for antiplatelet therapy, as it (i) is the major signal integrator for Ca2+ in platelets, (ii) plays a crucial role in the near-immediate activation of platelets, and (iii) provides feedback to but is not required for signaling via the PKC/P2Y12-dependent pathway. Furthermore, its complex domain structure may allow targeting of a regulatory rather than the catalytic domain within CalDAG-GEFI. To validate CalDAG-GEFI as a new target for antiplatelet therapy, and to determine the mechanism(s) by which the CalDAG-GEFI/Rap1 signaling module contributes to thrombus formation under physiological flow conditions, we subjected CalDAG-GEFI−/− and clopidogrel-treated wild-type (WT) mice to several state-of-the-art mouse models of thrombosis and hemostasis. Our studies identify CalDAG-GEFI signaling as a crucial component of the initial phase of platelet adhesion and thrombus formation, particularly under arterial shear conditions. Inhibition of P2Y12, however, led to thrombus instability but had little effect on the initial thrombus growth. In addition, we observed that CalDAG-GEFI deficiency has less impact on hemostasis in mice than P2Y12 inhibition. Thus, our studies indicate that targeting the initial phase of integrin activation mediated by CalDAG-GEFI may be superior to blocking sustained αIIbβ3 activation as achieved by inhibitors of P2Y12. Furthermore, we provide the first evidence that the C1 domain is critical for CalDAG-GEFI function and that inactivation of this regulatory domain may provide a mechanism to further improve the safety of anti-CalDAG–GEFI therapy.
Methods
For information on materials, Western blotting, immunofluorescence, and aggregometry, see supplemental information (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mice
CalDAG-GEFI−/−14 and littermate control WT mice were bred in the mouse facility of Thomas Jefferson University. Where indicated, WT or CalDAG-GEFI−/− mice were treated with clopidogrel 24 and 3 hours before the experiment at a dosage of 75 mg/kg bodyweight. P2Y12 inhibition was confirmed by standard aggregometry (impaired response to ADP, not shown). Treatment with clopidogrel did not affect platelet counts or counts of other blood cells (data not shown). Experimental procedures were approved by the Animal Care and Use Committee of Thomas Jefferson University.
Generation of chimeric mice expressing CalDAG-GEFIΔC1 in blood cells only
Retrovirus construction
The cDNA of murine CalDAG-GEFI was kindly provided by Drs Jill Crittenden and Ann Graybiel (Massachusetts Institute of Technology, Cambridge, MA). The C1 deletion mutant was generated using overlap polymerase chain reaction. Primers used were as follows: C1 deletion forward 5′-GAGGCCGCATGGGATTCGTA. CGTCGCAGAGCTCAGAGTGTCA; C1 deletion reverse 5′–TGACACTCTGAGCTCTGCGACG.TACGAATCCCATGCGGCCTC. WT and mutant cDNAs were subcloned into the murine stem cell virus MigR1 viral vector with an internal ribosome entry site-green fluorescent protein (IRES-GFP) inserted before the polyadenylation signal as previously described (kindly provided by Mark Kahn, University of Pennsylvania).22
Bone marrow cell transplantation
Bone marrow cells were harvested from the tibia and femur of adult CalDAG-GEFI−/− mice, cultured overnight in Iscove modified Dulbecco medium with a supplement of murine stem cell factor, interleukin-3, and interleukin-6. The cells were transduced twice by spinfection (50′) at 1000g with retrovirus at a multiplicity of infection equal to 5 virions per cell. The transduction efficiency after the second spinfection was 20% to 50%. A total of 2 × 106 cells were transplanted by retro-orbital injection (100 μL per mouse) into recipient animals (8-10 weeks old) conditioned with a lethal dose of 2 × 600 cGy total body irradiation. Six to eight weeks after transplantation, bone marrow was harvested and sorted for GFP positivity at the Thomas Jefferson University flow cytometry core facility, followed by transplantation of GFP-positive bone marrow cells into another set of lethally irradiated recipient mice (CalDAG-GEFI−/−). Some of the GFP-expressing WT chimeric mice were generated by transplant of bone marrow cells isolated from C57Bl/6 mice transgenic for GFP (The Jackson Laboratory). The platelet counts in all chimeric mice recovered to levels > 70% of WT controls within 6 to 8 weeks after transplant.
Platelet preparation
Blood was drawn from the retro-orbital plexus into heparinized tubes. Platelet-rich plasma was obtained by centrifugation at 100g for 5 minutes. Platelet-rich plasma was centrifuged at 700g in the presence of PGI2 (2 μg/mL) for 5 minutes at room temperature. After 2 washing steps, pelleted platelets were resuspended at the concentration of 4 × 108 platelets/mL in modified Tyrode Buffer (137mM NaCl, 0.3mM Na2HPO4, 2mM KCl, 12mM NaHCO3, 5mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5mM glucose, pH 7.3) containing 0.35% bovine serum albumin and 1mM CaCl2.
Flow cytometry
GFP expression in blood platelets
Heparin-anticoagulated whole blood from WT control and CalDAG-GEFIΔC1 chimeric mice was diluted in phosphate-buffered saline (PBS) and analyzed on a FACScan (BD Biosciences).
αIIbβ3 activation
Washed platelets were diluted in Tyrode buffer containing 1mM CaCl2, activated with convulxin or PAR4-activating peptide (PAR4p) in the presence of R-phycoerythrin-conjugated JON/A antibody (JON/A-PE)23 for 10 minutes and studied immediately by flow cytometry. In studies with CalDAG-GEFIΔC1 platelets, 2-MesAMP (2-methylthioadenosine 5′-monophosphate triethylammonium salt; 75μM) was added before platelet stimulation to eliminate feedback by P2Y12 receptor signaling.
Flow chamber studies
In vitro flow studies were performed in a microfluidic device fabricated in poly dimethylsiloxane. Fabrication of microfluidic devices and microfluidic collagen patterning were performed as previously described.24 Briefly, a 100-μm strip of fibrillar collagen type I (200 μg/mL) was deposited and immobilized by microfluidic patterning along the length of a glass slide. A poly dimethylsiloxane device with 10 flow channels (width: 250 μm, height: 60 μm, length: 6 mm) was oriented perpendicularly to the patterned collagen. Murine whole blood was drawn from the retro-orbital plexus into heparinized tubes (30 U/mL Lovenox), incubated with 1.5 μg/mL of anti-GPIX (glycoprotein IX)–Alexa488 and infused at arterial (2000−S) or venous (400−S) wall shear rates for 5 minutes. In some experiments, ADP and the TxA2 analog, U46619, were added to CalDAG-GEFI−/− whole blood immediately before the beginning of the perfusion. Adhesion of platelets was monitored continuously with a Nikon Ti-U inverted microscope (Nikon Instruments Inc) equipped with a Retiga EXL monochrome camera (QImaging; objective lenses, Plan fluor (air) 20×/0.45). Images were analyzed using Nikon NIS Elements Version 3.0 software (NIS-Elements Advanced Research).
Platelet depletion
Mice were injected retro-orbitally with a commercially available antibody to mouse GPIbα.25 Successful platelet depletion (peripheral platelet counts < 2% of untreated control mice) was confirmed by flow cytometry 1 hour after infusion of anti-GPIbα antibody.
Tail bleeding time and estimated blood loss measurement
Mice (approximately 8 weeks old) were matched for weight and anesthetized with 100 mg/kg ketamine (Forte Dodge Animal Health) and 10 mg/kg xylazine (Lloyd Laboratories) 10 minutes before tail transection. Mice were placed on a heating pad, and tails were transected 2 mm from the tip with a razor blade and immediately immersed into a 20-mL scintillation vial filled with 5 mL PBS (37°C). The time to cessation of bleeding was recorded, and after 10 minutes the bleeding was stopped by cauterization. To measure blood loss volume, the collected blood sample was frozen at −80°C overnight, thawed, and 5 mL of red blood cell lysis buffer (155mM NH4Cl, 10mM KHCO3, 0.1mM Na2 EDTA [disodium ethylenediamine tetraacetate[, pH = 7.3) was added. The optical density (490) was determined and compared with a standard curve. Statistical significance for the blood loss volume was determined by Mann-Whitney test.
Laser injury in the cremaster muscle
The studies on laser-induced thrombosis in the cremaster muscle were performed as previously described.26 Briefly, male mice (12-14 weeks of age) were anesthetized with pentobarbital (11 mg/kg; Abbott Laboratories), and platelet adhesion was studied in arterioles and venules of the cremaster muscle (10-20 μm) using an Olympus BX61WI microscope (Olympus) with a ×40/0.8 numeric aperture water-immersion objective lens. Laser injuries were done using an SRS NL100 Nitrogen Laser system (Photonic Instruments) at 65% energy level. Injuries were initiated 5 minutes after the intravenous injection of Alexa488-coupled Fab fragments of MWReg30, an antibody directed against murine αIIbβ3 (0.2 μg/g body weight, BD Pharmingen). Data were collected over 3 minutes at 5 frames/s and then averaged at each time point. Videos were analyzed using Slidebook Version 5.0.0.17 software (Intelligent-Imaging-Solutions).
FeCl3-induced thrombosis in mesenteric venules.
Three- to 5-week-old anesthetized male mice were retro-orbitally injected with Alexa488-conjugated antibodies to GPIX (0.1 μg/g, labeling of circulating platelets) or JON/A-PE (0.15 μg/g, labeling of activated αIIbβ3). The mesentery was exposed through a midline abdominal incision. Arterioles and venules were examined with a Nikon Eclipse Ti-U inverted microscope (Nikon) equipped with a Retiga EXL monochrome camera (QImaging; objective lenses, Plan fluor (air) 20×/0.45 and Plan Apo 10×/0.45). Vessel injury was generated by placing a filter paper (1 × 4 mm) soaked with 10% FeCl3 on the vessel for 3.5 minutes. The filter paper was then removed, and the vessel was superfused with saline. Vessels were monitored for 40 minutes after FeCl3 treatment or until blood flow stopped for longer than 10 seconds (identified as occlusion time). Videos were analyzed using Nikon NIS Elements Version 3.0 software (NIS-Elements, Advanced Research).
FeCl3-induced thrombosis in the carotid artery.
The right carotid artery of an anesthetized adult mouse (6-10 weeks of age, 100 mg pentobarbital per kg body weight) was exposed to a strip of filter paper saturated with FeCl3 (10% for 4 minutes or 40% for 2.5 minutes), then rinsed with PBS. Arterial flow rate was recorded for 30 minutes with a Doppler flow probe (Transonic). Stable occlusive thrombi were scored as complete cessation of blood flow, which remained for the 30-minute duration of the assay. Thrombi were scored as unstable if flow resumed before the end of the 30-minute time period or if the flow rate changed repeatedly by > 30% compared with the initial flow rate. The animal was scored as having no detectable thrombus if the flow rate never decreased by > 30% of the initial flow rate during the term of the assay. The mice were killed at the end of the procedure.
Statistical analysis
Results are reported as mean ± SEM, and statistical significance was assessed by unpaired 2-tailed Student t test, unless otherwise indicated. A P value less than .05 was considered significant.
Results
Thrombotic profile of CalDAG-GEFI−/− and clopidogrel-treated WT mice
We first performed flow chamber studies to determine the shear dependency of CalDAG-GEFI– and P2Y12-mediated thrombus formation on a collagen surface (Figure 1A and supplemental Videos 1-4). Platelets in whole blood were labeled with an Alexa488-conjugated antibody to GPIX, and fluorescence accumulation on the collagen surface was used to quantify the adhesion process. Compared with controls, thrombus formation at arterial shear rates (2000 s−1, Figure 1A left panel) was completely abolished in blood isolated from both CalDAG-GEFI−/− and clopidogrel-treated WT mice (WT/clopidogrel). In both cases, firmly adherent platelets were observed on the collagen surface at the end of the perfusion period (not shown). Under venous shear conditions (400 s−1, Figure 1A right panel), WT/clopidogrel platelets formed 3-dimensional thrombi (supplemental Video 2). However, thrombi were unstable and frequently embolized, resulting in a significant reduction in platelet coverage of the collagen surface compared with WT controls (supplemental Video 1). In contrast, CalDAG-GEFI−/− platelets were not able to form thrombi on the collagen surface (supplemental Video 3) but eventually formed a monolayer of firmly adherent cells similar to that observed in WT/clopidogrel blood at the end of the perfusion period. Clopidogrel treatment of CalDAG-GEFI−/− mice further reduced platelet adhesion to collagen (supplemental Video 4), confirming the importance of both pathways for integrin-mediated platelet adhesion. These studies demonstrate that, although both CalDAG-GEFI−/− and WT/clopidogrel platelets can form stable aggregates in a standard aggregometry assay, both pathways have to be active to enable formation of stable thrombi under flow conditions on collagen.
Platelet adhesion to fibrillar collagen under physiological flow conditions. (A) Whole blood from WT (WT, black lines and bars), CalDAG-GEFI−/− (knockout [KO], red), clopidogrel-treated WT (WT + clop., blue), or clopidogrel-treated KO (KO + clop., green) mice was perfused over collagen at arterial (2000 s−1, left) or venous (400 s−1, right) shear conditions. Platelets in whole blood were labeled with Alexa488-labeled antibodies to GPIX before perfusion. The top graphs represent time traces of the mean fluorescence intensity ± SEM expressed as a percentage of the maximal fluorescence observed (WT blood, 400 s−1). The bar graphs show the area coverage by fluorescent platelets after 5 minutes of blood perfusion, expressed as percentage of the collagen-coated area. Data are shown as mean ± SEM (n = 4-6, 3 independent experiments). *P < .05, **P < .01, ***P < .001. See supplemental Videos 1 to 4 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. (B-C) Effect of exogenous ADP and TxA2 (U46619) on the adhesion of CalDAG-GEFI−/− platelets. WT and CalDAG-GEFI−/− (KO) whole blood was perfused over collagen at 400 s−1 or 2000 s−1 in the presence (KO + ADP/U46) or absence (KO) of exogenous ADP (25μM) and U46619 (5μM). (B) Bar graphs for area coverage (top) and fluorescence intensity (bottom) measured after 5 minutes of perfusion with the following blood samples: WT (black bar), KO (red bar), and KO reconstituted with 25μM ADP and 5μM U46619 (KO + ADP/U46, red checkered bar). Data are shown as mean ± SEM (n = 5, 3 independent experiments). *P < .05; **P < .01; ***P < .001. (C) Representative images. Images were obtained after 5 minutes of perfusion on a Nikon Eclipse Ti-U inverted microscope (equipped with a Retiga EXL monochrome camera [QImaging] and Nikon NIS Elements software [NIS-Elements Advanced Research]).
Platelet adhesion to fibrillar collagen under physiological flow conditions. (A) Whole blood from WT (WT, black lines and bars), CalDAG-GEFI−/− (knockout [KO], red), clopidogrel-treated WT (WT + clop., blue), or clopidogrel-treated KO (KO + clop., green) mice was perfused over collagen at arterial (2000 s−1, left) or venous (400 s−1, right) shear conditions. Platelets in whole blood were labeled with Alexa488-labeled antibodies to GPIX before perfusion. The top graphs represent time traces of the mean fluorescence intensity ± SEM expressed as a percentage of the maximal fluorescence observed (WT blood, 400 s−1). The bar graphs show the area coverage by fluorescent platelets after 5 minutes of blood perfusion, expressed as percentage of the collagen-coated area. Data are shown as mean ± SEM (n = 4-6, 3 independent experiments). *P < .05, **P < .01, ***P < .001. See supplemental Videos 1 to 4 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. (B-C) Effect of exogenous ADP and TxA2 (U46619) on the adhesion of CalDAG-GEFI−/− platelets. WT and CalDAG-GEFI−/− (KO) whole blood was perfused over collagen at 400 s−1 or 2000 s−1 in the presence (KO + ADP/U46) or absence (KO) of exogenous ADP (25μM) and U46619 (5μM). (B) Bar graphs for area coverage (top) and fluorescence intensity (bottom) measured after 5 minutes of perfusion with the following blood samples: WT (black bar), KO (red bar), and KO reconstituted with 25μM ADP and 5μM U46619 (KO + ADP/U46, red checkered bar). Data are shown as mean ± SEM (n = 5, 3 independent experiments). *P < .05; **P < .01; ***P < .001. (C) Representative images. Images were obtained after 5 minutes of perfusion on a Nikon Eclipse Ti-U inverted microscope (equipped with a Retiga EXL monochrome camera [QImaging] and Nikon NIS Elements software [NIS-Elements Advanced Research]).
We recently demonstrated that CalDAG-GEFI is central to various aspects of Ca2+-dependent platelet activation such as integrin activation, TxA2 generation, and granule release.11,12 To establish if impaired release of second wave mediators contributes to the adhesion defect observed in CalDAG-GEFI−/− platelets, we co-infused ADP and the TxA2 analog, U46619, with CalDAG-GEFI−/− blood. At both arterial and venous shear rates, we observed that co-infusion of ADP and U46619 led to a marked increase in the surface area covered by fluorescent platelets (Figure 1B top panel, and Figure 1C). In contrast, only a small increase in the overall fluorescence on the collagen strip was detected (Figure 1B bottom panel, and Figure 1C), suggesting that the addition of exogenous mediators increased the adhesion of single platelets to the collagen surface while it did not restore 3-dimensional thrombus growth.
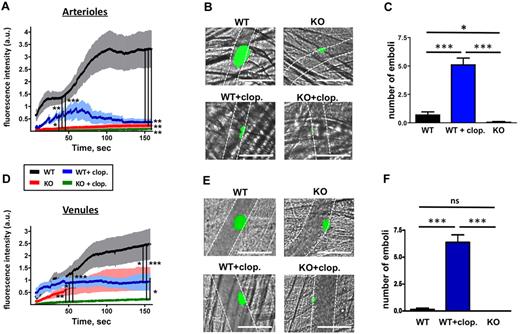
A limitation of the flow chamber assay is that it is performed with anticoagulated blood; that is, it does not account for the generation of thrombin, which is a central component of the thrombogenic response in vivo. Our previous in vitro studies, however, demonstrated that CalDAG-GEFI−/− platelets respond better to thrombin than to collagen.11,12,14 To investigate whether CalDAG-GEFI deficiency also protects from thrombin-driven thrombosis, we studied thrombus formation at a site of vascular injury, both in arterioles and venules (Figure 2 and supplemental Videos 5-8). As previously shown, laser-induced thrombosis in the cremaster microcirculation represents a model of localized, thrombin-driven thrombosis.27,28 When subjected to this model, CalDAG-GEFI−/− mice showed decreased platelet accumulation compared with WT mice in both arterioles (Figure 2A-B) and to a lesser extent in venules (Figure 2D-E). Thrombi in venules of CalDAG-GEFI−/− mice grew slowly but remained stably anchored to the vessel wall. Treatment of WT mice with clopidogrel significantly reduced thrombus size in arterioles and venules. Initial thrombus growth under these conditions occurred with similar kinetics as in WT controls, but thrombi were unstable and frequently embolized (Figure 2C,F). Platelet adhesion was almost completely inhibited in venules and arterioles of clopidogrel-treated CalDAG-GEFI−/− mice.
Laser-induced thrombosis in the cremaster muscle microcirculation. Mice were injected with Alexa488-labeled Fab fragments of MWReg30. (A,D) Changes in fluorescence intensity over time measured after laser injury in cremaster muscle arterioles (A) or venules (D) of the following mouse groups: WT (black line), CalDAG-GEFI−/− (KO, red), WT/clopidogrel (WT + clop., blue), CalDAG-GEFI−/− / clopidogrel (KO + clop., green). Results represent the mean fluorescence intensity ± SEM measured in 3 independent experiments (n = 9-27 vessels for each group). Only significant differences (*P < .05; **P < .01; ***P < .001) are shown. (B,E) Representative images taken at t = 150” after laser injury in arterioles (B) and venules (E). See supplemental Videos 5-8 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. Images were obtained on a Olympus BX61WI microscope (Olympus) with a ×40/0.8 numeric aperture water-immersion objective lens, using Slidebook software (Intelligent-Imaging-Systems). Dotted lines mark the vessel wall. White bar = 100 μm. (C,F) Number of emboli with a diameter of more than 10 μm forming after laser injury in arterioles (C) or venules (F) of WT, WT/clopidogrel, and CalDAG-GEFI−/− mice. n = 7-9. Mean venule diameter: WT: 63.4 ± 8.5 μm; WT + clopidogrel: 69.1 ± 8.8 μm; CalDAG-GEFI−/−: 55.9 ± 6.1 μm; CalDAG-GEFI−/− + clopidogrel: 60.2 ± 4.2 μm; P = nonsignificant (NS) for all comparisons. Mean arteriole diameter: WT: 37.3 ± 3.0 μm; WT + clopidogrel: 33.7 ± 1.7 μm; CalDAG-GEFI−/−: 38.1 ± 2.5 μm; CalDAG-GEFI−/− + clopidogrel: 37.1 ± 1.9 μm; P = NS for all comparisons.
Laser-induced thrombosis in the cremaster muscle microcirculation. Mice were injected with Alexa488-labeled Fab fragments of MWReg30. (A,D) Changes in fluorescence intensity over time measured after laser injury in cremaster muscle arterioles (A) or venules (D) of the following mouse groups: WT (black line), CalDAG-GEFI−/− (KO, red), WT/clopidogrel (WT + clop., blue), CalDAG-GEFI−/− / clopidogrel (KO + clop., green). Results represent the mean fluorescence intensity ± SEM measured in 3 independent experiments (n = 9-27 vessels for each group). Only significant differences (*P < .05; **P < .01; ***P < .001) are shown. (B,E) Representative images taken at t = 150” after laser injury in arterioles (B) and venules (E). See supplemental Videos 5-8 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. Images were obtained on a Olympus BX61WI microscope (Olympus) with a ×40/0.8 numeric aperture water-immersion objective lens, using Slidebook software (Intelligent-Imaging-Systems). Dotted lines mark the vessel wall. White bar = 100 μm. (C,F) Number of emboli with a diameter of more than 10 μm forming after laser injury in arterioles (C) or venules (F) of WT, WT/clopidogrel, and CalDAG-GEFI−/− mice. n = 7-9. Mean venule diameter: WT: 63.4 ± 8.5 μm; WT + clopidogrel: 69.1 ± 8.8 μm; CalDAG-GEFI−/−: 55.9 ± 6.1 μm; CalDAG-GEFI−/− + clopidogrel: 60.2 ± 4.2 μm; P = nonsignificant (NS) for all comparisons. Mean arteriole diameter: WT: 37.3 ± 3.0 μm; WT + clopidogrel: 33.7 ± 1.7 μm; CalDAG-GEFI−/−: 38.1 ± 2.5 μm; CalDAG-GEFI−/− + clopidogrel: 37.1 ± 1.9 μm; P = NS for all comparisons.
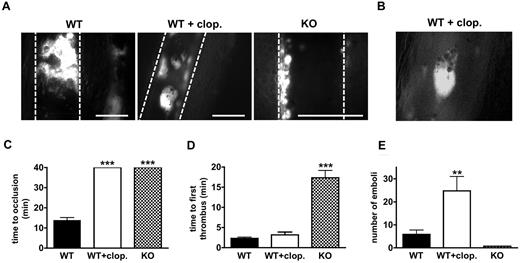
Similar results were obtained in a second microvascular thrombosis model, ferric chloride (FeCl3)–induced thrombosis in the mesentery. Compared with laser injury, FeCl3 induces a more severe injury to the endothelium, resulting in exposure of subendothelial collagen and the generation of thrombin.29 In WT animals, occlusive thrombi formed in arterioles (not shown) and venules within 9-15 minutes after FeCl3 application (Figure 3A left panel, and Figure 3C). Clopidogrel-treated WT animals were protected from vessel occlusion (Figure 3C), but thrombi of considerable size (up to 70 μm in diameter) formed in both arterioles (not shown) and venules (Figure 3A middle panel). Thrombi in clopidogrel-treated mice grew rapidly but embolized more frequently throughout the entire observation period (Figure 3D-E). The emboli stained positive for activated αIIbβ3 (Figure 3B), excluding the possibility that they consisted of passively agglutinated, nonactivated platelets. In contrast, small but stable mural thrombi formed with a delay in venules (Figure 3A right panel, and Figure 3D) of CalDAG-GEFI−/− animals, while thrombus formation was not observed in injured arterioles.30 As observed in the laser injury model, thrombus formation was completely inhibited in FeCl3-injured arterioles and venules of clopidogrel-treated CalDAG-GEFI−/− mice (not shown).
FeCl3-induced thrombosis in the mesentery. (A) Images of mesenteric venules taken 13 minutes after FeCl3 injury. Platelets were labeled by infusion of fluorophore-labeled antibodies to platelet receptor GPIX. White bar = 100 μm. Dotted lines mark the vessel wall. Representative of 5 independent experiments. (B) Embolizing thrombi in clopidogrel-treated WT animals stained positive for JON/A-PE, a probe that selectively detects the activated form of αIIbβ3 integrin.23 Representative of 3 experiments. (C) Mean occlusion time in FeCl3-injured venules of mice of WT (solid black bar), WT/clopidogrel (WT + clop., solid white), and CalDAG-GEFI−/− (KO, checkered) mice (n = 7-9). Note that none of the WT/clopidogrel or CalDAG-GEFI−/− mice occluded within the 40 minutes observation period. (D) Average time required to form a first thrombus of more than 20 μm in diameter. (E) Number of emboli with a diameter of more than 20 μm forming 8-13 minutes after FeCl3 injury. Mean venule diameter: WT: 254.9 ± 19.5 μm; WT + clopidogrel: 305 ± 28.7 μm; CalDAG-GEFI−/−: 239 ± 13.7 μm; P = NS for all comparisons. All images were obtained on a Nikon Eclipse Ti-U inverted microscope (Nikon) equipped with a Retiga EXL monochrome camera (QImaging) and the Nikon NIS Elements software (NIS-Elements Advanced Research).
FeCl3-induced thrombosis in the mesentery. (A) Images of mesenteric venules taken 13 minutes after FeCl3 injury. Platelets were labeled by infusion of fluorophore-labeled antibodies to platelet receptor GPIX. White bar = 100 μm. Dotted lines mark the vessel wall. Representative of 5 independent experiments. (B) Embolizing thrombi in clopidogrel-treated WT animals stained positive for JON/A-PE, a probe that selectively detects the activated form of αIIbβ3 integrin.23 Representative of 3 experiments. (C) Mean occlusion time in FeCl3-injured venules of mice of WT (solid black bar), WT/clopidogrel (WT + clop., solid white), and CalDAG-GEFI−/− (KO, checkered) mice (n = 7-9). Note that none of the WT/clopidogrel or CalDAG-GEFI−/− mice occluded within the 40 minutes observation period. (D) Average time required to form a first thrombus of more than 20 μm in diameter. (E) Number of emboli with a diameter of more than 20 μm forming 8-13 minutes after FeCl3 injury. Mean venule diameter: WT: 254.9 ± 19.5 μm; WT + clopidogrel: 305 ± 28.7 μm; CalDAG-GEFI−/−: 239 ± 13.7 μm; P = NS for all comparisons. All images were obtained on a Nikon Eclipse Ti-U inverted microscope (Nikon) equipped with a Retiga EXL monochrome camera (QImaging) and the Nikon NIS Elements software (NIS-Elements Advanced Research).
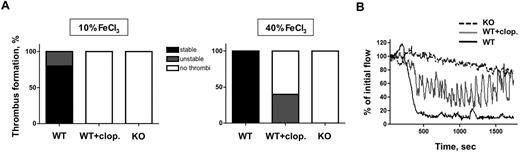
To validate our findings in a macrovascular thrombosis model, we studied the thrombotic response of CalDAG-GEFI−/− and WT/clopidogrel mice in FeCl3-injured carotid arteries. Thrombus formation in the carotid artery was detected by a reduction in the blood flow rate. Compared with WT controls, both study groups were completely protected from the formation of occlusive thrombi, both under mild (10% FeCl3) and severe (40% FeCl3) injury conditions (Figure 4A). Upon mild injury, no marked reduction in blood flow occurred in either CalDAG-GEFI−/− or clopidogrel-treated WT mice, although occlusive thrombi formed in all untreated WT mice. Upon severe injury, however, unstable thrombi were observed in 40% of the WT/clopidogrel mice but not in CalDAG-GEFI−/− mice. Characteristic flow traces showing stable occlusion in a WT mouse, unstable thrombus formation in a WT/clopidogrel mouse, and protection from thrombosis in a CalDAG-GEFI−/− mouse are shown in Figure 4B.
FeCl3-induced thrombosis in the carotid artery. (A) Percentage of mice showing stable, unstable, or no thrombi in response to exposure of the carotid artery to 10% FeCl3 for 4 minutes (left) or 40% FeCl3 for 2.5 minutes (right). n = 5 for all of the indicated study groups. (B) Flow traces recorded after exposure of the carotid artery of a WT (WT, black line), WT + clopidogrel (WT + clop., gray line), or CalDAG-GEFl−/− mouse (KO, dotted black line) to 40% FeCl3 for 2.5 minutes. Note the repeated changes in blood flow in the clopidogrel-treated WT mouse, indicative of the generation of near-occlusive thrombi followed by embolization.
FeCl3-induced thrombosis in the carotid artery. (A) Percentage of mice showing stable, unstable, or no thrombi in response to exposure of the carotid artery to 10% FeCl3 for 4 minutes (left) or 40% FeCl3 for 2.5 minutes (right). n = 5 for all of the indicated study groups. (B) Flow traces recorded after exposure of the carotid artery of a WT (WT, black line), WT + clopidogrel (WT + clop., gray line), or CalDAG-GEFl−/− mouse (KO, dotted black line) to 40% FeCl3 for 2.5 minutes. Note the repeated changes in blood flow in the clopidogrel-treated WT mouse, indicative of the generation of near-occlusive thrombi followed by embolization.
Role of CalDAG-GEFI and P2Y12 in hemostasis in mice
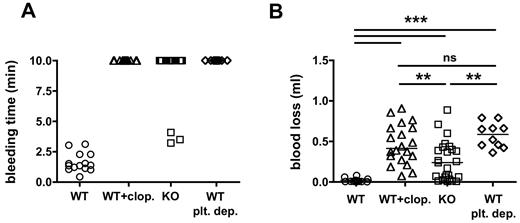
An increased risk of bleeding is the most serious side effect of antiplatelet agents like clopidogrel. To assess the bleeding tendency in mice, we subjected CalDAG-GEFI−/− and clopidogrel-treated WT mice to a tail-bleeding assay, and we compared their bleeding phenotype to that of thrombocytopenic mice (antibody-induced depletion of more than 98% of circulating platelets; Figure 5). The majority of clopidogrel-treated WT mice (20/20), CalDAG-GEFI−/− mice (22/25), and platelet-depleted WT mice (6/6) bled for the entire observation period (Figure 5A). To more quantitatively evaluate the bleeding risk in the various groups, we determined the amount of blood lost from the severed vessels (Figure 5B). Animals in all study groups lost significantly more blood than WT control mice. However, CalDAG-GEFI−/− mice lost significantly less blood than platelet-depleted or clopidogrel-treated WT mice, suggesting that signaling via P2Y12 supports the formation of hemostatically functional thrombi. In contrast, no significant difference in blood loss was observed between clopidogrel-treated and platelet-depleted WT mice.
CalDAG-GEFI–deficient mice show a similar bleeding time but a reduced amount of blood loss compared with clopidogrel-treated WT mice. (A) Tail-bleeding time in WT (circle, n = 14), clopidogrel-treated WT (WT + clop., triangle, n = 20), CalDAG-GEFI−/− (KO, square, n = 25), and platelet-depleted mice (WT plt. dep., diamond, n = 10). Three independent experiments. (B) Blood volume lost from the severed tails. Each dot represents the bleeding time or blood loss volume determined in individual mice.
CalDAG-GEFI–deficient mice show a similar bleeding time but a reduced amount of blood loss compared with clopidogrel-treated WT mice. (A) Tail-bleeding time in WT (circle, n = 14), clopidogrel-treated WT (WT + clop., triangle, n = 20), CalDAG-GEFI−/− (KO, square, n = 25), and platelet-depleted mice (WT plt. dep., diamond, n = 10). Three independent experiments. (B) Blood volume lost from the severed tails. Each dot represents the bleeding time or blood loss volume determined in individual mice.
Role of the regulatory C1 domain for CalDAG-GEFI function
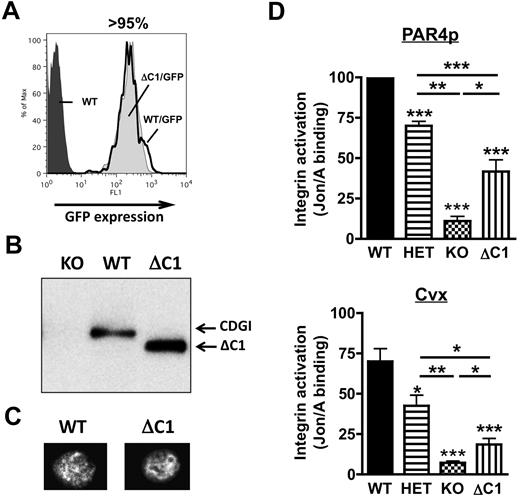
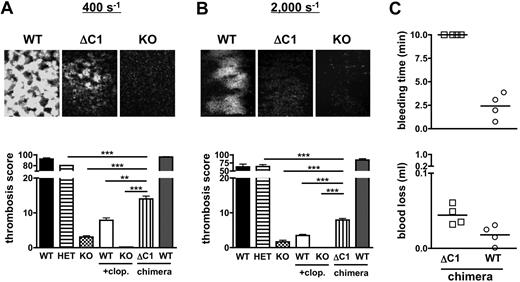
As part of ongoing structure-function studies on CalDAG-GEFI, we generated chimeric mice expressing CalDAG-GEFI that lacks the C1 regulatory domain (CalDAG-GEFIΔC1) in circulating blood cells. Chimeric mice were generated by retroviral transduction of bone marrow cells and subsequent transplant of the transduced cells into lethally irradiated mice. As evaluated by flow cytometry, > 95% of circulating platelets in the chimeric mice (both WT/GFP, expressing intact CalDAG-GEFI, and ΔC1/GFP, expressing CalDAG-GEFIΔC1) showed similar levels of GFP (Figure 6A) and stained positive for CalDAG-GEFI (not shown). Compared with WT controls, CalDAG-GEFIΔC1 platelets expressed a lower molecular weight CalDAG-GEFI protein, confirming the deletion of the C1 domain in these platelets (Figure 6B). Immunofluorescence studies demonstrated cytosolic expression for both CalDAG-GEFIΔC1 and intact CalDAG-GEFI in resting platelets (Figure 6C). To evaluate the ability of CalDAG-GEFIΔC1 to support αIIbβ3 activation in platelets, we studied JON/A-PE23 binding to WT, CalDAG-GEFI−/−, CalDAG-GEFI+/−, and CalDAG-GEFIΔC1 platelets stimulated in the presence of the P2Y12 inhibitor, 2-MesAMP. αIIbβ3 activation in CalDAG-GEFIΔC1 platelets was significantly stronger than in CalDAG-GEFI−/− platelets but weaker than that observed in CalDAG-GEFI+/− platelets (ie, in platelets with a 50% reduction in CalDAG-GEFI expression; Figure 6D). CalDAG-GEFI–dependent αIIbβ3 activation in CalDAG-GEFIΔC1 platelets activated via the thrombin receptor PAR4 (PAR4p) or the collagen receptor glycoprotein VI (convulxin) was reduced by 60%-70% compared with WT controls. This defect in integrin activation also led to impaired platelet aggregation in standard aggregometry (supplemental Figure 1). In the flow chamber assay, adhesion of CalDAG-GEFIΔC1 platelets at venous (400 s−1) and arterial shear rates (2000 s−1) was significantly increased compared with CalDAG-GEFI−/− or WT/clopidogrel platelets (Figure 7A-B). However, 3-dimensional thrombi were only observed in CalDAG-GEFIΔC1 blood perfused under venous shear stress. To determine the pathophysiological impact of reduced CalDAG-GEFI function, we evaluated the bleeding time and the blood loss volume in CalDAG-GEFIΔC1 mice (Figure 7C). While the chimeric mice still bled for the entire observation period, expression of CalDAG-GEFIΔC1 limited the blood loss to levels only slightly higher than those observed in WT chimeras. In contrast, thrombus formation under flow (Figure 7A-B) and the tail-bleeding time14 were normal in CalDAG-GEFI+/− mice.
Reduced integrin activation in platelets expressing CalDAG-GEFIΔC1. (A) Representative flow cytometric analysis of the GFP expression in platelets from chimeric mice expressing intact CalDAG-GEFI (WT/GFP, solid black line) or CalDAG-GEFIΔC1 (ΔC1/GFP, light gray shaded area) in comparison to WT control platelets (dark gray shaded area). (B) Western blot analysis of CalDAG-GEFI expression in CalDAG-GEFI−/− (KO), WT, and CalDAG-GEFIΔC1 (ΔC1) platelets. Mutant CalDAG-GEFI was detected at a lower molecular weight due to the deletion of the C1 domain (49 amino acids≈5.5 kDa). (C) Immunofluorescence staining for CalDAG-GEFI in WT and CalDAG-GEFIΔC1 (ΔC1) platelets. Results shown in panels A-C are representative of 3 independent experiments. (D) αIIbβ3 integrin activation (JON/A-PE binding23 ) was determined in WT (solid bars), CalDAG-GEFI−/− (KO, checkered), CalDAG-GEFIΔC1 (ΔC1, vertically striped), or CalDAG-GEFI+/− (HET, horizontally striped) platelets activated with PAR4 peptide (1mM, top) or convulxin (Cvx, 750 ng/mL, bottom) in the presence of the P2Y12 inhibitor, 2-MesAMP. n = 6, 3 independent experiments.
Reduced integrin activation in platelets expressing CalDAG-GEFIΔC1. (A) Representative flow cytometric analysis of the GFP expression in platelets from chimeric mice expressing intact CalDAG-GEFI (WT/GFP, solid black line) or CalDAG-GEFIΔC1 (ΔC1/GFP, light gray shaded area) in comparison to WT control platelets (dark gray shaded area). (B) Western blot analysis of CalDAG-GEFI expression in CalDAG-GEFI−/− (KO), WT, and CalDAG-GEFIΔC1 (ΔC1) platelets. Mutant CalDAG-GEFI was detected at a lower molecular weight due to the deletion of the C1 domain (49 amino acids≈5.5 kDa). (C) Immunofluorescence staining for CalDAG-GEFI in WT and CalDAG-GEFIΔC1 (ΔC1) platelets. Results shown in panels A-C are representative of 3 independent experiments. (D) αIIbβ3 integrin activation (JON/A-PE binding23 ) was determined in WT (solid bars), CalDAG-GEFI−/− (KO, checkered), CalDAG-GEFIΔC1 (ΔC1, vertically striped), or CalDAG-GEFI+/− (HET, horizontally striped) platelets activated with PAR4 peptide (1mM, top) or convulxin (Cvx, 750 ng/mL, bottom) in the presence of the P2Y12 inhibitor, 2-MesAMP. n = 6, 3 independent experiments.
Thrombosis and hemostasis in mice expressing CalDAG-GEFIΔC1 in circulating blood cells. (A-B) Platelet adhesion to collagen at 400 s−1 (A) or 2000 s−1 (B). (Top) Representative images (KO: CalDAG-GEFI−/−, ΔC1: CalDAG-GEFIΔC1). All images were obtained on a Nikon Eclipse Ti-U inverted microscope (Nikon) equipped with a Retiga EXL monochrome camera (QImaging) and the Nikon NIS Elements software (NIS-Elements Advanced Research). (Bottom)Thrombosis score (fluorescence intensity multiplied with area coverage after 5 minutes of perfusion) for the indicated genotypes (WT: wild-type; HET: CalDAG-GEFI+/−; KO: CalDAG-GEFI−/−; ΔC1: CalDAG-GEFIΔC1 chimera). n = 5-6, 3 independent experiments. (C) Bleeding time and blood loss volume determined in WT/GFP (circle) or CalDAG-GEFIΔC1/GFP (ΔC1/GFP, square) chimeric mice.
Thrombosis and hemostasis in mice expressing CalDAG-GEFIΔC1 in circulating blood cells. (A-B) Platelet adhesion to collagen at 400 s−1 (A) or 2000 s−1 (B). (Top) Representative images (KO: CalDAG-GEFI−/−, ΔC1: CalDAG-GEFIΔC1). All images were obtained on a Nikon Eclipse Ti-U inverted microscope (Nikon) equipped with a Retiga EXL monochrome camera (QImaging) and the Nikon NIS Elements software (NIS-Elements Advanced Research). (Bottom)Thrombosis score (fluorescence intensity multiplied with area coverage after 5 minutes of perfusion) for the indicated genotypes (WT: wild-type; HET: CalDAG-GEFI+/−; KO: CalDAG-GEFI−/−; ΔC1: CalDAG-GEFIΔC1 chimera). n = 5-6, 3 independent experiments. (C) Bleeding time and blood loss volume determined in WT/GFP (circle) or CalDAG-GEFIΔC1/GFP (ΔC1/GFP, square) chimeric mice.
Discussion
The present study was based on our recent observations showing that synergistic but independent signaling by CalDAG-GEFI and P2Y12 leads to Rap1-dependent platelet activation. While both pathways are able to induce Rap1 activation and platelet aggregation in vitro, we hypothesized that the different kinetics with which they mediate Rap1 activation would translate to marked differences in thrombus growth and stability in vivo. Our data demonstrate delayed platelet adhesion and formation of thrombi in CalDAG-GEFI–deficient mice compared with WT, confirming that CalDAG-GEFI is crucial for the near-immediate activation of Rap1 and αIIbβ3 in circulating platelets (Figures 1,Figure 2,Figure 3–4). In agreement with this observation, we also detected that thrombus formation in the absence of CalDAG-GEFI was more prevalent in venules than in arterioles (ie, under less stringent low shear conditions). In contrast, thrombi in WT mice treated with clopidogrel formed with similar kinetics to those in untreated WT mice, both in arterioles and venules. Impaired P2Y12 signaling, however, led to frequent embolization of growing thrombi, a phenotype that was not observed in CalDAG-GEFI−/− mice.
Our recent studies demonstrated that CalDAG-GEFI plays a critical role for various aspects of platelet activation, including integrin activation and the generation and release of autocrine mediators such as TxA2 and ADP.11,12,14 To evaluate if CalDAG-GEFI−/− mice are protected from thrombosis due to the defect in the release of second wave mediators, we studied their adhesion in the presence of exogenous ADP and TxA2. While addition of exogenous mediators significantly increased the adhesion of CalDAG-GEFI−/− platelets to a collagen surface, particularly when blood was perfused at venous shear rates, it did not restore growth of 3-dimensional thrombi (Figure 1B-C). Thus, our data suggest that the delayed kinetics of integrin activation rather than the defect in the release of autocrine mediators is the main reason for the marked antithrombotic phenotype of CalDAG-GEFI−/− platelets.
We also observed significantly better hemostasis in CalDAG-GEFI−/− mice compared with clopidogrel-treated WT mice (Figure 5). While nearly all CalDAG-GEFI−/− mice bled for the entire observation period, > 40% of these mice lost only marginally more blood than WT controls. Both platelet-depleted and clopidogrel-treated WT mice lost significantly more blood than CalDAG-GEFI−/− mice. It is likely that stable mural thrombi forming in venules of CalDAG-GEFI−/− mice support limited hemostasis. Confirming this hypothesis, interventions that predominantly inhibit thrombosis under arterial shear conditions, such as inhibitors of the GPIb–von Willebrand factor interaction, do not markedly affect hemostasis in laboratory animals or humans.31-34
Based on these studies, we propose inhibition of CalDAG-GEFI (or other molecules that are important for the first phase of αIIbβ3 activation) as a new strategy for antiplatelet therapy. CalDAG-GEFI plays a unique and critical role downstream of all agonist receptors that stimulate the activation of phospholipase C. Thus, inhibition of 1 molecule will affect multiple activation pathways. Our studies in mice demonstrate that inhibition of CalDAG-GEFI will provide better protection against thrombosis than inhibitors of P2Y12, especially under arterial shear conditions such as found in the setting of athero-thrombosis. We acknowledge the difficulty of extrapolating results from mouse models of thrombosis to human athero-thrombosis. However, we have used various microvascular and macrovascular thrombosis models, which all demonstrated virtually complete inhibition of arterial thrombosis in CalDAG-GEFI−/− mice. Clopidogrel treatment also protected mice against the formation of occlusive thrombi. In concordance with previously published results,10 it did not abolish thrombus growth but significantly impaired thrombus stabilization. In fact, some of the fast growing thrombi observed in WT/clopidogrel mice grew large enough to affect blood flow in the injured carotid artery (Figure 4), suggesting that similar near-occlusive events may occur on a ruptured atherosclerotic plaque in clopidogrel-treated humans. Thus, intervention in the first phase of Rap1-dependent integrin activation, mediated by CalDAG-GEFI, may be superior to inhibition of sustained activation of Rap1/αIIbβ3.
Its restricted expression profile is another favorable factor for CalDAG-GEFI as a target for antiplatelet therapy. Compared with other critical platelet signaling molecules such as phosphoinositide 3-kinase,35 Rap1,36 talin,37,38 or kindlin-3,39 which are abundantly expressed in many cell types, CalDAG-GEFI is predominantly expressed in platelets/megakaryocytes and neutrophils in the hematopoietic system as well as certain neurons in the brain.13,14 Although CalDAG-GEFI is potentially important for motor control,40 no brain-related abnormalities have been reported for the knockout mice. In our own studies, we showed impaired integrin activation in neutrophils from CalDAG-GEFI−/− mice,30 resulting in protection of the mice in models of inflammatory disease. Neutrophil extravasation, however, was only partially impaired in CalDAG-GEFI−/− mice, suggesting that an alternative pathway(s) can support neutrophil integrin activation in the absence of CalDAG-GEFI. Thus, CalDAG-GEFI inhibition may provide strong antithrombotic protection without major side effects.
To prevent thrombosis yet minimize therapy-related bleeding complications, partial inhibition of a critical player of platelet activation may be required. In this study, we provide the first evidence that targeting the regulatory C1 domain within CalDAG-GEFI may be a unique means to reduce but not abolish its function, independent of the dose of antagonist administered. Using a retroviral approach,22 we generated chimeric mice expressing CalDAG-GEFIΔC1 in blood cells only (Figures 6–7). Deletion of the C1 regulatory domain reduced CalDAG-GEFI–dependent αIIbβ3 activation by approximately 70%, resulting in significantly impaired thrombus formation under low and high shear conditions. In the tail bleed assay, mice expressing CalDAG-GEFIΔC1 still bled for the entire observation period, but the amount of blood loss in all tested chimeras was only marginally higher than in WT controls. In contrast, a 50% reduction in CalDAG-GEFI expression did not affect platelet adhesion to collagen and hemostasis in mice. This is the first report demonstrating an important role of the C1 domain for CalDAG-GEFI–mediated platelet activation. We currently do not know how the C1 domain contributes to CalDAG-GEFI function. Previous studies in cell lines demonstrated that the C1 domain in CalDAG-GEFI is atypical and thus binds DAG with very low affinity.16,41,42 C1 domains, however, are also known as protein interaction modules.43 Their binding partners include adapter proteins such as 14-3-344 and Ras guanosine triphosphtases,45 both of which are important in integrin inside-out activation. Further studies are required to elucidate the underlying mechanisms.
In summary, our studies are the first to characterize how differences in the kinetics of integrin activation, as observed in CalDAG-GEFI−/− or clopidogrel-treated WT mice, affect the growth and stability of pathophysiological platelet-rich thrombi. Based on these studies, we propose that targeting CalDAG-GEFI, a critical player in the first phase of integrin activation, has the potential to advance antiplatelet therapy in multiple ways. First, CalDAG-GEFI inhibition will have an antithrombotic effect similar to or greater than that achieved with P2Y12 inhibitors like clopidogrel but weaker than that of inhibitors of αIIbβ3 integrin. Second, the antithrombotic effect of CalDAG-GEFI inhibitors will be more profound under arterial than venous shear conditions. Third, CalDAG-GEFI–independent but P2Y12-dependent platelet activation will allow for the formation of hemostatically active thrombi in venules. Lastly, targeting one of the regulatory domains within CalDAG-GEFI, such as the C1-like domain, will potentially further improve the safety profile of such a new therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mark Kahn for support with retroviral technology, Constantine Daskalakis for help with the statistical analysis of data, Jill Crittenden and Ann Graybiel for providing CalDAG-GEFI cDNA and knockout mice, and the members of the Platelet Interest Group at TJU for helpful discussions and support.
This work was supported by the American Heart Association (SDG0630044N, W.B.), the American Society of Hematology (W.B.), and National Heart, Lung, and Blood Institute, National Institutes of Health grants R01 HL094594 (W.B.), R01 HL056621 (S.L.D.), R01 HL081241 (D.S.W.), and T32HL007971 (S.F.M.).
National Institutes of Health
Authorship
Contribution: M.S. and L.S. designed the study, performed most of the experiments, and wrote the paper; R.C.R. and T.D.O. maintained the mouse colony and helped with experiments; M.C., J.H., T.G., and S.F.M. performed experiments; S.L.D., M.P., and D.S.W. wrote the manuscript; and W.B. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang Bergmeier, 1015 Walnut St, 702 Curtis, Thomas Jefferson University, Philadelphia, PA 19107; e-mail: wolfgang.bergmeier@jefferson.edu.
References
Author notes
M.S. and L.S. contributed equally to this study.

![Figure 1. Platelet adhesion to fibrillar collagen under physiological flow conditions. (A) Whole blood from WT (WT, black lines and bars), CalDAG-GEFI−/− (knockout [KO], red), clopidogrel-treated WT (WT + clop., blue), or clopidogrel-treated KO (KO + clop., green) mice was perfused over collagen at arterial (2000 s−1, left) or venous (400 s−1, right) shear conditions. Platelets in whole blood were labeled with Alexa488-labeled antibodies to GPIX before perfusion. The top graphs represent time traces of the mean fluorescence intensity ± SEM expressed as a percentage of the maximal fluorescence observed (WT blood, 400 s−1). The bar graphs show the area coverage by fluorescent platelets after 5 minutes of blood perfusion, expressed as percentage of the collagen-coated area. Data are shown as mean ± SEM (n = 4-6, 3 independent experiments). *P < .05, **P < .01, ***P < .001. See supplemental Videos 1 to 4 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. (B-C) Effect of exogenous ADP and TxA2 (U46619) on the adhesion of CalDAG-GEFI−/− platelets. WT and CalDAG-GEFI−/− (KO) whole blood was perfused over collagen at 400 s−1 or 2000 s−1 in the presence (KO + ADP/U46) or absence (KO) of exogenous ADP (25μM) and U46619 (5μM). (B) Bar graphs for area coverage (top) and fluorescence intensity (bottom) measured after 5 minutes of perfusion with the following blood samples: WT (black bar), KO (red bar), and KO reconstituted with 25μM ADP and 5μM U46619 (KO + ADP/U46, red checkered bar). Data are shown as mean ± SEM (n = 5, 3 independent experiments). *P < .05; **P < .01; ***P < .001. (C) Representative images. Images were obtained after 5 minutes of perfusion on a Nikon Eclipse Ti-U inverted microscope (equipped with a Retiga EXL monochrome camera [QImaging] and Nikon NIS Elements software [NIS-Elements Advanced Research]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/3/10.1182_blood-2010-07-297713/4/m_zh89991064170001.jpeg?Expires=1769085690&Signature=LpWn32wLGvtqRqL8VaHAQZOZDzIREiFrlB~2ej2GQ8w62RHalmi8QBfHD0Hi6MqIJ7QqeO89Ak9qqqEPs6Of67nn0TXH1DXHQEn2tee-HCCBJT64ZPNjHgfizGDe5lLNCzkqmlYL3-oufFI5GLjwhrzUfKm0sGTE6Tpx9DD8glZMQyd722zsgxNwbAedGqXws7pT3yn7z3zu1Hqp4azTGxipgkC3t8818pXC8VgqyGUq2zylaay9Vxm~n5FLrIn7~BPolXWWVvrDo0-nxQ4mAh2-3IpZA12XfL44FBvEoEsDxmZdC3TbALNOZc~OO-or5~gXNacpwg4Yzaae3c0aFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal