Abstract
Natural killer (NK) cell subsets can be defined by the differential expression of inhibitory receptors for MHC class I molecules. Early after congenic HSCT, we found that Ly49G2high single-positive NK cells repopulated, displayed an activated phenotype, and were highly cytolytic. Over time, this subset was replaced with NK cells with a normal pattern of Ly49 expression. Treatment of mice with IL-2 also resulted in the rapid expansion of these Ly49G2high single-positive NK cells. Only the Ly49g (Klra7) Pro1 transcript was highly induced in both HSCT- and IL-2–treated recipients. MHC-independent expansion of the Ly49G2+ subset was also observed after Listeria monocytogenes or mouse cytomegalovirus infection. Our data indicate that during reconstitution after HSCT and various activation stimuli, Ly49G2+ NK cells represent the “first-responder” NK cells, which occur independently of NK-cell licensing via Ly49-MHC interactions. These data suggest that the inhibitory Ly49G2 receptor represents an activation marker on mouse NK cells under various conditions.
Introduction
Natural killer (NK) cells represent a vital arm of innate immunity, mediating important antitumor and antiviral effects.1 Although classically viewed as effectors that exhibit MHC-unrestricted cytotoxicity toward target cells, NK cells also produce numerous cytokines and mediate immunoregulatory functions.2
The precise mechanisms by which NK cells recognize their targets have not been completely elucidated. NK cells use several receptor systems that recognize MHC for discrimination of abnormal cells from healthy cells. Both human and rodent NK cells exist as subsets bearing receptors that bind MHC class I molecules capable of inducing powerful inhibitory signals. Although the human killer-cell immunoglobulin-like receptors (KIRs) and rodent Ly49 receptors differ in structure, they are very similar in function. Both families are composed of members with either inhibitory or stimulatory activity that regulate the function of the NK cell after binding the appropriate MHC class I molecules.3,4 It has been postulated that these inhibitory receptors exist as a means to prevent autoreactive attack by the NK cell; however, there are NK-cell subsets that do not appear to bear receptors binding “self” MHC molecules. This suggests that whereas the abilities of these molecules to bind MHC may indeed play critical roles in governing NK-cell development and activity, other means exist for their control.
The concept of NK-cell education by “licensing” or “arming” the cells expressing the appropriate Ly49 inhibitory receptor has been proposed.5,6 This concept was supported by data showing that NK cells bearing inhibitory Ly49 receptors for self-MHC demonstrated an increased ability to respond to stimulation in vitro.7 This would provide the licensed NK cells with an advantage in the initial response to a stimulus and perhaps result in a more sustained response. However, this hypothesis has been brought into question recently with the demonstration that “unlicensed” Ly49+ NK cells were the predominant subset that expanded after mouse cytomegalovirus (MCMV) infection and provided the greatest protection after adoptive transfer.8 Therefore, the role of NK-cell licensing in vivo with regard to development and function remain unclear. Ly49 receptor expression occurs early in development and its distribution may or may not be driven by MHC.9,10
NK cells have been under intense study for their potential use in HSCT and cancer therapy after reports demonstrating that the use of the appropriate KIR or KIR ligand mismatches in allogeneic HSCT improved clinical outcome.11–13 However, very little is known regarding the role of mouse NK-cell subsets in reconstitution after HSCT or the response of the different subsets to activation stimuli induced by infection or administration of cytokines in vivo. In the present study, we show a preferential expansion of a Ly49 receptor in both reconstitution and activation models that is completely independent of the MHC haplotype.
Methods
Mice
Female C57BL/6 (B6, H-2b), Ly5.2 congenic B6 (B6, H-2b), C57BL/10 (B10, H-2b), and B10.D2 (H-2d) mice were purchased from The Jackson Laboratory. All mice were 8-12 weeks of age and were housed in the animal facility at the University of Nevada-Reno under specific pathogen-free conditions. All protocols involving animals were approved by the institutional laboratory animal care and use committee. Beginning 1 week before HSCT, the mice received antibiotic prophylaxis for 4 weeks with 100 mg of ciprofloxacin (Ciprobay; Bayer) per liter of drinking water.
Antibodies
The following fluorochrome-conjugated mAbs and streptavidin conjugates were purchased from eBioscience: Pacific blue–anti-CD45 (30-F11) and anti-CD45.1 (A20), PE–anti-CD11b (M1/70), PE–anti-CD94 (18d3), PE–NKG2A (16a11), PE–anti-CD49b/Pan-NK cells (DX5), PE–anti-CD43 (eBioR2/60), PE–CD90.2 (Thy1.2; 30-H12), PE–Cy7- or biotinylated anti-CD3 (145-2C11), biotinylated anti-CD122 (TM-β1), allophycocyanin (APC)- or Alexa Fluor 647–anti-NK1.1 (PK136), and biotinylated anti-Ly49H (3D10). The following were purchased from BD Biosciences: FITC- and PE–anti-Ly49CI (5E6), APC- or FITC–anti-Ly49G2 (4D11), FITC–anti-Ly49A (A1), Alexa Fluor 700- or FITC–anti-Ly49D (4E5), Qdot 605–anti-Ly49H (3D10), PerCP–Cy5-anti- NK1.1 (PK136). APC-Cy7–anti-TCRβ (H57-597) and Pacific blue–anti-Ly49A (YE/148) were purchased from BioLegend. PE–anti-Ly49A (YE1/48.10.6) and Texas Red–anti-CD19 (6D5) were purchased from Invitrogen. Isotype-matched mouse and rat IgG mAbs were used as negative staining controls. Indirect staining was performed using APC-conjugated streptavidin purchased from eBioscience. Anti–CD32/CD16 antibody (2.4G2) from eBioscience was used to block FcgII/III receptor–mediated unspecific binding.
HSCT
BM cells were flushed out from femurs and tibias of donors (B6 Ly5.2, B10, and B10.D2) under aseptic conditions. Donors were treated with 200 μg of anti-NK1.1 mAb (PK136 in 0.2 mL of PBS by intraperitoneal injection) 2 days before HSCT to deplete donor NK cells before harvest. Single-cell suspensions were prepared, followed by T-cell depletion using anti-Thy1.2 mAb (30H12) and rabbit complement, as described previously.14 Donor NK- and T-cell depletion from the BM was confirmed by flow cytometric assessment using CD122 and CD3 as discerning markers. The recipients were exposed to a lethal dose of 950 cGy of gamma irradiation from a 137Cs source. Immediately after irradiation, the recipients were injected intravenously into the tail veins with congenic or syngeneic BM cells (5 × 106 BM cells in 0.5 mL of PBS) from sex- and age-matched mice. Each experiment was performed with 3-4 mice per group per harvest time point. Absolute numbers of donor NK cells—CD45.1+NK1.1+CD3− (congenic) and CD45+NK1.1+CD3− (syngeneic)—were calculated by multiplying the percentage of NK cells by the number of leukocytes harvested from the organ. In some experiments, CD122 was used instead of NK1.1 in the CD3-negative population to determine total NK cell numbers. In other experiments, 200 μg of purified anti-Ly49G2 (4D11) or rat IgG was given to B6 recipients 7 days after HSCT to deplete the Ly49G2+ NK cells.
Cell preparation and flow cytometry
Recipient mice were killed at different time points after HSCT, IL-2 administration, and Listeria monocytogenes and MCMV infection. For flow cytometry, splenocyte single-cell suspensions were first incubated with 2.4G2 mAb to block nonspecific antibody binding, and then stained with combinations of the indicated fluorochrome-conjugated or biotinylated mAbs, as described previously.14 When biotinylated mAbs were used, cells were further incubated with APC-streptavidin. Stained cells were analyzed with an FC500 (Beckman Coulter), LSR II (BD Biosciences), or S1000 (Stratedigm) flow cytometer. Data analysis was performed using FlowJo Version 8.8.7 software (TreeStar). The mean fluorescence intensity (MFI) of each receptor was defined for the subset of NK cells expressing the relevant receptor, with MFI values ranging from 0-30 and 0-30 000 depending on the flow cytometer used.
Cell culture and cytotoxicity assays
The NK-sensitive tumor cell line YAC-1 was cultured in RPMI 1640 medium supplemented with 10% FBS, 2mM l-glutamine, 5 × 10-5M 2-ME, 1mM sodium pyruvate, 10mM HEPES, 1% penicillin/streptomycin, and 1× nonessential amino acids. The rat myeloma cell line YB transfected with H2Dd or H2Db was provided by Dr Stephen K. Anderson (National Cancer Institute, National Institutes of Health, Bethesda, MD), and cultured in complete medium plus G418 (1 mg/mL). Single-cell suspensions from spleens of control, day 14 post-HSCT, or IL-2 treated mice were stained using specific mAbs (CD45/CD45.1, CD3, CD19, CD122, Ly49G2, and Ly49C/I) for CD122+ or Ly49C/I+Ly49G2− or Ly49C/I−Ly49G2+ NK cells and sorted using a FACSAria II flow cytometer (BD Biosciences). Sorted splenic CD122+ NK cells were tested for NK cytolytic activity against YAC-1 cells in a 4-hour killing assay. To determine the inhibitory function of Ly49G2 NK cells, enriched NK cells (EasySep Negative Selection Mouse NK Cell Enrichment Kit; StemCell Technologies) from HSCT mice or Ly49C/I+Ly49G2− and Ly49C/I−Ly49G2+ sorted NK cells from IL-2 treated mice were cultured at different effector to target (E:T) ratios with YB H2Dd-transfected and H2Db-transfected cell lines in an 8-hour killing assay. 51Cr release was used to measure NK-cell–mediated lysis, as described previously.15
IL-2 administration
B6 and B10.D2 mice received 1 × 106 IU of recombinant human IL-2 (a gift from the National Cancer Institute repository) intraperitoneally for 2 consecutive days. Splenocyte single-cell suspensions were prepared and stained for CD45, NK1.1, CD3, Ly49CI, Ly49G2, Ly49A, and Thy1.2.
L monocytogenes
B6 mice were infected with 106 colony-forming units of L monocytogenes (ΔactA strain Lm-OVA 44) in 200 μL of PBS via intravenous injection, as described previously.16 Three days after infection, splenocyte single-cell suspensions were stained for CD45, NK1.1, CD3, Ly49CI, and Ly49G2.
MCMV infection
Mice were infected with 5 × 104 pfu MCMV (Smith strain) via intraperitoneal injection, as described previously.8 Peripheral blood lymphocytes from 7- to 8-week-old female B6 or B10.D2 mice were stained for NK1.1, TCRβ, Ly49C/I, Ly49G2, and Ly49A at days 0 and 6 after infection.
Ly49 transcript detection by PCR
RNA from the spleen, BM, and liver of HSCT- and IL-2–treated recipients was extracted using the RNeasy Mini Kit (QIAGEN) following the manufacturer's instructions. PCR to detect the Pro1 transcripts of the genes encoding Ly49a, Ly49c, Ly49i, and Ly49G2 was performed using cDNA and the Platinum PCR SuperMix (Invitrogen) for 35 cycles of 94° for 20 seconds, 58° for 30 seconds, and 73° for 20 seconds. Primers were specific for the Pro1 transcript of each gene. The specificity of each primer set was confirmed by sequencing the PCR products. The sequence of the primers was as follows: Ly49G2 exon-1a forward: AGCAAGTGATCAGCCTATTCTTGTG and Ly49G2 exon 2 reverse: CTAGTTTCTGCAACCTTGAAGACTC (predicted product size: 326 bp); Ly49i exon-1a forward: GATAGCACTATAGTCCAACGGGCTG and Ly49i exon 1 reverse: TCCACCGAGTGATACTCAACCTCG (predicted product size: 368 bp); Ly49c exon-1a forward: GATAGCACTATCGTCCAACAGGCTG and Ly49c exon 1 reverse: TCCACCGGGTGATACTCAACCTCA (predicted product size: 368 bp); and Ly49a exon-1a forward: CAGTCCAAGGGTGTGACTGGAAG and Ly49a exon 1 reverse: AGAAGTAACGTATTGTGCCAGATG (predicted product size: 296 bp). PCR products were electrophoresed in an agarose gel, as described previously.17
Statistical analysis
Statistical significance was tested using the 2-tailed Student t test and 1-way or 2-way ANOVA. P < .05 was considered statistically significant.
Results
Ly49G2high NK cells emerge as the distinct and predominant NK-cell subset after HSCT regardless of MHC haplotype
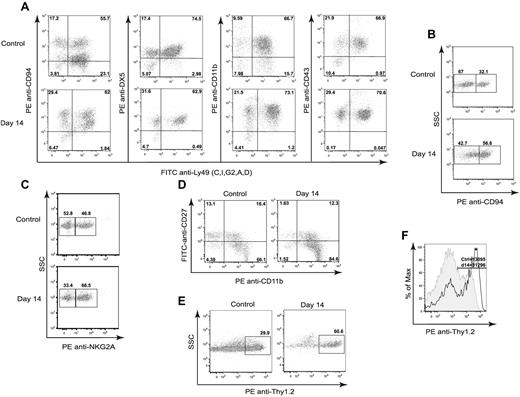
We performed congenic HSCT in mice using BM depleted of mature NK and T cells and characterized donor NK-cell development in lethally irradiated H2b recipients. We found that, analogous to clinical HSCT, NK cells were the first lymphoid cell to repopulate—as early as 14 days after transplantation—in the spleens of recipient mice. Intriguingly, the majority of the cells displayed a mature phenotype. NK cells bearing Ly49, CD94, NKG2A, CD43, CD27, and CD11b, as well as the activation marker Thy1.2, predominated in the spleens of recipients (Figure 1A-E). Total splenic NK-cell numbers were reduced compared with mice that did not receive transplantation (3.43 × 105 ± 0.89 vs 10.5 × 105 ± 2.8, P < .001). In addition, the MFI values of Thy1.2 mice were significantly higher than those of control mice (23 718 ± 1896 vs 11 124 ± 810, P < .001), indicating higher expression levels of this receptor (Figure 1F). These results indicate a rapid reconstitution of mature NK cells with an activated phenotype after HSCT.
Phenotypic characterization of developing NK cells in CD45-congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of B6 (H-2b) recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, Ly49G2, Ly49A, Ly49D, CD94, DX5, NKG2A, CD27, Thy1.2, CD11b, and CD43 at day 14 after congenic BM transplantation and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− cells are shown. (A) Frequencies of NK cells positive for CD94, DX5, CD11b, and CD43. (B) Frequencies of CD94dim and CD94bright NK cells. (C) Frequencies of NK cells positive for NKG2A. (D) Proportions of NK cells expressing CD27 and CD11b. (E) Frequency of Thy1.2+ NK cells. (F) Thy1.2 density shown as MFI on control (tinted gray) and transplanted (solid dark line) mice. The numbers represent the percentages of cells positive for the mentioned markers or MFI values. Data are representative of 5 experiments using 3-4 mice per group in each experiment.
Phenotypic characterization of developing NK cells in CD45-congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of B6 (H-2b) recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, Ly49G2, Ly49A, Ly49D, CD94, DX5, NKG2A, CD27, Thy1.2, CD11b, and CD43 at day 14 after congenic BM transplantation and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− cells are shown. (A) Frequencies of NK cells positive for CD94, DX5, CD11b, and CD43. (B) Frequencies of CD94dim and CD94bright NK cells. (C) Frequencies of NK cells positive for NKG2A. (D) Proportions of NK cells expressing CD27 and CD11b. (E) Frequency of Thy1.2+ NK cells. (F) Thy1.2 density shown as MFI on control (tinted gray) and transplanted (solid dark line) mice. The numbers represent the percentages of cells positive for the mentioned markers or MFI values. Data are representative of 5 experiments using 3-4 mice per group in each experiment.
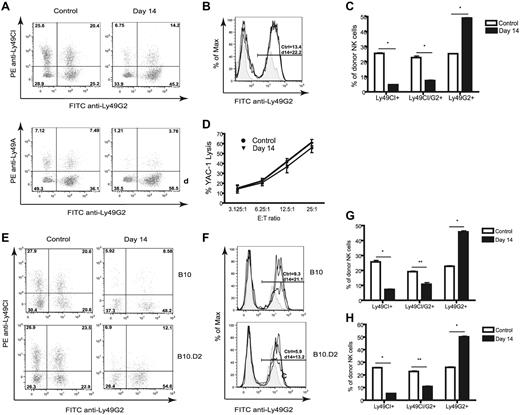
We then assessed the expression of individual NK-cell subsets, focusing on the inhibitory receptors Ly49A, Ly49C/I, and Ly49G2 to determine whether MHC and licensing played a role in reconstitution with regard to Ly49 subset selection. In H2b strain mice, NK cells expressing Ly49C and/or Ly49I have been reported to be licensed because of their ability to bind H2b, whereas Ly49G2 and Ly49A were said to be unlicensed based on their inability to bind H2b.7,18 Surprisingly, it was the Ly49G2 single-positive subset and not NK cells expressing Ly49C/I+ or Ly49A+ that dominated repopulation after HSCT (Figure 2A). Ly49G2 was also the only Ly49 that showed a significantly increased MFI compared with the expression of Ly49A and Ly49C/I subsets, as well as increased expression of Ly49G2 on NK cells in resting mice (24.7 ± 0.8 vs 11.7 ± 0.5, P < .01; Figure 2B). The expansion of this subset was marked, because as much as 60% of the total NK-cell population expressed Ly49G2 (with the majority being single-positive) 14 days after HSCT (Figure 2C).
Increased frequency of Ly49G2high NK cells in congenic and syngeneic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, Ly49A, and Ly49G2 at day 14 after congenic HSCT and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− are shown. (A) Frequencies of NK cells positive for Ly49G2, Ly49CI, and Ly49A. (B) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and transplanted (solid dark line) mice. (C) Frequencies of NK-cell subsets. *P < .001. (D) NK cells (CD45.1+CD122+CD3− CD19−) were sorted from spleen cells of control or transplanted B6 (H-2b) mice (day 14) and used in a 4-hour killing assay against YAC-1 to assess cytotoxicity. Lethally irradiated B10 (H-2b) and B10.D2 (H2d) mice were transplanted with 5 × 106 syngeneic BMCs depleted of NK and T cells. Splenocytes of recipients were stained for CD45, NK1.1, CD3, Ly49C/I, and Ly49G2 at day 14 after syngeneic HSCT and compared with untreated control mice. Gated CD45+NK1.1+CD3− cells are shown. (E) Frequencies of NK cells positive for Ly49G2 and Ly49C/I. (F) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and transplanted (solid dark line) mice. Distribution of NK-cell subsets in B10 (G) and B10.D2 (H) are shown. *P < .001; **P < .01. The numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
Increased frequency of Ly49G2high NK cells in congenic and syngeneic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, Ly49A, and Ly49G2 at day 14 after congenic HSCT and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− are shown. (A) Frequencies of NK cells positive for Ly49G2, Ly49CI, and Ly49A. (B) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and transplanted (solid dark line) mice. (C) Frequencies of NK-cell subsets. *P < .001. (D) NK cells (CD45.1+CD122+CD3− CD19−) were sorted from spleen cells of control or transplanted B6 (H-2b) mice (day 14) and used in a 4-hour killing assay against YAC-1 to assess cytotoxicity. Lethally irradiated B10 (H-2b) and B10.D2 (H2d) mice were transplanted with 5 × 106 syngeneic BMCs depleted of NK and T cells. Splenocytes of recipients were stained for CD45, NK1.1, CD3, Ly49C/I, and Ly49G2 at day 14 after syngeneic HSCT and compared with untreated control mice. Gated CD45+NK1.1+CD3− cells are shown. (E) Frequencies of NK cells positive for Ly49G2 and Ly49C/I. (F) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and transplanted (solid dark line) mice. Distribution of NK-cell subsets in B10 (G) and B10.D2 (H) are shown. *P < .001; **P < .01. The numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
To determine whether the NK cells were functional, we sorted CD122+ splenic NK cells after HSCT and found that these cells lysed tumor targets in vitro to a degree similar to that of control mice (Figure 2D), confirming that the NK-cell population was indeed mature and active early after HSCT. Next we sought to determine whether the Ly49G2+ NK subset preserves its inhibitory properties against H2d19–21 targets early after HSCT. NK cells obtained early after HSCT showed reduced killing capabilities against the rat myeloma cell line YB H2Dd-transfected compared with YB H2Db-transfected targets (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), demonstrating that the Ly49G2+ NK cells, which are predominant early after HSCT, are still inhibited by H2Dd. Therefore, in contrast to a selective effect of MHC and licensing on NK-cell subset development, the subset that predominated after HSCT possessed an unlicensed NK-cell phenotype because it was restricted to the Ly49G2 and not Ly49C/I. Interestingly, even Ly49A was not expanded, which is correlated with selection of the unlicensed phenotype.
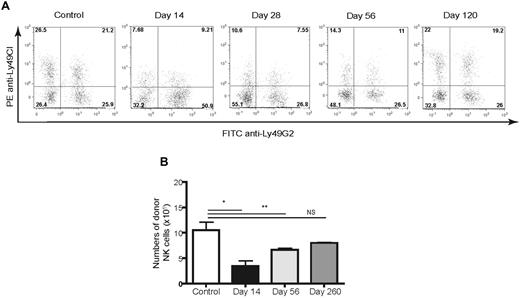
We addressed whether the MHC haplotype of the recipient influenced Ly49 acquisition, because the majority of previous NK cell studies had used H2b strain mice. We performed syngeneic HSCT in B10 (H2b) and B10.D2 (H2d) strain mice, which differ only at the MHC. Surprisingly, we found again that only the Ly49G2high single-positive subset predominated early during reconstitution in H2d strain mice and was similar to that observed with H2b recipients (Figure 2E-H). The Ly49G2 MFI values in B10.D2 mice after HSCT were again higher compared with NK cells in control B10.D2 mice. The MFI values were lower than observed with B10 HSCT recipients, likely because of the ability of Ly49G2 to bind self-H2d molecules and to block antibody binding (Figure 2F). These results demonstrate that reconstitution of NK cells after HSCT was dominated by the Ly49G2high subset, and this occurred completely independently of MHC haplotype. Interestingly, this Ly49G2high subset was observed for weeks after HSCT, but was gradually replaced by NK cells displaying Ly49G2 with a reduced MFI (6.4 ± 0.9), increased percentages of NK cells expressing Ly49C/I (Figure 3A) and Ly49A, and eventually had a distribution pattern similar to that of unirradiated control recipients. By days 28 and 56 after HSCT, an increased frequency of NK cells negative for Ly49C/I/G2 was observed, although this was not correlated with Ly49A acquisition (supplemental Figure 2). The Ly49G2high NK subset was markedly reduced by day 120 after HSCT with the emergence of the Ly49C/I/Ly49G2 or Ly49A/Ly49G2 double-positive subsets. Therefore, the phenotypic profile of NK-cell subsets early after HSCT appears skewed to Ly49G2, is independent of MHC haplotype, and is gradually (months later) replaced with an NK-cell Ly49 subset distribution similar to that of control mice.
Long-term course of Ly49C/I and Ly49G2 expression on developing NK cells in CD45-congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of B6 (H-2b) recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, and Ly49G2 at different time points after congenic HSCT and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− cells are shown. (A) Frequencies of NK-cell subsets. The numbers represent the percentages of cells in each quadrant. (B) Numbers of donor-derived NK cells after HSCT. *P < .001; **P < .01. NS indicates not significant. Data are representative of 3 experiments using 3-4 mice per group per time point in each experiment (means ± SEM).
Long-term course of Ly49C/I and Ly49G2 expression on developing NK cells in CD45-congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. Splenocytes of B6 (H-2b) recipients were stained for CD45.1, NK1.1, CD3, Ly49C/I, and Ly49G2 at different time points after congenic HSCT and compared with untreated control mice. Gated CD45.1+NK1.1+CD3− cells are shown. (A) Frequencies of NK-cell subsets. The numbers represent the percentages of cells in each quadrant. (B) Numbers of donor-derived NK cells after HSCT. *P < .001; **P < .01. NS indicates not significant. Data are representative of 3 experiments using 3-4 mice per group per time point in each experiment (means ± SEM).
Ly49G2 depletion results in long-term deficits of total NK-cell recovery after HSCT
Despite the initial early recovery, total NK-cell numbers remained below control levels for months after congenic HSCT (Figure 3B), even when the T-cell repopulation had been restored (supplemental Figure 3). These results indicate that there are waves of NK-cell reconstitution, with the emergence of the Ly49G2high subset dominating early regardless of the MHC haplotype. Furthermore, despite the appearance of these cytolytically and phenotypically mature cells, normal Ly49 distribution patterns returned at 4 months, but total NK-cell numbers remained depressed for extended periods of time.
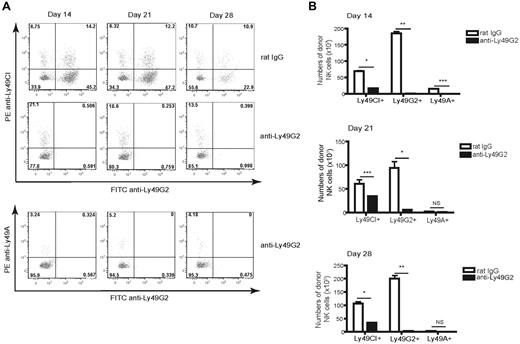
We assessed the effects of depletion of the Ly49G2+ NK subset during reconstitution to determine whether expansion of the Ly49A or Ly49C/I subsets would then occur. Depletion of Ly49G2+ NK cells using the 4D11 mAb 7 days after HSCT resulted in complete absence of the Ly49G2+ NK cells, along with a concomitant reduction in total donor-derived NK-cell numbers assessed at 14, 21, and 28 days after HSCT, indicating that the antibody was depleting this population (Figure 4A-B). However, the remaining NK subsets still failed to expand and total NK-cell numbers remained markedly lower for weeks after HSCT. These data reveal the importance of the Ly49G2+ subset in early NK-cell reconstitution and demonstrate that this subset was not out-competing the other subsets for cytokine consumption, thus inhibiting their expansion.
Ly49G2 depletion results in deficient NK-cell numbers after congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. A group of mice received purified anti-Ly49G2 (4D11) depleting Ab. Splenocytes were stained for CD45.1, CD122, Ly49C/I, Ly49G2, and Ly49A at days 14, 21, and 28 after congenic HSCT and compared with transplanted control mice. (A) Frequencies of NK-cell subsets. The numbers represent the percentages of cells in each quadrant. (B) Numbers of developing CD45.1+CD122+CD3− cells. *P < .001; **P < .0001; ***P < .01. NS indicates not significant. Data are representative of 2 experiments using 3-4 mice per group per time point in each experiment (means ± SEM).
Ly49G2 depletion results in deficient NK-cell numbers after congenic HSCT. Lethally irradiated B6 (H-2b, CD45.2) mice were transplanted with 5 × 106 Ly5.2 congenic (H-2b, CD45.1) BMCs depleted of NK and T cells. A group of mice received purified anti-Ly49G2 (4D11) depleting Ab. Splenocytes were stained for CD45.1, CD122, Ly49C/I, Ly49G2, and Ly49A at days 14, 21, and 28 after congenic HSCT and compared with transplanted control mice. (A) Frequencies of NK-cell subsets. The numbers represent the percentages of cells in each quadrant. (B) Numbers of developing CD45.1+CD122+CD3− cells. *P < .001; **P < .0001; ***P < .01. NS indicates not significant. Data are representative of 2 experiments using 3-4 mice per group per time point in each experiment (means ± SEM).
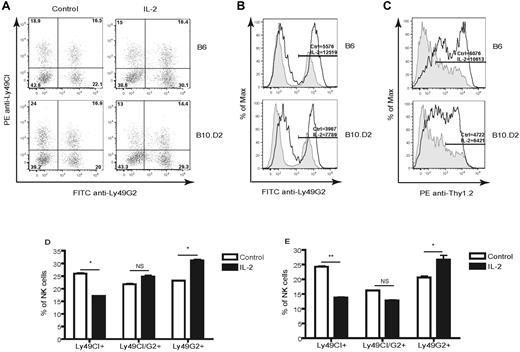
Ly49G2+ NK cells as primary responders to IL-2 in vivo
We conducted a series of experiments to determine whether the expansion of the Ly49G2+ subset is observed during activation situations in normal healthy mice with a normal repertoire of Ly49-expressing NK cells. B6 and B10.D2 mice were given IL-2, which has been shown to activate NK cells in vitro and in vivo,22 and assessed the effects on Ly49 receptor distribution in the NK-cell population. Similar to the HSCT studies, administration of recombinant human IL-2 resulted in a significant expansion of the Ly49G2high NK-cell subset (Figure 5A,D-E) expressing Thy1.2 (Figure 5B-C). These events were also independent of the H2 haplotype of the IL-2–treated mice. In addition, the MFI of Ly49A and Ly49C/I were not altered after IL-2 treatment (supplemental Figure 4A-B); only the Ly49G2+ NK-cell subset was affected (Figure 5B). We then isolated the Ly49G2+ subset by cell sorting and observed that the Ly49G2+ IL-2–treated NK cells lysed YB-H2Dd tumor cells to a greater extent than Ly49G2+ NK cells from untreated mice. This subset demonstrated significantly reduced lysis of YB-H2Dd tumor targets compared with YB-H2Db targets (supplemental Figure 1B), which is in agreement with the effects observed in NK cells after HSCT and demonstrates that Ly49G2 was functional. In addition, Ly49C/I+ IL-2–treated NK cells killed more YB-H2Dd targets than Ly49G2+ NK cells (data not shown), confirming that Ly49G2+ NK cells maintain some degree of inhibition despite their activation.
Increased frequency of Ly49G2high NK cells in IL-2–treated mice. B6 (H-2b) and B10.D2 (H-2d) mice received recombinant human IL-2. Gated splenic CD45+NK1.1+CD3− cells expressing Ly49s in IL-2–treated mice versus untreated mice are shown. (A) Frequencies of NK cells positive for Ly49G2 in B6 and B10.D2 mice. (B) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and treated (solid dark lines) mice. Distribution of NK-cell subsets in B6 (C) and B10.D2 (D) mice. *P < .01; **P < .001. NS indicates not significant. The numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
Increased frequency of Ly49G2high NK cells in IL-2–treated mice. B6 (H-2b) and B10.D2 (H-2d) mice received recombinant human IL-2. Gated splenic CD45+NK1.1+CD3− cells expressing Ly49s in IL-2–treated mice versus untreated mice are shown. (A) Frequencies of NK cells positive for Ly49G2 in B6 and B10.D2 mice. (B) Intensity levels of Ly49G2 expression as determined by MFI on control (tinted gray) and treated (solid dark lines) mice. Distribution of NK-cell subsets in B6 (C) and B10.D2 (D) mice. *P < .01; **P < .001. NS indicates not significant. The numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
Enhanced Ly49g Pro1 transcription early after HSCT and IL-2 administration
The Pro1 promoter of each Ly49 gene is active early in NK development and has been shown to function as a probabilistic switch that controls the variegated expression of Ly49 genes.17,23 To determine whether the Pro1 promoter of Ly49g was preferentially activated by HSCT or IL-2, the level of Ly49 Pro1 transcripts was assessed by RT-PCR using Ly49-specific primers. The results showed that a significant increase in Ly49g and not Ly49a or Ly49c occurred after both HSCT and IL-2 administration in vivo (Figure 6A-B). Ly49i Pro 1 transcripts were not detected under either situation (data not shown). Ly49a and Ly49c transcripts were slightly elevated, particularly after IL-2 treatment, but not nearly to the same extent as Ly49g, which was approximately 30-fold greater. Interestingly, the increase in Ly49g was also observed in the liver and BM of the HSCT-treated and IL-2–treated recipients, indicating activity where NK-cell development was occurring. These results are consistent with Ly49 Pro1 transcription playing a role in the initial activation of the Ly49 genes, as previously reported, and suggest that increased Ly49g Pro1 transcription drives the preferential expansion of the Ly49G2 subset.
Preferential induction of Ly49G2 transcripts by HSCT and IL-2. RT-PCR (35 cycles) of Ly49 Pro1 transcripts was performed with primers specific for Ly49G2, Ly49a, and Ly49c/i. The identity of all products was confirmed by DNA sequencing. (A) Detection of Ly49 Pro1 transcripts after HSCT. RNA was isolated from the spleen, BM, or liver of mice receiving hematopoietic stem cell transfer HSCT or control mice. The numbers under each panel (Δ+HCST) indicate the average -fold induction of the transcript by HCST as measured by SYBR-Green quantitative PCR. (B) Induction of Ly49 Pro1 transcripts by in vivo IL-2 treatment. Induction (fold) in response to IL-2 treatment is shown under each panel (Δ+IL-2). Data are representative of 3 experiments.
Preferential induction of Ly49G2 transcripts by HSCT and IL-2. RT-PCR (35 cycles) of Ly49 Pro1 transcripts was performed with primers specific for Ly49G2, Ly49a, and Ly49c/i. The identity of all products was confirmed by DNA sequencing. (A) Detection of Ly49 Pro1 transcripts after HSCT. RNA was isolated from the spleen, BM, or liver of mice receiving hematopoietic stem cell transfer HSCT or control mice. The numbers under each panel (Δ+HCST) indicate the average -fold induction of the transcript by HCST as measured by SYBR-Green quantitative PCR. (B) Induction of Ly49 Pro1 transcripts by in vivo IL-2 treatment. Induction (fold) in response to IL-2 treatment is shown under each panel (Δ+IL-2). Data are representative of 3 experiments.
Ly49G2high NK cells emerge during pathogen infection
We examined whether this pattern of Ly49G2 expression could be observed under pathologic situations such as during infection. B6 mice were infected with L monocytogenes and then assessed for NK-cell responses. Consistent with the HSCT and IL-2 experiments, significant expansion of the Ly49G2high NK-cell subset was observed in the spleens of the infected mice (Figure 7A-B). We have demonstrated previously that preferential expansion of the Ly49G2+ NK-cell subset occurs in B6 recipients after infection with MCMV.8 Furthermore, this subset was found to be protective when adoptively transferred into MCMV-infected H2b strain mice, indicating that the licensed subset had a subservient role with regard to in vivo protection.8 We extended these studies to determine whether the expansion of the Ly49G2+ subset after viral infection was influenced by MHC haplotype. B10 or B10.D2 mice were infected with MCMV and, similar to the reconstitution and activation models, we observed a marked expansion of the Ly49G2high NK subset in both strains (Figure 7C-D). In H2d strain mice, it is the Ly49C/I+ subset that lacks the optimal MHC ligand and would be considered unlicensed, confirming that Ly49G2 does not behave like other Ly49 members and represents a molecule that is induced and expanded regardless of MHC haplotype.
Increased frequency of Ly49G2high NK cells in mice infected with pathogens. B6 (H2b) mice were infected with L monocytogenes and splenocytes were stained for CD45, NK1.1, CD3, Ly49C/I, and Ly49G2 at day 3 after infection and compared with uninfected control mice. (A) Increase in the total numbers of NK cells and (B) frequencies of Ly49G2high cells in mice infected with L monocytogenes. B6 (H2b) and B10.D2 (H2d) mice were infected with MCMV and splenocytes were stained for NK1.1, TCRβ, Ly49C/I, and Ly49G2 at day 6 after infection and compared with uninfected control mice. Shown are increased percentages (C) and increased intensity levels (D) of Ly49G2 expression as determined by MFI on NK cells in MCMV-infected mice (solid dark line) compared with uninfected controls (tinted gray). *P < .001; **P < .01. The absolute numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
Increased frequency of Ly49G2high NK cells in mice infected with pathogens. B6 (H2b) mice were infected with L monocytogenes and splenocytes were stained for CD45, NK1.1, CD3, Ly49C/I, and Ly49G2 at day 3 after infection and compared with uninfected control mice. (A) Increase in the total numbers of NK cells and (B) frequencies of Ly49G2high cells in mice infected with L monocytogenes. B6 (H2b) and B10.D2 (H2d) mice were infected with MCMV and splenocytes were stained for NK1.1, TCRβ, Ly49C/I, and Ly49G2 at day 6 after infection and compared with uninfected control mice. Shown are increased percentages (C) and increased intensity levels (D) of Ly49G2 expression as determined by MFI on NK cells in MCMV-infected mice (solid dark line) compared with uninfected controls (tinted gray). *P < .001; **P < .01. The absolute numbers represent the percentages of cells or MFI values. Data are representative of 3 experiments using 3-4 mice per group in each experiment (means ± SEM).
Discussion
NK-cell licensing has been proposed as a means to allow increased responsiveness by Ly49 NK-cell subsets that have receptors for self-MHC, and suggests that MHC exerts critical control signals for their function and development. The results presented here indicate that a further hierarchy exists in which Ly49G2high NK cells represent the first responders in both development and activation states regardless of haplotype. Ly49G2 is relatively unique among the inhibitory Ly49 receptors (compared with Ly49A, Ly49C, and Ly49I) because of its lower affinity for binding MHC class I and its relative specificity for Dd in contrast to the other Ly49s.24 This high specificity for Dd would seem at variance with the observation of its universal presence in mice with different Ly49 and H2 haplotypes and with our data.25 Ly49G2-expressing NK cells also represent the largest Ly49 subset in B6 and B10 strain mice. In vivo depletion of Ly49G2+ NK cells has been shown to abrogate the rejection of H2b allografts by lethally irradiated H2d mice, but the opposite is not true, indicating the higher affinity of Ly49G2 for H2d versus H2b, which affects in vivo function.26 Despite this differential effect of Ly49G2+ NK cells on BM allograft rejection, which is contingent on the MHC haplotype of the recipient and has a strong affinity only for Dd, it appears that expression of Ly49G2 is increased and that this subset is expanded in reconstitution, pathogenic, or activation situations independently of MHC haplotype. BM allograft rejection occurs rapidly, within 24-48 hours after irradiation,27 suggesting that it may reflect the property of the Ly49G2 subset under less activating conditions. The increased expression observed in both reconstitution after HSCT and various activation states may also indicate that during these situations, increased expression (in percentage, total numbers, and surface expression) of an Ly49 with lower affinity but higher specificity for a MHC class I has been selected compared with the other Ly49 members, suggesting that the increased MFI either alters MHC-binding capabilities or another ligand exists that exerts regulatory pressure. Neither Ly49A nor Ly49C/I (both of which have been shown to mediate significant degrees of binding to other class I molecules) subsets expanded, indicating that there may be no requirements for an Ly49 capable of binding self-MHC in the initial response. Therefore, NK-cell licensing may not be critical for initial responses. Because the increased expression of Ly49G2 was observed for weeks after HSCT, it is unlikely that inflammation alone was responsible, although inflammation may contribute to the initiation events leading to high-level expression. This was also correlated with an increased population of Ly49A,G2,C/I negative subsets at day 28, although the nature of this population needs to be further examined (supplemental Figure 2). The observation that Ly49g Pro1 transcripts were induced to a greater extent than other Ly49 Pro1 transcripts indicates that Ly49g is innately more responsive in situations in which enhanced NK-cell expansion is occurring. Possibly because of the relatively long half-life of NK cells,28 it took considerable time for a normal Ly49 equilibrium to be reestablished. Although Ly49G2 binds H2d, its strength in licensing NK cells compared with other Ly49 receptors has not been directly addressed. However, we have found that Ly49G2 can indeed exhibit licensing in vivo with regard to BM rejection (manuscript in preparation), suggesting that it can perform dual roles (both licensing and nonlicensing properties) contingent on the stimulus and conditions. Our results suggests that Ly49 family members need to be reexamined with regard to their functional roles due to MHC, because there appear to be layers of reactivity based on the Ly49 used by the NK cell. The data demonstrating that the Ly49G2 single-positive NK cells preferentially expand after infection suggests that NK cells expressing other Ly49 receptors do not emerge as players in innate responses until later. This implies that licensing might not play a dominant role early after HSCT reconstitution or early in activation states. Consistent with this hypothesis, we have found that the Ly49G2+ NK cells were responsible for mediating critical resistance to MCMV infection in H2b and H2d mice, and our data indicate that this population is highly cytolytic.8 Recent studies have shown that the depletion of Ly49G2+ NK cells before infection abrogated MCMV resistance in H-2Dk mice,29 indicating that this subset and Dk recognition play a role in antiviral responses. However, our data imply that Ly49G2 may simply behave as a general activation marker on the NK cell and perform similar functions as the cytotoxic T-lymphocyte antigen 4 on T cells. This up-regulation of expression may therefore occur via any activation/homeostatic signaling through CD122, although this needs to be further elucidated.
There are significant differences between mouse and human NK cells, although the basic tenet of inhibitory receptors modulating NK activity through binding MHC exists in both. There is increasing interest in using NK cells and their subsets clinically, and there are data suggesting that KIR usage follows a similar paradigm in humans. Recent clinical data have shown that leukemia patients receiving NK cells from donors with unlicensed NK cells had better outcomes, suggesting that this paradigm may indeed exist.12 KIR2DL2/S2/2DL3–expressing NK cells have been shown to dominate early responses in HSCT,30 and a bias toward the expression of these receptors in human NK cells differentiated in vitro also exists.31 In addition, the presence of the HLA-C1 ligand for the early-expressed KIR receptors was associated with reduced survival if present in the donor but increased survival if present in the recipient.32
The emergence of Ly49-positive NK cells using in vitro stroma-dependent systems has shown that Ly49G2 is an early responder in the in vitro differentiation model.24,33 Although the investigators in these studies used these data to infer that Ly49 receptor expression occurs in a specific order, it may merely be a reflection of the higher probability of Ly49g activation that is further exaggerated under conditions of increased cell proliferation. The data presented here suggest that innate responses by NK cells during reconstitution after either HSCT or activation are layered with the emergence and predominance of particular Ly49 NK-cell subsets. Therefore, different Ly49 family members may play much more complex roles than initially thought with regard to development, function, and the effect of MHC.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Weihong Ma for excellent technical support and Cherish Agutos for assisting in the preparation of this manuscript. We also thank Dr John Ortaldo for helpful discussions and the NK group of the Murphy Laboratory for critical review of the manuscript.
This study was supported by National Institutes of Health (NIH) grants R01HL089905, P20 RR016464, and P01CA067493. M.T.O. is an Irvington Fellow of the Cancer Research Institute. L.L.L. is an American Cancer Society Professor and is supported by NIH grant A1068129. This project has been funded in whole or in part with federal funds from the National Cancer Institute (NCI), NIH, under contract HHSN261200800001E. This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: I.B., M.A., S.K.A., D.R., and W.J.M. had experimental oversight, analyzed data, and assisted in the writing of the manuscript; B.R.B. and H.E.S. conducted the L monocytogenes experiments; L.L.L. and M.T.O. conducted the MCMV experiments; S.K.A. conducted the RT-PCR experiments; and I.B., M.A., and E.A. conducted all other experiments and assisted with preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr William J. Murphy, Professor and Vice Chair of Research, Departments of Dermatology and Internal Medicine, University of California-Davis School of Medicine, CTSC-IRC Suite 1630, 2921 Stockton Blvd, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
References
Author notes
I.B. and M.A. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal