Abstract
The evolutionarily conserved immune system of the zebrafish (Danio rerio), in combination with its genetic tractability, position it as an excellent model system in which to elucidate the origin and function of vertebrate immune cells. We recently reported the existence of antigen-presenting mononuclear phagocytes in zebrafish, namely macrophages and dendritic cells (DCs), but have been impaired in further characterizing the biology of these cells by the lack of a specific transgenic reporter line. Using regulatory elements of a class II major histocompatibility gene, we generated a zebrafish reporter line expressing green fluorescent protein (GFP) in all APCs, macrophages, DCs, and B lymphocytes. Examination of mhc2dab:GFP; cd45:DsRed double-transgenic animals demonstrated that kidney mhc2dab:GFPhi; cd45:DsRedhi cells were exclusively mature monocytes/macrophages and DCs, as revealed by morphologic and molecular analyses. Mononuclear phagocytes were found in all hematolymphoid organs, but were most abundant in the intestine and spleen, where they up-regulate the expression of inflammatory cytokines upon bacterial challenge. Finally, mhc2dab:GFP and cd45:DsRed transgenes mark mutually exclusive cell subsets in the lymphoid fraction, enabling the delineation of the major hematopoietic lineages in the adult zebrafish. These findings suggest that mhc2dab:GFP and cd45:DsRed transgenic lines will be instrumental in elucidating the immune response in the zebrafish.
Introduction
In recent years, the zebrafish (Danio rerio) has proven to be a unique vertebrate model for the study of hematopoiesis.1 The use of the zebrafish to study the ontogeny of leukocyte subsets,2 immune cell migration,3,4 and host-pathogen interactions5 has provided new insights into our understanding of innate immunity in the developing vertebrate embryo. A major focus of previous studies was on the neutrophil response, because several transgenic reporter lines have been generated that mark this granulocyte subset. Whereas neutrophils generally constitute the first line of defense against invading pathogens, the role of other immune cell subsets in the innate immune response has received less attention. In addition to neutrophils, macrophages are key in the response to pathogen challenge. In the zebrafish embryo, primitive macrophages have been demonstrated to be capable of clearing injected bacteria by phagocytosis.6 However, the absence of markers specific to macrophages has limited the study of this myeloid cell subset in the zebrafish.
The mononuclear phagocyte system (MPS) comprises monocytes, tissue macrophages, and dendritic cells (DCs), as well as their lineage-committed progenitors.7 The primary function of mature MPS cells is the clearance of pathogens by phagocytosis. This activity is crucial during immune challenge to clear invasive pathogens. Mononuclear phagocytes also play an important role in the removal of apoptotic cell corpses, especially during embryonic development. In mice, embryonic macrophages colonize several structures to be removed during development, including interdigital tissues, the hyaloid vasculature, and pupillary membranes.8,9 In addition, macrophages residing in hematopoietic tissues support erythroblast proliferation and differentiation and engulf dying erythrocytes in the spleen.10,11 The capacity to engulf a wide array of particles relies on the existence of different surface receptors, such as scavenger receptors (Marco; CD163) or TLRs that recognize pathogen-associated molecular patterns.12,13 After phagocytosis, macrophages and DCs can activate antigen-specific T lymphocytes, a process dependent on MHCII molecules.
Monocytes and macrophages have been identified in zebrafish based on morphology, cytochemistry, and gene expression.1 During development, the first macrophages originate from the anterior lateral plate mesoderm at approximately 20 hours postfertilization (hpf) and migrate over the yolk ball before colonizing other tissues.6,14 These primitive macrophages can be visualized in vivo using a variety of transgenic lines in which green fluorescent protein (GFP) expression is driven by myeloid-specific promoter sequences, including pu.1:eGFP, lyz:eGFP, and mpx:eGFP.15–19 However, these promoters are only specific to macrophages during a short, defined window of embryogenesis; each is also subsequently expressed in granulocytic cells, precluding the prospective isolation of mononuclear phagocytes after 48 hpf. Although a novel macrophage-specific reporter line was recently generated using the mpeg1 promoter, it appears that the transgene is active only in the embryo and larvae, precluding its use for the study of macrophages in adult fish.20 Another important subset of the MPS is the DC. DCs are professional APCs and constitute a rare leukocyte population in mammals. We and others recently described the identification of DCs in adult zebrafish and medaka (Oryzias latipes).21,22 Zebrafish DCs, detected through their phagocytic ability and further enriched by their affinity for the lectin peanut agglutinin, displayed morphologic and cytochemical characteristics reminiscent of those exhibited by their mammalian counterparts. However, the presence of a high proportion of immature myeloid cells within peanut agglutinin–positive cells demonstrated the need for improved enrichment strategies.22
To address these issues and to fully characterize the MPS of zebrafish, we generated a transgenic line that specifically marks APCs using the regulatory elements from the MHCII dab gene to drive GFP. We demonstrate that, when used in combination with a previously generated cd45:DsRed transgenic animal (also referred to as ptprc:DsRed), the mhc2dab:GFP transgenic line can be used to identify and isolate a pure population of mononuclear phagocytes. This has enabled characterization of gene expression, tissue distribution, and functional properties of monocytes, macrophages, and DCs in the zebrafish. We also show that, when combined with light-scattering characteristics, the expression of the mhc2dab:GFP and cd45:DsRed transgenes delineates each of the major hematopoietic lineages in the adult zebrafish.
Methods
Zebrafish strains and maintenance
Generation of Tg(mhc2dab:GFP-LT)sd6 and Tg(mhc2dab:mCherry)sd7 transgenic animals
A 3.8-kb fragment upstream of the mhc2dab transcriptional start site was amplified from the bacmid AL928944 using the following primers: FP-GAGCGGCCGCCTTAGTGTATGTACGAGTGTATAGATGTTTCCC and RP-GAGGATCCGAGTCTTTGAATGTGTCAAATGAAGAACTTTC (with 5′-NotI and 3′-BamHI cloning sites added [underlined sequences]). This fragment was cloned into GFP-LT/Tol2 and mCherry/Tol2 vectors, and the resulting constructs were coinjected with transposase mRNA into zygotes to generate transgenic founders.25 Throughout the text, transgenic animals are referred to without allele designations for clarity.
Fluorescent microscopy
Transgenic lines were imaged using an inverted confocal microscope (SP5; Leica). GFP and DsRed were excited by 488- and 543-nm laser lines, respectively.
Flow cytometry
Hematopoietic cells isolated from adult organs were processed as described previously.26 Sytox Red (Invitrogen) was used to exclude dead cells and debris. FACS was performed using a FACSAria flow cytometer (Becton Dickinson), flow cytometry was performed with an LSRII flow cytometer (Becton Dickinson), and data analyses were performed using FlowJo Version 9.2 software (TreeStar). For in vivo phagocytosis assays, 2 μg of pHrodo E. coli BioParticles (Molecular Probes) was injected intravenously in mhc2dab:GFP adult fish. Splenocytes were collected 18 hours after injection for analysis.
Real-time quantitative PCR
For real-time quantitative PCR (Q-PCR) analyses, RNA was isolated from sorted cells using the RNeasy Kit (QIAGEN), and cDNA was obtained using qScript cDNA Supermix (Quanta BioSciences). Q-PCR was performed with the Mx3000P System (Stratagene) according to the manufacturer's instructions. Each sample was tested in duplicate. For each independent experiment, elongation-factor 1-α (ef1a) expression was scored for each population. The signals detected for each transcript were normalized to ef1a, and data were analyzed by the ΔΔCt method according to the manufacturer's recommended protocol (Stratagene). Primers are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Generation of the mhc2dab:GFP reporter line
Our previous studies revealed the presence of APCs, including DC cells, in adult zebrafish.22 Because further biologic characterization has been hindered by our inability to specifically mark these cells, we speculated that the promoters of genes orthologous to mammalian APC-specific genes would constitute potential candidates for the generation of novel zebrafish transgenic lines marking all APC populations. Among others, MHCII genes are required for antigen presentation and T-cell activation and are constitutively expressed by APCs.27 The structure of MHCII proteins is highly conserved among vertebrates. Each MHCII molecule is a heterodimer composed of an α and a β chain. In mice, 4 class II genes, Aα, Aβ, Eα, and Eβ, pair to form the classic MHCII molecules I-A and I-E, respectively. The human MHCII locus is more complex and encodes α and β chains for HLA-DP, HLA-DQ, and HLA-DR. Understanding the genomic organization of the MHC locus in zebrafish has been complicated by the fact that, unlike in mammals, it appears to be fragmented,28 a trait shared among other teleosts, including trout, stickleback, guppy, and cichlids.29 Consequently, the exact number of MHC genes in the zebrafish is unknown. One class II α (mhc2daa) and 6 class II β (mhc2dab to mhc2dfb) genes have been identified by genomic library screens.30,31 Most of these appear to be pseudogenes, however, because only the mhc2daa and mhc2dab genes are expressed.31 Preliminary analysis of the zebrafish mhc2dab gene suggested that it might be expressed in APCs, because robust expression was observed in adult organs relevant to immunologic function, such as the thymus, gut, and spleen (data not shown).
To generate a transgenic zebrafish line that specifically marks APCs, we analyzed the upstream regulatory elements of the mhc2dab gene. In mammals, mhc2 expression is regulated at the transcriptional level by the class II transactivator, which is recruited to the mhc2 promoter by proteins selectively bound to the X1, X2, and Y promoter sequences that are required for optimal constitutive and cytokine-induced expression in both humans and mice.27 Analysis of the zebrafish mhc2dab proximal promoter revealed the presence of these conserved elements within a 4-kb fragment upstream of the transcriptional start site (data not shown). This 4-kb regulatory domain of the mhc2dab gene was cloned upstream of GFP-LT and flanked by Tol2 recombination sequences. The mhc2dab:GFP construct was injected into zygotes and the resulting potential founders were raised to maturity and mated to produce F1 embryos.
Characterization of mhc2dab:GFP transgenic embryos
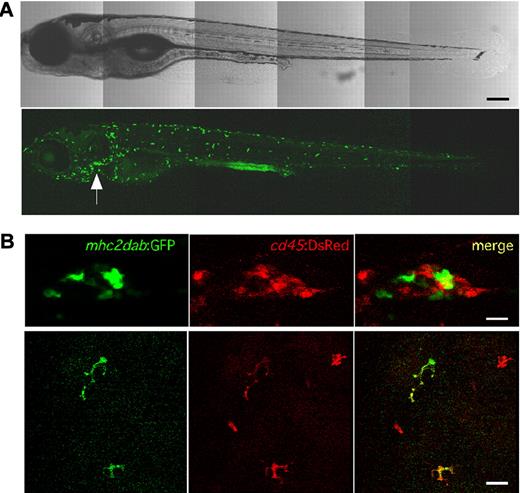
Transgenic progeny of mhc2dab:GFP founders were identified by fluorescent microscopy, in which reporter gene expression was first observed at 5 days postfertilization (dpf) within the thymus and 4 days later in scarce cells within the skin (data not shown). Widespread transgene expression was not observed until after 12 dpf, when GFP+ cells increased in number and became brightly fluorescent (Figure 1A). To assess which mhc2dab:GFP+ cells were of hematopoietic origin, mhc2dab:GFP animals were mated to cd45:DsRed transgenic fish, which we described previously to label definitive blood cells at 72 hpf.23 Confocal analyses performed on cd45:DsRed; mhc2dab:GFP double-transgenic animals at 12 dpf showed that most thymic mhc2dab:GFP+ cells did not express the hematopoietic-specific cd45:DsRed transgene (Figure 1B top panel). This finding, along with the morphology and expression of the forkhead box N1 and E-cadherin genes, suggests that early thymic mhc2dab+ cells are thymic epithelial cells (supplemental Figure 1), a result that is consistent with observations in mammals.27 Interestingly, both cd45:DsRed and mhc2dab:GFP expression colocalized to stellate cells within the skin (Figure 1B bottom panel), suggesting that the mhc2dab promoter also marked APCs derived from the blood-forming system. Fluorescent microscopic analyses demonstrated persistent transgene expression in thymic epithelial cells and skin leukocytes throughout adulthood. However, at approximately 45 dpf, GFP expression also became evident in keratinocytes (data not shown). This may have resulted from a lack of regulatory sequences in the 4-kb mhc2 promoter or it may accurately reflect expression in epithelial cells, which can serve as “nonprofessional” APCs in mammals.27 Epithelial GFP expression did not impair the ability to visualize GFPhi skin leukocytes by confocal imaging.
Expression of the mhc2dab:GFP and cd45:DsRed transgenes during zebrafish development. (A) Lateral view of a 14-dpf mhc2dab:GFP transgenic fish showing reporter expression in cells scattered throughout the skin and in the thymus (arrowhead). The scale bar indicates 200μm. (B) Thymic (top panel) and skin (bottom panel) reporter expression in a double cd45:DsRed; mhc2dab:GFP transgenic fish at 12 dpf. For each location, the GFP (left), DsRed (middle), and merged (right) images are shown. The scale bar indicates 20μm.
Expression of the mhc2dab:GFP and cd45:DsRed transgenes during zebrafish development. (A) Lateral view of a 14-dpf mhc2dab:GFP transgenic fish showing reporter expression in cells scattered throughout the skin and in the thymus (arrowhead). The scale bar indicates 200μm. (B) Thymic (top panel) and skin (bottom panel) reporter expression in a double cd45:DsRed; mhc2dab:GFP transgenic fish at 12 dpf. For each location, the GFP (left), DsRed (middle), and merged (right) images are shown. The scale bar indicates 20μm.
Mhc2dab expression was not observed earlier during embryogenesis, particularly within primitive macrophages specified by 20 hpf.6 Analysis of cd45:DsRed animals between 24 and 28 hpf showed transgene expression in hematopoietic cells on the yolk ball and within surrounding tissues, including the retina and brain (supplemental Figure 2). Based on their tissue distribution and behavior, these cells likely represent primitive macrophages.6 DsRed+ cells were purified by flow cytometry at 24 hpf and analyzed for gene expression by Q-PCR. Sorted cells expressed high levels of canonical macrophage-associated genes, including csf1r, mpeg1, and marco (data not shown). In contrast, the expression of mhc2dab and mhc2daa was undetectable. These findings are consistent with murine embryonic macrophages.32 Although highly phagocytic, macrophages in the early mammalian embryo do not appear to be capable of antigen presentation via MHCII molecules; rather, it appears that these cells exist primarily to remodel developing tissues,7,8 serve as immune sentries,6 and seed nascent sites of the MPS, including microglia of the brain, with phagocytes.33
Expression of mhc2dab:GFP in the adult animal
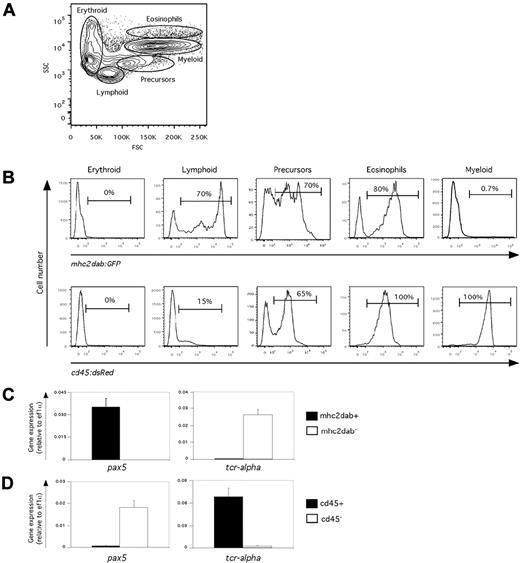
Our initial results indicated that the mhc2dab:GFP transgenic line marked developing APCs in the zebrafish embryo, making it a suitable tool for the study of APC ontogeny and early function. To assess the potential of our transgenic line for immunobiologic investigations in adult animals, we examined the persistence of transgene expression in 3-month-old mhc2dab:GFP animals using flow cytometry. Because all hematopoietic lineages are produced in the adult kidney, we analyzed whole kidney marrow (WKM). Using light-scattering characteristics, we and others have previously shown that each of the major hematopoietic lineages segregate into unique subsets.26,34 Figure 2A shows the gates used to discriminate between erythroid, lymphoid, precursor, myeloid, and eosinophilic cells in the WKM. In the mammalian hematopoietic system, expression of MHCII proteins is mainly confined to B lymphocytes, macrophages, and DCs.27 Whereas no expression was observed in erythroid cells, 70% of kidney lymphoid cells expressed the mhc2dab:GFP transgene (Figure 2B top panels). To determine the cell types marked by the mhc2dab:GFP transgene, we purified GFP+ and GFP− subsets from the lymphoid fraction and surveyed each for B- and T-lymphocyte markers. Lymphoid mhc2dab:GFP+ cells specifically expressed pax5, a master regulator of the B-cell lineage,35 whereas expression of the T-cell receptor gene tcr-a was restricted to the mhc2dab:GFP− fraction (Figure 2C). These results suggested that B cells, but not T cells, are marked with the mhc2dab:GFP transgene. Within the precursor fraction, mhc2dab:eGFP marked approximately 70% of cells. Purified mhc2dab:GFP+ precursor cells expressed both B-cell and myeloid genes, suggesting that precursors of both lineages are present in this fraction. Within the eosinophil gate, approximately 80% of cells were mhc2dab:GFP+, which is consistent with our previous observation that gata-2:eGFPhigh eosinophils expressed high levels of mhc2dab36 and with studies showing MHCII expression in mammalian eosinophils.37 Finally, within the myeloid scatter fraction, < 1% of cells expressed mhc2dab:GFP. Because the majority of cells found within this gate are neutrophils, we crossed mhc2dab:GFP animals with the neutrophil-specific lyz:DsRed line to more precisely assess the lineal affiliation of mhc2dab. Expression of these 2 transgenes was largely mutually exclusive, demonstrating that neutrophils do not express the mhc2dab:GFP transgene (supplemental Figure 3). Our results demonstrate that the mhc2dab promoter is active in adult zebrafish, driving expression in B lymphocytes, eosinophils, and a minor population of myeloid cells that is distinct from neutrophils.
Expression of the mhc2dab:GFP and cd45:DsRed transgenes in adult WKM. (A) Gating strategy to isolate the main hematopoietic lineages—erythroid, lymphoid, precursor, myeloid, and eosinophils—using light-scattering characteristics (B) For each gate, the expression of mhc2dab:GFP (top panels) and cd45:DsRed (bottom panels) is presented as a histogram plot; percentages are indicated. (C-D) Gene expression in mhc2dab:GFP (C) and cd45:DsRed (D) kidney lymphoid cells examined for the presence of B-cell (pax5) and T-cell (tcr-a)–specific transcripts. Units on the y-axis represent transcript expression normalized to ef1a transcript levels. Error bars indicate SD.
Expression of the mhc2dab:GFP and cd45:DsRed transgenes in adult WKM. (A) Gating strategy to isolate the main hematopoietic lineages—erythroid, lymphoid, precursor, myeloid, and eosinophils—using light-scattering characteristics (B) For each gate, the expression of mhc2dab:GFP (top panels) and cd45:DsRed (bottom panels) is presented as a histogram plot; percentages are indicated. (C-D) Gene expression in mhc2dab:GFP (C) and cd45:DsRed (D) kidney lymphoid cells examined for the presence of B-cell (pax5) and T-cell (tcr-a)–specific transcripts. Units on the y-axis represent transcript expression normalized to ef1a transcript levels. Error bars indicate SD.
We similarly analyzed expression of the cd45:DsRed transgene to further refine our studies. Whereas no expression was observed in erythrocytes, 100% of cells within the myeloid and eosinophil gates expressed high levels of DsRed (Figure 2B bottom panels); however, only 65% and 15% of precursor and lymphoid cells, respectively, expressed the DsRed transgene. Based on the presence of erythrocyte precursors, we did not expect the cd45:DsRed transgenic line to mark the entire precursor fraction.26,34 However, it was surprising that only a subset of lymphoid cells was positive. We therefore sorted DsRed-positive and DsRed-negative cells from the lymphoid fraction for gene-expression analysis. Q-PCR showed that lymphoid cd45:DsRed+ cells expressed high levels of the tcr-a gene but were negative for pax5 (Figure 2D). Purified lymphoid cd45:DsRed− cells showed the opposite expression pattern. These results demonstrate that the cd45:DsRed reporter line efficiently marks all myeloid cells and T lymphocytes in the WKM, but is not expressed in the B-cell lineage. The lack of transgene expression in B lymphocytes is not reflective of the endogenous expression of cd45, because lymphoid cd45:DsRed− cells are positive for cd45 transcript expression (data not shown).
Isolation of a pure population of mononuclear phagocytes using combined expression of mhc2dab:GFP and cd45:DsRed transgenes
Because we expected the mhc2dab promoter to mark cells of the MPS, we investigated the nature of the rare mhc2dab:GFP+ population within the myeloid scatter fraction. To better define this population, we used a combination of mhc2dab:GFP and cd45:DsRed reporter lines. As described in the previous paragraph, the combined expression pattern permits exclusion of B cells (mhc2dab+; cd45−). Therefore, we used this combination to focus on candidate APCs within the myeloid lineage. As shown in Figure 3Ai, separation of cells based on both transgenes revealed distinct populations of myeloid cells: approximately 87% were cd45:DsRed+ only, whereas 0.7% expressed high levels of both mhc2dab:GFP and cd45:DsRed transgenes. To confirm our hypothesis that the myeloid mhc2dab:GFP+ population represents a leukocyte subset distinct from neutrophils, we performed May-Grünwald-Giemsa staining on myeloid cd45:DsRed+; mhc2dab:GFP+ cells isolated by flow cytometry. Morphologic analyses revealed that the majority of cells exhibited the characteristics of monocytes/macrophages, namely low nuclear to cytoplasm ratios and a high number of cytoplasmic vacuoles (Figure 3B). Interestingly, a few cd45:DsRed+; mhc2dab:GFP+ mononuclear phagocytes presented branched projections emanating in all directions from the cell body and a clear cytoplasm devoid of large granules (Figure 3B). These cytologic features resemble those we described previously for zebrafish DCs.22
Isolation of a pure population of mononuclear phagocytes in WKM. (A) Distribution of cd45:DsRed and mhc2dab:GFP fluorescence in the myeloid gate (i), in the precursor gate (ii), and in a gate encompassing the myeloid and precursor gates (iii). cd45:DsRedhigh; mhc2dab:GFPhigh mononuclear phagocytes (red gate) were reanalyzed by forward and side scatter, showing that these cells overlap the myeloid and precursor fractions in WKM (iv). (B) Morphology of cd45:DsRed+; mhc2dab:GFP+ double-positive cells isolated from the myeloid fraction in WKM. Cells were cytospun and stained with May-Grünwald-Giemsa. The majority of the cells show the characteristics of macrophages, with kidney-shaped nuclei and vacuoles. Rare cells presented a different morphology with dendrites and a higher nuclear/cytoplasm ratio. The scale bar indicates 20 μm. (C) Q-PCR expression for genes specific to the neutrophil lineage (top panels) and the MPS (bottom panels). Whereas cd45:DsRed+; mhc2dab:GFP− cells were highly enriched for neutrophil genes (top panels), cd45:DsRed+; mhc2dab:GFP+ cells expressed macrophage-specific genes only (bottom panels). Units on the y-axis represent changes (fold) above WKM. Error bars indicate SD.
Isolation of a pure population of mononuclear phagocytes in WKM. (A) Distribution of cd45:DsRed and mhc2dab:GFP fluorescence in the myeloid gate (i), in the precursor gate (ii), and in a gate encompassing the myeloid and precursor gates (iii). cd45:DsRedhigh; mhc2dab:GFPhigh mononuclear phagocytes (red gate) were reanalyzed by forward and side scatter, showing that these cells overlap the myeloid and precursor fractions in WKM (iv). (B) Morphology of cd45:DsRed+; mhc2dab:GFP+ double-positive cells isolated from the myeloid fraction in WKM. Cells were cytospun and stained with May-Grünwald-Giemsa. The majority of the cells show the characteristics of macrophages, with kidney-shaped nuclei and vacuoles. Rare cells presented a different morphology with dendrites and a higher nuclear/cytoplasm ratio. The scale bar indicates 20 μm. (C) Q-PCR expression for genes specific to the neutrophil lineage (top panels) and the MPS (bottom panels). Whereas cd45:DsRed+; mhc2dab:GFP− cells were highly enriched for neutrophil genes (top panels), cd45:DsRed+; mhc2dab:GFP+ cells expressed macrophage-specific genes only (bottom panels). Units on the y-axis represent changes (fold) above WKM. Error bars indicate SD.
We performed Q-PCR on sorted cells to assess the gene-expression profile of the 2 different cell subsets isolated from the myeloid scatter fraction. As expected, myeloid cd45:DsRed+; mhc2dab:GFP− cells expressed high levels of neutrophilic genes, including csf3r, mpx, and lyz (Figure 3C). In contrast, purified myeloid cd45:DsRed+; mhc2dab:GFP+ cells showed little or no expression of mpx, csf3r, or lyz. Rather, they expressed high levels of marco, csf1r, b7r, and interleukin-12p40 transcripts, suggesting that these cells were mononuclear phagocytes. We also investigated the expression of ptpn6, mpeg1, and cxcr3.2, which were previously reported as being macrophage specific in the zebrafish embryo.20,38 Whereas we confirmed the specific expression of mpeg1 in macrophages, cxcr3.2 and ptpn6 transcripts were present in both myeloid populations (supplemental Figure 4).
The existence of cells expressing high levels of mhc2dab:GFP within the precursor fraction (Figure 2) suggested that mononuclear phagocytes may not exclusively localize within the myeloid fraction, so we aimed to better define the scatter profile of this novel population. Indeed, mature cd45:DsRed+; mhc2dab:GFP+ mononuclear phagocytes found within the myeloid fraction were also detected in the precursor fraction, demonstrating double-positive cells overlapping the standard myeloid and precursor gates in WKM (Figure 3Aii). This was in contrast to mature lyz:DsRed+ or mpx:eGFP+ neutrophils, which localized entirely within the myeloid scatter fraction (data not shown). Interestingly, the precursor fraction of cd45:DsRed; mhc2dab:GFP double-transgenic animals also contained 2 additional populations with lower levels of transgene expression (Figure 3Aii). These populations, defined as cd45:DsRed−; mhc2dab:GFPlow and cd45:DsRedlow; mhc2dab:GFPlow, likely represent less differentiated leukocyte precursor subsets. Finally, because kidney mononuclear phagocytes displayed a specific scatter profile overlapping the myeloid and precursor fractions (Figure 3Aiv), we determined that the most effective way to isolate these cells was to include both scatter fractions. As shown in Figure 3Aiii, kidney mononuclear phagocytes, referred to as cd45:DsRedhigh; mhc2dab:GFPhigh cells, accounted for 3% of the cells in the combined myeloid and precursor fractions. Attempts at sorting mononuclear phagocytes using this purification strategy reproducibly resulted in the recovery of approximately 3500 cells per adult WKM.
Distribution of mononuclear phagocytes among adult tissues
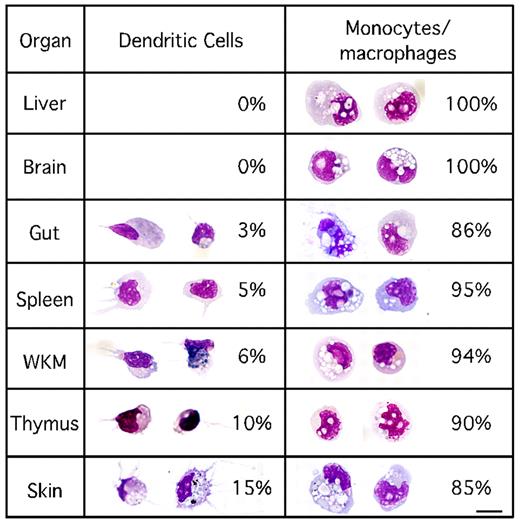
Because the mhc2dab:GFP; cd45:DsRed transgene combination enabled the isolation of mononuclear phagocytes, we initiated a detailed examination of their tissue distribution in the adult zebrafish. Using flow cytometry, we performed cell counts of the myeloid mhc2dab:GFP+; cd45:DsRed+ cells within each hematolymphoid organ to establish the abundance and types of mononuclear phagocytes present. These analyses demonstrated that mononuclear phagocytes are abundant in the kidney, spleen, gut, and skin (Table 1). Consistent with other vertebrate animals, mononuclear phagocytes accounted for a small fraction of the leukocyte population in all organs investigated (1%-2%). Myeloid cd45:DsRed+; mhc2dab:GFP+ cells were sorted from each tissue presented in Table 1 and stained with May-Grünwald-Giemsa to assess morphology. In the liver and brain, sorted cells uniformly exhibited the characteristics of monocytes/macrophages (Figure 4). Whereas monocytes and macrophages were the predominant cell types isolated from gut, spleen, kidney, thymus, and skin, we also detected DCs within the myeloid cd45:DsRed+; mhc2dab:GFP+ fraction of these organs (Figure 4). Dendritic cells were scarce within the gut, spleen, and kidney, but accounted for approximately 10% and 15% of the mononuclear phagocytes in the thymus and skin, respectively. Morphologic analyses showed that sorted cells from all organs constituted a pure population of mononuclear phagocytes, with the exception of the gut. Indeed, 10% of the gut myeloid cd45:DsRed+; mhc2dab:GFP+ population displayed classic morphologic features of mast cells, including single-lobed nuclei and multiple dense granules in the cytoplasm (supplemental Figure 5).
Mononuclear phagocyte cell counts in adult hematolymphoid organs
| Organ . | Mononuclear phagocytes, % . | Isolated cell number . |
|---|---|---|
| Liver | 0.6 ± 0.2 | 560 ± 182 |
| Brain | 0.3 ± 0.1 | 3503 ± 542 |
| Gut | 2.8 ± 0.9 | 3492 ± 97 |
| Spleen | 2.2 ± 1.1 | 3210 ± 830 |
| WKM | 1.4 ± 0.2 | 3669 ± 142 |
| Thymus | 1.4 ± 1.0 | 1102 ± 376 |
| Skin | 0.9 ± 0.3 | 4858 ± 1402 |
| Organ . | Mononuclear phagocytes, % . | Isolated cell number . |
|---|---|---|
| Liver | 0.6 ± 0.2 | 560 ± 182 |
| Brain | 0.3 ± 0.1 | 3503 ± 542 |
| Gut | 2.8 ± 0.9 | 3492 ± 97 |
| Spleen | 2.2 ± 1.1 | 3210 ± 830 |
| WKM | 1.4 ± 0.2 | 3669 ± 142 |
| Thymus | 1.4 ± 1.0 | 1102 ± 376 |
| Skin | 0.9 ± 0.3 | 4858 ± 1402 |
Numbers are presented as means ± SD. Percentages were obtained by analyzing cell suspensions from each organ by flow cytometry (n = 3). Cell numbers correspond to the number of mononuclear phagocytes obtained following cell sorting (n = 3), which, in the case of WKM, is approximately 50% of the total cells.
Distribution of DCs and monocytes/macrophages in adult tissues. For each tissue investigated, cd45:DsRed, mhc2dab:GFP double-positive cells were sorted, cytospun, and stained with May-Grünwald-Giemsa. Differential cell counts were obtained by identifying at least 200 cells per organ. Except for the gut, in which presumptive mast cells were also found, 2 different and separable morphologies were observed: those of DCs and those of monocytes/macrophages. The scale bar indicates 20 μm.
Distribution of DCs and monocytes/macrophages in adult tissues. For each tissue investigated, cd45:DsRed, mhc2dab:GFP double-positive cells were sorted, cytospun, and stained with May-Grünwald-Giemsa. Differential cell counts were obtained by identifying at least 200 cells per organ. Except for the gut, in which presumptive mast cells were also found, 2 different and separable morphologies were observed: those of DCs and those of monocytes/macrophages. The scale bar indicates 20 μm.
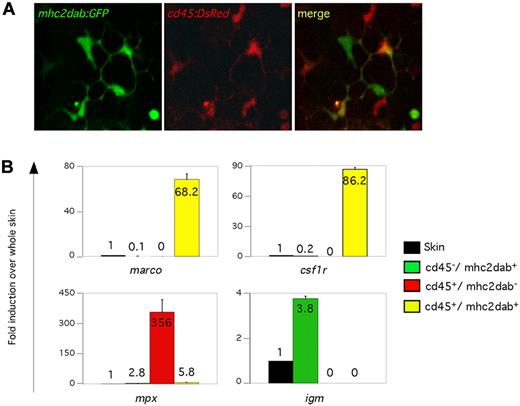
The skin contained the highest number of mononuclear phagocytes and the highest ratio of DCs. To visualize how these cells are spatially organized under steady-state conditions, we performed confocal analyses on the skin of mhc2dab:GFP; cd45:DsRed adult animals. Imaging revealed a network of double-positive DC-like cells reminiscent of the Langerhans cell network found in the mammalian epidermis (Figure 5A). In addition to double-positive cells, single-positive cells were also observed (Figure 5A). Purification of each of the 3 subsets by flow cytometry showed that mhc2dab:GFP+; cd45:DsRed+ cells specifically expressed marco and csf1r by Q-PCR (Figure 5B), which is consistent with our results suggesting that double-positive cells are mononuclear phagocytes. In contrast, purified mhc2dab:GFP−; cd45:DsRed+ cells appeared to be neutrophils, as evidenced by the high expression of mpx (Figure 5B). Finally, mhc2dab:GFP+; cd45:DsRed− cells appeared to be B lymphocytes based on their unique expression of igm. These results demonstrate that the combined expression of mhc2dab:GFP; cd45:DsRed transgenes uniquely marks mononuclear phagocytes regardless of the tissue source. Furthermore, double-transgenic animals can be used to discriminate between additional leukocyte populations, including B lymphocytes and neutrophils.
Visualization of DC-like cells in adult skin. (A) Skin from a cd45:DsRed, mhc2dab:GFP double-transgenic adult fish imaged with confocal microscopy. The GFP (left), DsRed (middle), and merged (right) images are shown. Three populations of cells could be isolated based on their expression of the 2 transgenes and their different morphologies. (B) Q-PCR expression for genes specific to macrophages and DCs (marco, csf1r), neutrophils (mpx), and B cells (igm) for sorted cells from the skin. Units on the y-axis represent changes (fold) above whole skin. Error bars indicate SD.
Visualization of DC-like cells in adult skin. (A) Skin from a cd45:DsRed, mhc2dab:GFP double-transgenic adult fish imaged with confocal microscopy. The GFP (left), DsRed (middle), and merged (right) images are shown. Three populations of cells could be isolated based on their expression of the 2 transgenes and their different morphologies. (B) Q-PCR expression for genes specific to macrophages and DCs (marco, csf1r), neutrophils (mpx), and B cells (igm) for sorted cells from the skin. Units on the y-axis represent changes (fold) above whole skin. Error bars indicate SD.
Zebrafish mononuclear phagocytes become activated upon antigen challenge
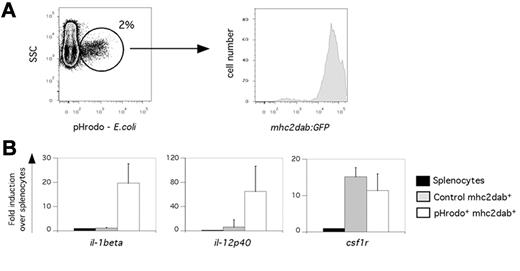
A key feature of APCs is their ability to recognize and uptake pathogen-derived antigens for presentation to T lymphocytes. To begin to functionally characterize zebrafish APCs, we investigated their phagocytic properties in vivo using pHrodo-marked, heat-inactivated bacteria in which the rhodamine derivative pHrodo fluoresces only upon acidification of the phagosome, making it a useful phagocytosis sensor.39 We injected labeled bacteria intravenously into adult mhc2dab:GFP zebrafish and collected spleens 18 hours after injection for analysis by flow cytometry. As presented in Figure 6A, approximately 2% of total splenocytes were pHrodo+ phagocytes. Interestingly, all pHrodo+ phagocytes expressed the mhc2dab:GFP transgene (Figure 6A). These cells were myeloid, based on their light-scattering characteristics (data not shown). We then isolated these phagocytic pHrodo+, mhc2dab:GFP+ cells and unchallenged myeloid mhc2dab:GFP+ splenocytes by flow cytometry. Both populations were compared for gene expression by Q-PCR. Zebrafish APCs specifically up-regulated the expression of inflammatory cytokines after phagocytosis. As shown in Figure 6B, the expression of il-1beta and il-12p40 was increased 20- and 60-fold, respectively, whereas csf1r expression was unchanged. These results demonstrate that zebrafish MPS cells display functional properties similar to their mammalian counterparts.
Phagocytosis induces a strong inflammatory response in splenic mononuclear phagocytes. (A) Heat-killed pHrodo bacteria were injected into the circulation of mhc2dab:GFP adults. Eighteen hours later, spleens were harvested and analyzed by cytometry. As shown on the FACS plot, 2% of the splenocytes phagocytosed bacteria, with all of them expressing the mhc2dab:GFP reporter. (B) Q-PCR expression for inflammatory genes (il-1beta and il-12p40) was performed on phagocytic and nonphagocytic control mhc2dab+ myeloid splenocytes. Whereas expression of csf1r was not changed after phagocytosis, the expression of il-1beta and il-12p40 was up-regulated. Units on the y-axis represent changes (fold) above whole splenocytes. Error bars indicate SD.
Phagocytosis induces a strong inflammatory response in splenic mononuclear phagocytes. (A) Heat-killed pHrodo bacteria were injected into the circulation of mhc2dab:GFP adults. Eighteen hours later, spleens were harvested and analyzed by cytometry. As shown on the FACS plot, 2% of the splenocytes phagocytosed bacteria, with all of them expressing the mhc2dab:GFP reporter. (B) Q-PCR expression for inflammatory genes (il-1beta and il-12p40) was performed on phagocytic and nonphagocytic control mhc2dab+ myeloid splenocytes. Whereas expression of csf1r was not changed after phagocytosis, the expression of il-1beta and il-12p40 was up-regulated. Units on the y-axis represent changes (fold) above whole splenocytes. Error bars indicate SD.
Discussion
Until recently, there have been limited studies investigating the morphologic and functional characterization of immune cells in the zebrafish. Recent efforts, facilitated by the generation of lineage-specific transgenic reporter lines, have permitted the characterization of neutrophils and eosinophils.15,17,18,36 The biology of other hematopoietic lineages, however, remains unclear because of the lack of specific reporter lines. Monocytes/macrophages and DCs, collectively referred to as mononuclear phagocytes, were previously identified in zebrafish based on their morphology and ultrastructural characteristics.1,22 However, our attempts to isolate mononuclear phagocytes to purity using basic methodologies have been unsuccessful so far. In the present study, we demonstrate that the combined expression of cd45:DsRed and mhc2dab:GFP, along with light-scattering characteristics, can be used to isolate these lineages to purity in the zebrafish. This advance now enables the thorough characterization of mononuclear phagocyte identity, localization, and function.
Our results have demonstrated that all cell types comprising the mammalian MPS were identified within the myeloid cd45:DsRed+; mhc2dab:GFP+ double-positive population: monocytes, macrophages, and DCs. Cytochemical analyses demonstrated that with the exception of the gut, this marker combination was sufficient to isolate pure populations of mononuclear phagocytes from any adult organ. Examination of myeloid cd45:DsRed+; mhc2dab:GFP+ cells in immune-related tissues showed that mononuclear phagocytes were the most abundant in the intestine and spleen, followed by the kidney and thymus. Although the sites of antigen presentation to lymphocytes have yet to be defined in the zebrafish, these observations are in agreement with these tissues functioning as secondary lymphoid organs in mammals.40 Quantification of the different mononuclear phagocyte subsets by cytochemical staining indicated that zebrafish DCs are scarce among adult tissues, a trait shared with their mammalian counterparts. We estimate that the number of DCs isolated by flow cytometry ranges from 100-700 DCs per tissue, with the spleen and the skin presenting the lowest and highest yields, respectively. In the skin, DCs accounted for up to 15% of the mononuclear phagocyte population. Confocal imaging of transgenic adult skin showed the presence of a dense network of double-positive cells with a dendriform morphology. These cells were likely Langerhans cells, a DC subtype found in the mammalian epidermis, which we recently characterized in the zebrafish.22
In the present study, we performed the first gene-expression analyses in purified mononuclear phagocytes and observed expression of genes associated with macrophage and DC development and function. It was recently reported that embryonic macrophages and neutrophils could be separated in the mpx:eGFP line on the basis of transgene expression levels, macrophages being GFPlow.41 In the adult animal, our results indicate that mononuclear phagocytes lack expression of myeloid peroxidase. Rather, we found that mpx:GFPlow cells in the adult kidney exhibited the morphology of myeloid precursors (data not shown), an observation consistent with the early expression of mpx during mammalian myelopoiesis.42 The lyz:DsRed transgenic line was originally described to mark both neutrophils and macrophages.15 However, subsequent reports have suggested that the transgene becomes restricted to neutrophils after 2 dpf.43 In support of these findings, we show herein that mononuclear phagocytes do not express lyz transcripts and that the expression of mhc2dab:GFP is mutually exclusive to lyz:DsRed in the adult kidney. Finally, because our transgenic lines allow for the distinction between macrophages and neutrophils, we have investigated the expression of several genes recently reported to be macrophage specific in the zebrafish embryo.38 Whereas mpeg1 was found to be macrophage specific, cxcr3.2 and ptpn6 were equally expressed in both kidney neutrophils and macrophages. Although we cannot exclude the possibility that cxcr3.2 and ptpn6 are differentially expressed during development, our findings are in agreement with mammalian studies demonstrating expression in lineages outside of the MPS.44
Our analyses indicated that mhc2dab:GFP marked the 3 hematopoietic lineages that act as APCs in mammals: macrophages, DCs, and B lymphocytes. The identification of B cells within the mhc2dab:GFP+ fraction is of great relevance for the study of zebrafish immunity because there are no transgenic lines that mark the B-cell lineage. In addition, these findings strongly suggest that the function of antigen presentation by B lymphocytes may be evolutionarily conserved in zebrafish. Combined with existing transgenic lines, including rag2:DsRed,45 the mhc2dab:GFP reporter will allow a better understanding of B-cell differentiation in teleosts. In particular, the ability to track and study the ontogeny of B cells during embryonic development will likely lead to new insights into B-cell biology in the zebrafish, an issue that remains to be thoroughly investigated.
We also observed expression of mhc2dab:GFP within the WKM eosinophil fraction, a result consistent with our previous findings that gata-2:eGFP+ eosinophils express mhc2 transcripts.36 The role of eosinophils as APCs to induce Th2 responses in mammals has been documented previously.37 In addition to eosinophils, we observed the expression of mhc2dab:GFP in another granulocyte subset in the intestine. Purified mhc2dab:GFP+ cells from the gut demonstrated similar morphologic characteristics to mast cells described previously in the zebrafish.46 Whereas the exact nature of these cells needs to be validated, the expression of mhc2 has been described previously in mammalian mast cells.47
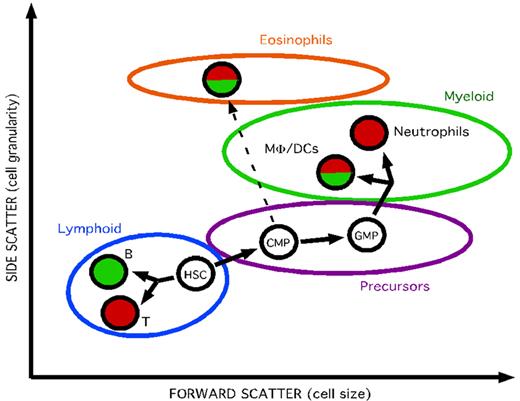
The cd45:DsRed transgenic line that we describe in this study marks the majority of leukocytes. Gene-expression and morphologic analyses showed that all myeloid cells—monocytes/macrophages, DCs, eosinophils, neutrophils, and mast cells—expressed the transgene. However, we have determined that within the lymphoid lineages, only T cells were marked. Whereas B cells express at least one variant of the endogenous cd45 gene, the promoter fragment that we used to generate our transgenic line was not expressed in this lineage. Accordingly, it was shown that the murine cd45 promoter contains 3 distinct transcriptional initiation start sites that are differentially active in distinct hematopoietic lineages.48 Overall, the combined unique expression patterns of cd45:DsRed and mhc2dab:GFP transgenic lines now permits the specific identification and purification of the major immune cell lineages in the zebrafish by flow cytometry: monocytes/macrophages/DCs (FSChi, SSCint, cd45+, mhc2dab+), neutrophils (FSChi, SSCint, cd45+, mhc2dab−), eosinophils (FSChi, SSChi, cd45+, mhc2dab+), T cells (FSClow, SSClow, cd45+, mhc2dab−), and B cells (FSClow, SSClow, cd45−, mhc2dab+; Figure 7). Because these identification criteria are applicable to a variety of different tissues, our transgenic lines will allow a more precise characterization of the cellular composition of each hematolymphoid organ in the zebrafish.
Separation of the major hematopoietic lineages from kidney using the cd45:DsRed and mhc2dab:GFP transgenic lines. By combining light-scattering characteristics and expression of the fluorescent reporter genes, each of the major blood cell lineages could be resorted and purified by flow cytometry.
Separation of the major hematopoietic lineages from kidney using the cd45:DsRed and mhc2dab:GFP transgenic lines. By combining light-scattering characteristics and expression of the fluorescent reporter genes, each of the major blood cell lineages could be resorted and purified by flow cytometry.
The generation of an adaptive immune response in mammals requires interactions between APCs and lymphocytes. These interactions occur in specialized secondary lymphoid organs such as the spleen, lymph nodes, and Peyer patches.49 Despite the presence of a lymphatic system, zebrafish (and teleosts in general) do not appear to possess organized secondary lymphoid structures, and the sites of immune recognition remain to be determined. Because the localization of different APC subsets within immune tissues is an important determinant of their function, our transgenic lines will be instrumental in defining the sites of immune recognition in teleosts. Our observations that mononuclear phagocytes are abundant in the spleen and intestine suggest that these organs might serve as the preeminent sites for antigen presentation. Because histologic analyses have indicated that zebrafish do not form germinal centers in the spleen, and because fish have gut-associated lymphoid tissues scattered throughout their intestinal mucosa,50 it is generally believed that the gut may act as the predominant site of immune cell interaction in teleosts. The ability to mark and follow zebrafish APCs now permits elucidation of this open question.
In conclusion, the ability to specifically identify and isolate cells of the MPS will advance studies of immunology in the zebrafish. The identification of gene-regulatory elements targeting expression to cells of the MPS now enables lineage tracing of the first mononuclear phagocytes to appear in the embryo via fate-mapping approaches. Recent studies have demonstrated that murine microglia are seeded by primitive macrophages that first appear in the extra-embryonic yolk sac.33 It will be interesting to determine whether other adult phagocyte subsets initiate from this population during embryogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank David Stachura for critical evaluation of the manuscript, Konstantin Stoletov and Richard Klemke for help with supplemental Figure 1B, Roger Rainville and Lisa Phelps for animal care, and Kerstin Richter for laboratory management.
This work was supported by fellowships from the Belgian American Education Foundation (to V.W.), the European Molecular Biology Organization (to V.W.), the Fonds de la Recherche Scientifique (FNRS; to V.W.), and the American Society of Hematology (to J.Y.B).
Authorship
Contribution: V.W., J.Y.B., and D.T. designed the experiments; V.W. and J.Y.B. performed the research and analyzed the data; P.W.G. performed aspects of the research; and V.W., J.Y.B., and D.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Traver, University of California San Diego, Department of Cellular and Molecular Medicine, Division of Biological Sciences, La Jolla, CA 92093-0380; e-mail: dtraver@ucsd.edu.
References
Author notes
V.W. and J.Y.B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal