Xenotransplantation of acute myeloid leukemia (AML) into immunodeficient mice has been critical for understanding leukemogenesis in vivo and defining self-renewing leukemia-initiating cell subfractions (LICs). Although AML-engraftment capacity is considered an inherent property of LICs, substrains of NOD/SCID mice that possess additional deletions such as the IL2Rγcnull (NSG) have been described as a more sensitive recipient to assay human LIC function. Using 23 AML-patient samples, 39% demonstrated no detectable engraftment in NOD/SCID and were categorized as AMLs devoid of LICs. However, 33% of AML patients lacking AML-LICs were capable of engrafting NSG recipients, but produced a monoclonal T-cell proliferative disorder similar to T-ALL. These grafts demonstrated self-renewal capacity as measured by in vivo serial passage and were restricted to CD34-positive fraction, and were defined as LICs. Molecular analysis for translocations in MLL genes indicated that these AML patient-derived LICs all expressed the MLL-AFX1 fusion product. Our results reveal that the in vivo human versus xenograft host microenvironment dictates the developmental capacity of human LICs residing in a small subset of patients diagnosed with AML harboring MLL mutations. These findings have implications both for the basic biology of CSC function, and for the use of in vivo models of the leukemogenic process in preclinical or diagnostic studies.
Introduction
One of the most provocative observations in the field of human cancer biology has been the demonstration that some types of cancer are initiated and sustained by a rare population of cells that possess stem cell characteristics.1,2 These cells, termed cancer stem cells (CSCs), are best described in human acute myeloid leukemia (AML), representing an aggressive hematopoietic malignancy where an impaired differentiation program results in the accumulation of immature myeloid blasts.3,4 The identification of a rare self-renewing cell population termed leukemia-initiating cells (LICs) capable of initiating and sustaining growth of human leukemia was based on transplantation of primary AML cells into immunodeficient NOD/SCID mice.3,4 LICs can be prospectively isolated based on surface-marker expression in the BM and peripheral blood of AML patients.4,5 Because of their characteristics that include self-renewal and disease initiation, it has been proposed that these cells possess “stem cell–like” properties and are responsible for the occurrence of minimal residual disease (MRD) and related leukemia relapse.6,,–9 As such, characterization of the LICs in human leukemia has become an important pursuit for understanding the human leukemogenic process to design new therapies capable of targeting LICs with the overarching goal of eradicating the disease.
Although, AML-derived LICs are defined functionally in xenotransplantation models, different strains of immunodeficient mice have been studied to detect human AML-LICs. Initially SCID recipients3 and subsequently NOD/SCID mice4 have been widely used to model human leukemia and define the LIC fraction that is restricted to the CD34+ population.3,4 Further modification of the NOD/SCID, especially the absence of β2 microglobulin in NOD/SCID β2−/− mice lacking the expression of MHC class I, diminishes host innate response and permits enhanced human leukemia engraftment.10 More recently, IL2Rγc (CD132) deficient NOD/SCID mice have impaired signaling through multiple cytokine receptors, resulting in blocked NK-cell development and severely decreased immune response, providing a unique environment for growth and development of human cells.11,–13
While differences in human AML LIC frequency and phenotype have been reported between various strains of immunodeficient mice,3,4,10,11 the role of the in vivo microenvironment in controlling the self-renewal and differentiation capacity of LICs is poorly understood. Here, we identified AML patient samples devoid of AML-LICs as detected in NOD/SCID or NOD/SCID β2−/− mice, but capable of human T-lymphoid engraftment in NOD/SCID IL2Rγcnull recipients. The LIC population was restricted to the CD34+ subfraction, demonstrated self-renewal capacity as measured by serial transplantation and monoclonality for the T-cell receptor (TCR), and uniquely harbored a rare MLL-AFX1 fusion mRNA shared between myeloid and lymphoid leukemias. We suggest that the xenograft microenvironment influences both the process of leukemogenic initiation and lineage differentiation leading to the detection of developmentally distinct subclasses of patient AML-LICs harboring MLL mutations with lymphoid leukemia-initiating capacity in xenografts.
Methods
Primary human samples
For AML specimens, peripheral blood and/or BM was collected at the time of clinical presentation (supplemental Table 2, available on Blood Web site; see the Supplemental Materials link at the top of the online article). All samples were obtained after informed consent following the Declaration of Helsinki, according to Research Ethics Board–approved protocols at McMaster University and the London Health Sciences Centre. Mononuclear cells were prepared using Ficoll-Paque Premium (GE Healthcare).
Antibodies
To detect human hematopoietic cells, APC- or FITC-labeled anti–human CD45 was used (BD Biosciences). FITC anti-CD33, PE anti-CD13, FITC anti-CD2, APC anti-3, PE anti-CD3, PE anti-CD56, PE anti-CD8, FITC anti-CD4, and PE anti-CD19 antibodies were obtained from BD Pharmingen.
CFU assay
AML cells were plated at 50 000 cells/mL in Methocult GF H4434 (StemCell Technologies). Colonies were scored after 14 days of culture according to their morphology using an Olympus 1 × 18 microscope, and images were captured with a Olympus Q-color 3 camera (Q Capture 2.98.2 software).
Xenotransplantation assay
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ adult mice (NOD/SCID IL2Rγcnull, NSG), NOD.Cg-Prkdcscid adult mice (NOD/SCID, NS) or NOD.Cg-Prkdcscid B2mtm1Unc/J adult mice (NOD/SCID β2−/−, NSB) were sublethally irradiated with 315, 325 or 365 rads, respectively, 24 hours before transplantation. AML MNCs (10 × 106) were injected via tail vein. Alternatively, newborn NOD/SCID IL2Rγcnull mice were irradiated with 100 rads and transplanted with 10 × 106 AML MNCs via intrahepatic injections. After 8 weeks, animals were killed, the BM and spleen were analyzed for the presence of human cells by flow cytometry (FACSCalibur; BD Biosciences) and data were analyzed using FlowJo Version 9.2 software (TreeStar Inc). For secondary transplants, unsorted engrafted AML cells in the BM were transplanted intravenously in adult irradiated NOD/SCID IL2Rγcnull or NOD/SCID β2−/− mice as described for primary transplants.
Prospective isolation of leukemic-initiating cells
AML MNCs were sorted based on the expression of CD34 and absence of CD3 using a FACSAria II (BD Biosciences). Isolated populations were injected intravenously into irradiated adult NOD/SCID IL2Rγcnull or NOD/SCID β2−/− mice. Graft analysis was done as described for primary transplantations.
RT-PCR for screening MLL chromosomal aberrations
Total RNA was extracted using a Total RNA extraction kit (Norgen Biotek). RT and following PCRs were performed as described previously.14
Clonality of the TCR
Genomic DNA was extracted using phenol and TCRG gene clonality was assayed as described previously15 (InVivoScribe Technologies). PCR products detection was performed by gel (heteroduplex analysis) or fluorescence (ABI3100, Applied Biosystems).
FISH
Engrafted NSG-AML sample #22 was negative selected for human cells (mouse CD45− cells) using an ARIA II (BD Biosciences). Cells were fixed with 2:1 methanol:acetic acid, dropped onto slides and allowed to dry overnight. Cellular DNA and FIP1L1/CHIC2/PDGFRA deletion/fusion probe (Aquarius) were codenatured at 75°C for 2 minutes then hybridized at 37°C overnight. Residual probe was washed from slide with 0.4× SSC at 72°C for 2 minutes, followed by 2×SSC/0.05% Tween-20 at RT for 2 minutes. Nuclei were counterstained with DAPI antifade. Fifty interphase nuclei were scored for the presence of a translocation of PDGFRA.
Statistical analysis
Significant differences between groups were determined by unpaired 2-way Student t test using GraphPad Prism Version 4 software.
Results
Identification of AML patient samples devoid of NOD/SCID β2−/−-detectable AML-LICs that possess engraftment capacity in NOD/SCID IL2Rγcnull recipients
The recently developed NOD/SCID IL2Rγcnull mouse has been shown to provide substantial differences compared with previous xenotransplantation models such as the NOD/SCID and NOD/SCID β2−/− for the study of human acute leukemic disease progression.11,16 NOD/SCID IL2Rγcnull recipients not only support a more rapid and more robust-established leukemic graft, but also allow the study of both human myeloid and lymphoblastic leukemias.17 Although NOD/SCID IL2Rγcnull mice support higher levels of AML engraftment compared with NOD/SCID or NOD/SCID β2−/− mice, a comparable frequency of AML patient samples give rise to myeloid grafts in all 3 of these models (ranging between 49%-70%).10,16,18
A total of 23 individual AML patient samples were transplanted into NOD/SCID or NOD/SCID β2−/− mice where 14 (61%) patient samples gave rise to a myeloid leukemia (CD45+CD33+ and/or CD45+CD13+ population in transplanted BM cells) phenotypically similar to the patient's disease (supplemental Table 1 and supplemental Figure 1A) indicating the presence of AML-LICs (myeloid-LICs). However, as described previously not all AML samples contain LICs and are capable of engrafting immunodeficient recipients.10 A total of 9 AML patient samples (39%) failed to produce a myeloid human graft (absence of CD45+ in the BM or presence of lymphoid populations; no-LICs; supplemental Table 1 and illustrated in supplemental Figure 1A).
The inability to initiate human leukemia from some AML patient samples could be attributed to an inability of human cells to respond to host microenvironment signals, host immune response to the graft (as NOD/SCID and NOD/SCID β2−/− retain some NK cell activity),19 or simply the absence of LICs in specific AML patients. To investigate the role of murine environment in the engraftment capacity, the same 23 AML samples were transplanted in NOD/SCID IL2Rγcnull recipient mice. Myeloid leukemic grafts were detected in NOD/SCID IL2Rγcnull mice transplanted with the same 14 AML samples tested in NOD/SCID-NOD/SCID β2−/− (61%). Twenty-six percent (6/23) of the samples did not produce any myeloid human graft in transplanted NOD/SCID IL2Rγcnull mice (supplemental Table 1 and supplemental Figure 1A). Interestingly, the majority of those AML samples injected in NOD/SCID-NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice generated the same type of human graft after transplantation (14 AML samples produced myeloid leukemia; 6 samples did not generate leukemic grafts). However, 3 AML patient samples were revealed to engraft in NOD/SCID IL2Rγcnull mice, but were unable to reconstitute a human graft in NOD/SCID or NOD/SCID β2−/− mice (supplemental Table 1, AML samples #22, #23, and #24). Instead, the human graft was comprised only of T cells (supplemental Table 1 and supplemental Figure 1A). None of these AML samples displayed clonogenic capacity in myeloid-specific colony forming assays for normal progenitors or AML-blasts (supplemental Figure 2). These results reveal that the inability of these samples to produce a myeloid graft in NOD/SCID or NOD/SCID β2−/− mice is not because of a host-immune response, but rather was a function of the host marrow microenvironment, and therefore the engraftment capacity was recipient-dependent.
Phenotype of leukemic disease in NOD/SCID IL2Rγcnull vs NOD/SCID β2−/− mice
As reported previously,11,16 AML-LICs transplanted into NOD/SCID β2−/− or NOD/SCID IL2Rγcnull mice phenocopy patient's disease, as shown in the representation example in Figure 1A where human grafts mainly comprisemyeloid cells. B and T cells, if detected, were represented as a small proportion of total human graft (Figure 1A). Normal human lymphoid grafts produced by AML samples were composed of CD19+ B cells and/or CD3+ T cells with the presence of both CD4+ and CD8+ subpopulations indicative of normal lymphopoietic development (supplemental Figure 3) and are the result of normal HSC engraftment and/or mature lymphoid cells adoptively transferred from the AML patient sample to the recipient mouse. In contrast, transplantation of T-ALL samples generates T-lymphoid leukemia, phenotypically similar to the patient disease presentation (Figure 1B). However, the phenotypic characterization of NOD/SCID IL2Rγcnull (NSG)–engrafting AML samples (AML patient samples unable to engraft in NSG or NOD/SCID β2−/− mice but capable of generating human lymphoid engraftment in NOD/SCID IL2Rγcnull mice) revealed that within these grafts only human CD4+ T cells were present, while CD8+ T cells and B cells were undetected (Figure 1C). Whereas most AML samples mainly generated myeloid cells in both BM and spleen of transplanted NOD/SCID IL2Rγcnull mice (Figure 1D), NSG-engrafting AMLs produced human grafts composed of T cells in both organs with a low frequency of other human cell types (Figure 1D).
NSG-engrafting AMLs produce a lymphoid graph in NOD/SCID IL2Rγcnull mice. BM cells from (A) AML- (AML#2), (B) T-ALL-, and (C) NSG-engrafting AML (AML#23)-transplanted NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice were analyzed by flow cytometry. Surface expression of myeloid-cell (CD33 and CD13 inside CD45-positive population), B-cell (CD19 inside CD45-positive population) and T-cell (CD3, and CD4/CD8 inside CD3+ population) markers is represented. Phenotype of de novo isolated (A) AML, (B) T-ALL, and (C) NSG-AML is represented in the left panel. (D) Summary of the human graft composition (inside human CD45-positive population) in BM (left) and spleen (right) of AML-transplanted NOD/SCID IL2Rγcnull mice. Myeloid, B and T cells are defined by the expression of CD13/CD33; CD19; and CD3, respectively. Black indicates NSG-AML samples; and white, AML samples. Each symbol represents a specific AML sample. Individual mice are graphed. NSG-AML: AML#22, #23, #24; 9 mice. AML: AML#2, #4, #5, #6, #11, #20, #21; 22 mice. ***P ≤ .00001.
NSG-engrafting AMLs produce a lymphoid graph in NOD/SCID IL2Rγcnull mice. BM cells from (A) AML- (AML#2), (B) T-ALL-, and (C) NSG-engrafting AML (AML#23)-transplanted NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice were analyzed by flow cytometry. Surface expression of myeloid-cell (CD33 and CD13 inside CD45-positive population), B-cell (CD19 inside CD45-positive population) and T-cell (CD3, and CD4/CD8 inside CD3+ population) markers is represented. Phenotype of de novo isolated (A) AML, (B) T-ALL, and (C) NSG-AML is represented in the left panel. (D) Summary of the human graft composition (inside human CD45-positive population) in BM (left) and spleen (right) of AML-transplanted NOD/SCID IL2Rγcnull mice. Myeloid, B and T cells are defined by the expression of CD13/CD33; CD19; and CD3, respectively. Black indicates NSG-AML samples; and white, AML samples. Each symbol represents a specific AML sample. Individual mice are graphed. NSG-AML: AML#22, #23, #24; 9 mice. AML: AML#2, #4, #5, #6, #11, #20, #21; 22 mice. ***P ≤ .00001.
As unfractionated total mononuclear AML cells were used for xenotransplantation in both NOD/SCID β2−/− and NOD/SCID IL2Rγcnull recipient mice, it is possible that the T-lymphoid graft detected in AML samples exclusively engrafting NSG mice may have resulted from adoptive transfer of existing mature T cells within the transplanted samples. Similar to total AML MNC transplants, CD33-enriched AML cells transplanted into recipient NSG mice produced a myeloid graft with the absence of T and B cells (supplemental Figure 4); however, passive transfer of the purified T-cell fraction generated a graft that phenotypically resemble the population transplanted (mixture of CD4+ and CD8+ CD3+ T cells) and had no self-renewal capacity as measured by secondary transplantation (supplemental Figure 4). Accordingly, the lymphoid graft detected in NOD/SCID IL2Rγcnull mice transplanted with AML patient samples is not because of mature T-cell transfer.
Despite a clinical presentation and diagnosis of human AML, our results indicate that a subset of primary human AML samples produce abnormal T-lymphoid grafts in NOD/SCID IL2Rγcnull mice. Based on side-by-side in vivo transplant comparisons between NOD/SCID, NOD/SCID β2−/− and NOD/SCID IL2Rγcnull recipients, it seems that this effect is host-dependent.
NOD/SCID IL2Rγcnull-restricted AML samples contain leukemic stem cells capable of in vivo self-renewal capacity
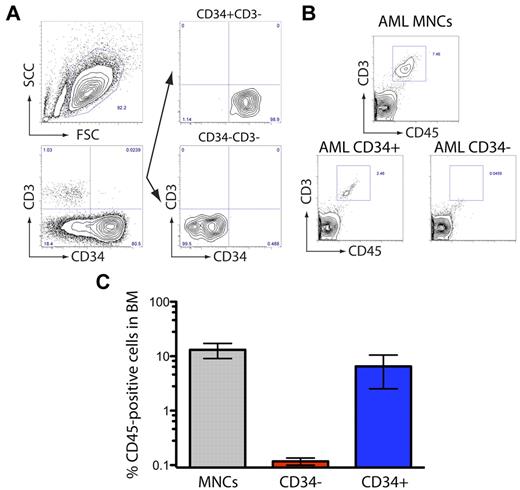
Populations enriched for CSCs such as LICs can be prospectively isolated based on the expression of known phenotypic cell surface markers. Expression of the primitive cell-surface antigen CD34 has been widely associated with both normal and transformed human stem cell populations, and has been used as a cancer stem cell marker that distinguishes human tumor-initiating cells from the bulk tumor population.20 To investigate if the engrafting population in NSG-engrafting AML patient samples expressed this common stem cell marker, T cell–depleted NSG-engrafting AML was fractionated based on the expression of CD34 and transplanted into NOD/SCID IL2Rγcnull recipients (Figure 2A). These experiments indicated that only the CD34+CD3− population contained T-cell LICs from AML patients, in contrast to CD34−CD3− population (Figure 2B-C). These results indicate that the LIC fraction responsible for leukomogenesis in NOD/SCID IL2Rγcnull recipients resides in the compartment of leukemic blasts that express the stem cell marker CD34.
NSG-engrafting AML-LICs express CD34. (A) An NSG-engrafting AML sample (AML#22) was sorted based on the surface expression of CD34 and the absence of CD3 and transplanted in NOD/SCID IL2Rγcnull mice. (B) Representative flow cytometry of human (CD45) T-cell population (CD3) present in the BM of transplanted mice. (C) Human engraftment levels in BM are represented.
NSG-engrafting AML-LICs express CD34. (A) An NSG-engrafting AML sample (AML#22) was sorted based on the surface expression of CD34 and the absence of CD3 and transplanted in NOD/SCID IL2Rγcnull mice. (B) Representative flow cytometry of human (CD45) T-cell population (CD3) present in the BM of transplanted mice. (C) Human engraftment levels in BM are represented.
As serial transplantation provides the only in vivo experimental assay to functionally measure stem cell self-renewal capacity,21 human cells isolated from the BM of AML-transplanted NOD/SCID IL2Rγcnull mice were injected in secondary recipients, and the presence of human cells in the BM and spleen was analyzed by flow cytometry (Figure 3A). As shown in Figure 1, NSG-engrafting AML samples containing T-cell LICs only gave rise to a human graft when injected in NOD/SCID IL2Rγcnull mice (Figure 3B) in contrast to the absence of reconstitution in NOD/SCID β2−/− recipients (Figure 3B). BM cells recovered from primary recipients were transplanted in either NOD/SCID β2−/− or NOD/SCID IL2Rγcnull mice. Similar to our observations in primary recipients, secondary grafts were only detected in NOD/SCID IL2Rγcnull mice; whereas NOD/SCID IL2Rγcnull-engrafted cells were unable to reconstitute NOD/SCID β2−/− secondary mouse recipients (Figure 3B). Both primary and secondary grafts were phenotypically composed by CD3+CD4+CD8− T cells, suggesting a clonal T-lymphopoiesis in BM, spleen and lymph nodes (Figure 3C and supplemental Figure 5). No CD13+ or CD33+ myeloid, CD19+ B, or CD2+CD56+ NK cells were detected in engrafted BM (Figure 3C). As a control, human cells isolated from the BM of AML-transplanted NOD/SCID IL2Rγcnull mice were injected in secondary NOD/SCID IL2Rγcnull recipients and the grafts observed were phenotypically similar to the primary and comprised only by myeloid leukemic cells (CD33, CD13) without detectable B (CD19) or T cells (CD3; Figure 3D). As a positive control, secondary NOD/SCID IL2Rγcnull recipient mice transplanted with T-ALL engrafted samples also produced a graft similar to the primary graft where only T cells were detected (Figure 3D). These findings demonstrate that LICs derived from NSG-engrafting AML samples express CD34 and possess functional stem cell properties of self-renewal in these specific hosts.
NSG-engrafting AML cells reconstitute secondary recipient. (A) NSG-engrafting AML MNCs were injected in NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice. Engrafted BM cells from NOD/SCID IL2Rγcnull-transplanted mice were injected in secondary irradiated NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice. Mice were analyzed 8-weeks after transplantation by flow cytometry. (B) Representative flow cytometry plots of human engraftment in primary and secondary recipients after NSG-engrafting AML injection (AML#23). (C) Phenotype analysis of primary (left panel) and secondary (central panel) BM grafts of NSG-engrafting AML (AML#23)-transplanted mice. Left panel corresponds to primary spleen engrafted cells. Myeloid (CD33 and CD13 inside CD45-positive cells), B (CD19 inside CD45-positive cells), NK (CD2 and CD56 inside CD45-positive cells), T cells (CD3, and CD4/CD8 inside CD3-positive cells) are represented. (D) Analysis of primary (left panel) and secondary (right panel) graft of AML (AML#21)- and T-ALL–transplanted mice based on the myeloid (CD33 and CD13 inside CD45-positive cells), B (CD19 inside CD45-positive cells) and T (CD3, and CD2/CD7 inside CD45-positive cells) surface markers.
NSG-engrafting AML cells reconstitute secondary recipient. (A) NSG-engrafting AML MNCs were injected in NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice. Engrafted BM cells from NOD/SCID IL2Rγcnull-transplanted mice were injected in secondary irradiated NOD/SCID β2−/− and NOD/SCID IL2Rγcnull mice. Mice were analyzed 8-weeks after transplantation by flow cytometry. (B) Representative flow cytometry plots of human engraftment in primary and secondary recipients after NSG-engrafting AML injection (AML#23). (C) Phenotype analysis of primary (left panel) and secondary (central panel) BM grafts of NSG-engrafting AML (AML#23)-transplanted mice. Left panel corresponds to primary spleen engrafted cells. Myeloid (CD33 and CD13 inside CD45-positive cells), B (CD19 inside CD45-positive cells), NK (CD2 and CD56 inside CD45-positive cells), T cells (CD3, and CD4/CD8 inside CD3-positive cells) are represented. (D) Analysis of primary (left panel) and secondary (right panel) graft of AML (AML#21)- and T-ALL–transplanted mice based on the myeloid (CD33 and CD13 inside CD45-positive cells), B (CD19 inside CD45-positive cells) and T (CD3, and CD2/CD7 inside CD45-positive cells) surface markers.
NSG-engrafting AMLs produce a monoclonal T-cell lymphoproliferative disorder and express MLL-AFX1
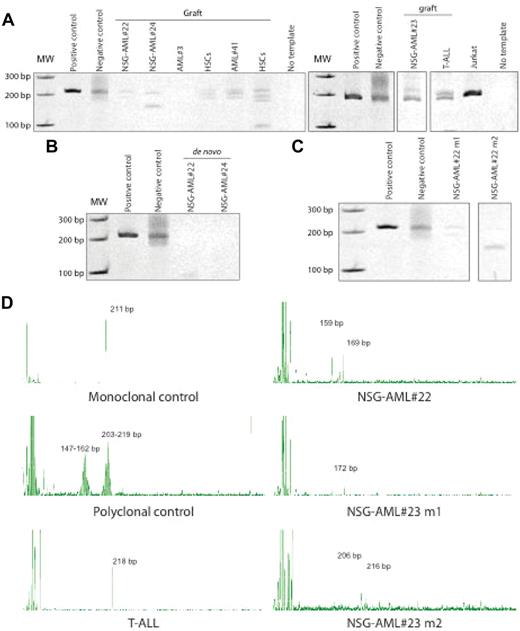
By definition, neoplastic lymphoid disorders, which are monoclonal, are derived from a single lymphocyte, which acquires uncontrolled proliferative, survival, and self-renewal properties.22,23 A single clonal population gives rise to the lymphocyte pool, thus the concept of polyclonality versus monoclonality is routinely applied in the diagnostic assessment of lymphoid neoplasm.24 To assess whether the lymphoid graft derived from AML patients observed in NOD/SCID IL2Rγcnull mice was monoclonal or polyclonal in origin, clonal rearrangements of TCR genes, which reflect clonal expansion of T-lymphocytes, were measured in engrafted samples by PCR.25 NSG-engrafting AML patient samples showed monoclonality in the TCRG gene rearrangements, whereas no amplification was detected in any of the other engrafted AML samples (Figure 4A). As healthy controls for in vivo polyclonal lymphopoiesis, human cord blood was used; whereas samples from human T-ALL patients served as a positive control for clonal T-cell neoplasia in vivo. As expected, engrafted CB cells from healthy SCID-repopulating cells (SRCs)26 were polyclonal in nature19 (Figure 4A); whereas monoclonal T-cell leukemia was detected in mice transplanted with human T-ALL27 (Figure 4A-D). De novo isolated NSG-AML samples showed no TCRG recombination, indicating that TCRG gene has not been rearranged in the majority of the AML blasts (Figure 4B) suggesting the lack of a clonal T-cell population in the patient. Moreover, TCRG rearrangements were different in human grafts generated after transplantation of each NSG-AML sample and in mice injected with the same NSG-AML sample (Figure 4C-D). Based on these in vivo molecular analyses, NSG-engrafting AML samples give rise to a T-cell lymphoproliferative disorder based on the surface-marker expression and molecular clonality of TCR. These NSG-AML samples may contain a LIC population with lymphoid potential and once in the mouse, rearrangements of the TCR genes occur.
NSG-engrafting AML graft in NOD/SCID IL2Rγcnull mice is monoclonal. (A) Engrafted human cells and (B) de novo isolated NSG-AML samples were analyzed for TCRG gene rearrangements by heteroduplex formation. NOD/SCID IL2Rγcnull mice were transplanted with NSG-AML#22 and #23 and TCRG gene rearrangements were detected in engrafted mice by (C) heteroduplex formation and (D) PCR fragment size analysis.
NSG-engrafting AML graft in NOD/SCID IL2Rγcnull mice is monoclonal. (A) Engrafted human cells and (B) de novo isolated NSG-AML samples were analyzed for TCRG gene rearrangements by heteroduplex formation. NOD/SCID IL2Rγcnull mice were transplanted with NSG-AML#22 and #23 and TCRG gene rearrangements were detected in engrafted mice by (C) heteroduplex formation and (D) PCR fragment size analysis.
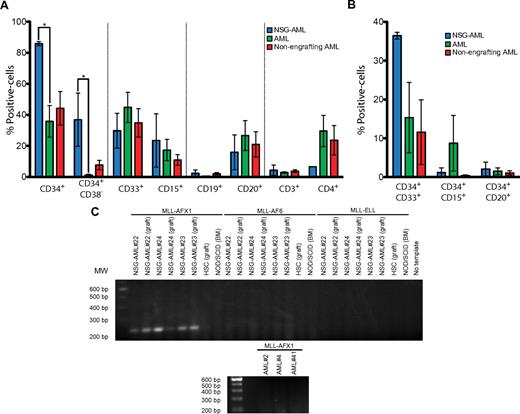
To further investigate the potential difference between NSG-engrafting AMLs and AMLs capable of engraftment in all of the mouse models used, cell-surface marker expression analysis was performed in patient samples by flow cytometry. Similar expression of myeloid (CD33, CD15), B-cell (CD20, CD19), and T-cell (CD3, CD4) markers were found in both types of samples; however, a significantly higher frequency of primitive CD34+ and CD34+CD38− cells was observed in NSG-engrafting AMLs (Figure 5A). Expression of myeloid and lymphoid markers in the CD34+ population was similar (Figure 5B). Therefore, based on the expression pattern of common antigens, NSG-engrafting AMLs contained a higher proportion of primitive cells than other AML patient samples examined.
NSG-AML samples express MLL-AFX1 mRNA. (A) Surface-marker expression in NSG-engrafting AML (blue), engrafting AML (green), and nonengrafting AML (red) samples. Percentage of positive population by flow cytometry is represented. NSG-AML: AML#22, #23, #24. AML: AML#1, #2, #3, #4, #5, #6, #8, #9, #11, #20, #21. Nonengrafting AML: AML#26, #27, #29, #37, #38, #39, #41, #42, #43, #44. *P ≤ .05. (B) Surface-marker expression in CD34-positive NSG-AML (blue), AML (green), and nonengrafting AML (red) samples analyzed as in panel A. (C) mRNA from de novo and engrafted samples was isolated and RT-PCR against MLL fusion proteins was performed.
NSG-AML samples express MLL-AFX1 mRNA. (A) Surface-marker expression in NSG-engrafting AML (blue), engrafting AML (green), and nonengrafting AML (red) samples. Percentage of positive population by flow cytometry is represented. NSG-AML: AML#22, #23, #24. AML: AML#1, #2, #3, #4, #5, #6, #8, #9, #11, #20, #21. Nonengrafting AML: AML#26, #27, #29, #37, #38, #39, #41, #42, #43, #44. *P ≤ .05. (B) Surface-marker expression in CD34-positive NSG-AML (blue), AML (green), and nonengrafting AML (red) samples analyzed as in panel A. (C) mRNA from de novo and engrafted samples was isolated and RT-PCR against MLL fusion proteins was performed.
MLL translocations span a unique group of leukemias, which are phenotypically characterized in the clinic and include AML, acute lymphoblastic leukemias (ALL), and acute leukemia of ambiguous lineage.28 Because MLL is implicated in hematologic disorders of both myeloid and lymphoid lineages, we analyzed MLL fusion transcripts in NSG-engrafting AML samples using a multiplex RT-PCR analysis system that facilitates the detection of more than 80 mRNA breakpoints or splice variants.14 MLL-AFX1 mRNA was uniquely detected in all NSG-engrafting AMLs in both de novo samples derived from patients directly and engrafted samples derived from NSG mice, while none of the MLL-fusion products tested in the assay were present in other AML samples that produce myeloid grafts or in normal HSCs (Figure 5C). NSG-engrafting AML samples taken directly from the patients carried the MLL translocation before transplantation into recipient mice and, therefore, MLL-AFX1 translocation is not a phenomenon because of the xenotransplantation assay itself and only LICs responsible for the human T-lymphoid leukemic graft carried the MLL translocation. Taken together, our findings show that transplantation of NSG-engrafting AML in NOD/SCID IL2Rγcnull mice produced a monoclonal lymphoproliferative disorder that originates from a self-renewing leukemia-initiating cell population that expresses the surface marker CD34, and that all of the NSG-engrafting AML samples carried an MLL translocation fused with AFX1.
Discussion
Our study identifies AML patients who possess LICs detected exclusively in NOD/SCID IL2Rγcnull host, but are devoid of putative cancer stem cells based on the inability to successfully reproduce the leukemic disease in NOD/SCID and NOD/SCID β2−/− recipient xenograft assays. Based on these data, we propose a model (illustrated in Figure 5) where AML patients harboring MLL-AFX1 mutations contain self-renewing CD34-expressing LICs with monoclonal T-cell lymphoproliferative disease potential. We suggest that the LIC compartment is heterogeneous beyond subclasses of LICs,29 and is equally dependent on host microenvironment for its developmental leukemic potential. AML-derived LICs possessing an MLL-AFX1-fusion product are plastic in nature and have both lymphoid and myeloid developmental capacity in mice and human hosts, respectively.
Our study indicates that specific LICs are detected in NOD/SCID IL2Rγcnull mice. Given the heterogeneity of the disease, AML may develop in permanently established subclones with subclone-specific stem cells. Therefore, the LIC pool may be composed of several different subtypes of LICs with variousbiologic properties that can contribute to disparate engrafting capacity.29 Similar to lymphoid leukemias,30,31 genetic diversity of the cancer propagating cells occurred in NSG-AML samples evidenced by the appearing of genetically distinct LICs in different mice transplanted with the same patient sample. Thus, in NSG-engrafting AML samples; at least 2 different types of LICs are present. One subtype maintains the patient disease, and lacks CFU and NSG-engrafting capacity. Another LIC population might be responsible for the T-cell leukemia detection in NOD/SCID IL2Rγcnull mice. As the success of developing effective treatments that eliminate LICs in leukemia patients relies on the ability to detect the presence of all subtypes of LICs in patient samples, specific attention should be placed on using xenograft models to detect heterogeneous human LICs where MLL mutations (MLL-AFX1 specifically) may provide a diagnostic marker. As AML patients with MLL translocations are 62% more likely to lack therapeutic response to pan-AML treatment approaches,32 we further suggest that therapeutic approaches that combat lymphoid leukemias33 may have remission-induction potential in AML patients with MLL-AFX mutations.
LIC heterogeneity in NSG-engrafting AML patient samples. NSG-engrafting AML samples contain at least 2 different subtypes of LSCs responsible for the patient myeloid disease and T-cell lymphoproliferative disorder in NOD/SCID IL2Rγcnull mice.
LIC heterogeneity in NSG-engrafting AML patient samples. NSG-engrafting AML samples contain at least 2 different subtypes of LSCs responsible for the patient myeloid disease and T-cell lymphoproliferative disorder in NOD/SCID IL2Rγcnull mice.
Chromosomal translocations leading to MLL gene fusions are a common event in patients with lymphoid and myeloid acute leukemias.34 MLL fusion partners are very diverse and the leukemogenic program developed is influenced by the identity of the fusion partner, as well as by microenvironmental signals.35,36 MLL-AFX1 is a rare translocation observed in both human AML37,,–40 and T-ALL41,42 ; in fact, it has been suggested that happens early in hematopoietic cells before commitment to distinct lineages.41 MLL-AFX1–expressing NSG-engrafting AMLs produce a myeloid leukemia in humans, but induce a T-lymphoproliferative disorder in NOD/SCID IL2Rγcnull mice, which could be explained based on the inability of human cells to recognize mouse myeloid factors, and by the existence of putative lymphoid signals in the NOD/SCID IL2Rγcnull BM microenvironment that NOD/SCID and NOD/SCID β2−/− mice may lack. Lineage plasticity in xenotransplantation mouse models has also been described in other MLL-fusion protein-transduced human primitive hematopoietic cell fraction.36,43 Nonetheless, the mechanism underlying this lineage plasticity remains unclear, as do the targets of MLL fusion products.44
Regardless of the mouse strain used, the ability to engraft immunodeficient mice seems to be inherent to transplanted cells or to some other unknown microenvironmental parameters. The successful differentiation of human T-cell populations in immunodeficient IL2Rγcnull mice19 could explain the lymphoid engraftment detected in NOD/SCID IL2Rγcnull mice and the lack of engraftment in NOD/SCID mice. Recently, a study using melanoma cells as a model has highlighted the effect of the mouse microenvironment in xenotransplantation assays, and suggested models such as the NOD/SCID mouse might underestimate the frequency of CSCs present in tumors.45 Our results, however, suggest that NSG-engrafting AML-CSCs may require a microenvironment similar to early-staged HSCs to initiate leukemogenesis, and therefore extrinsic microenvironmental parameters (present in NOD/SCID IL2Rγcnull BM stroma but absent in NOD/SCID β2−/− or NOD/SCID) may regulate the ability of CSCs to generate grafts in some cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Tracy Wynder, Uyen M. Dang, Lisa Gallagher, Andrew G. McFarlane, Simona Salati, and The Center of Applied Genomics (The Hospital for Sick Children, Toronto, ON) for their technical help; and Allison Boyd, Eleftherios Sachlos, and Eva Szabo for their invaluable insights during the preparation of the manuscript.
This work was supported by grants from the Ontario Institute of Cancer Research (OICR), Canadian Institute of Health Research (CIHR), and Canadian Cancer Society (CCS) to M.B. M.B. is supported by the Canadian Chair Program and holds the Canada Research Chair in human stem cell biology. R.M.R. is supported by a CCS-TFF fellowship.
Authorship
Contribution: R.M.R. designed and performed experiments, analyzed data, and wrote the paper; C.J.V.C., and S.D. performed experiments, analyzed data, and assisted with writing the paper; M.L.-M. sorted samples and assisted with flow analysis; B.L. and A.X. provided samples and conceptual advice; and M.B. designed experiments, supervised the project, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Mickie Bhatia, Stem Cell and Cancer Research Institute, McMaster University, Faculty of Health Sciences, 1280 Main St West, MDCL 5029, Hamilton, ON, L8S 4K1, Canada; e-mail: mbhatia@mcmaster.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal