Natural killer (NK) cells are innate lymphocytes capable of killing tumors and virus-infected cells. Their function is regulated through a variety of different receptors. In humans, the largest group of receptors belongs to the killer Immunoglobulin-like family, or KIRs. Although structurally different from KIRs, mice possess Ly49 receptors that share similar functional characteristics; both KIRs and Ly49 receptors are composed of members possessing inhibitory or activating properties that regulate the function of the NK cell on binding its cognate MHC class I ligand. Although cell subsets lacking inhibitory receptors for self-MHC have been observed in both man and mouse, in vitro studies have shown that these potentially auto-reactive NK-cell populations are in most cases hyporesponsive. In contrast, NK cells whose inhibitory receptors interact with self-MHC class I acquire functional competence, a process referred to as licensing.1 Such licensed NK cells are immunologically competent and are capable of responding to stimuli in vitro and in vivo.
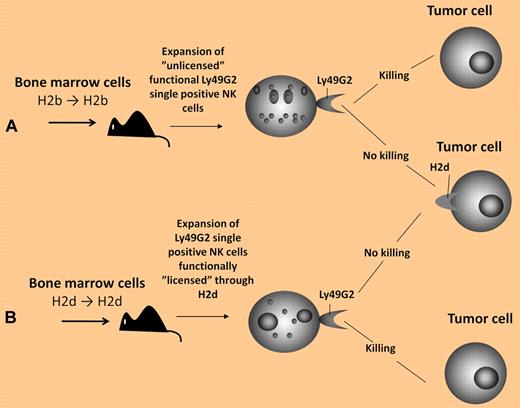
In this issue of Blood, Barao et al investigated whether licensing plays a role in the reconstitution of NK cells bearing different Ly49 receptors in mice undergoing HSCT.2 Initial experiments focused on transfer of bone marrow cells in congenic C57BL/6 mice (H-2b-positive). In these mice, Ly49G2 single-positive NK cells are considered unlicensed because of their inability to bind H2b. Contrary to expectations, early NK-cell repopulation after HSCT was dominated by Ly49G2 single-positive NK cells. Remarkably, these unlicensed NK cells were phenotypically mature and were not hyporesponsive, lysing tumor targets to a similar degree as licensed NK-cell controls. When experiments were repeated in mice on an H-2d background, a selective expansion of Ly49G2 single-positive NK cells was again observed. Thus, it appears that NK-cell repopulation early after HSCT is dominated by Ly49G2 single-positive NK cells that expand independent of the MHC haplotype (see figure). Barao and colleagues next investigated whether the emergence of Ly49G2 single-positive NK cells would occur in the context of host stress induced through inflammatory cytokines and/or infection. Consistent with results observed in recipients of HSCT, treatment with IL-2 or infection with Listeria monocytogenes induced a significant expansion of Ly49G2 single-positive NK cells. Taken altogether, these data suggest Ly49G2 single-positive NK cells represent a first responder NK-cell population that does not require licensing to mediate innate immunity against infection or cytotoxicity against tumors.
Reconstitution of Ly49G2 single-positive NK cells that (A) acquire function without the need for “licensing” versus (B) NK cells that acquire function through MHC-dependent “licensing.”
Reconstitution of Ly49G2 single-positive NK cells that (A) acquire function without the need for “licensing” versus (B) NK cells that acquire function through MHC-dependent “licensing.”
Several studies have investigated the acquisition of Ly49 receptors during NK-cell development. Two principal models (2-step and sequential) are used to describe how NK-cell receptor repertoires are shaped by interactions with cognate MHC class I molecules.3 Williams et al examined clonal expansion of NK cells and found that Ly49G2 was expressed first, followed by Ly49C, I and A.4 The findings of Barao et al could in part reflect such an orderly acquisition of Ly49 receptor expression. However, results observed after IL-2 treatment and L monocytogenes infection support the concept that Ly49G2 expression serves as a marker for NK-cell activation, with Ly49G2 single-positive cells representing an early emerging NK-cell population whose activation status may be MHC-independent. Whether differences in receptors for inflammatory cytokines or other activating or inhibitory receptors between Ly49G2 single-positive and other Ly49 expressing NK-cell subpopulations account for these differences remains to be elucidated.
The role NK cells play in HSCT has been extensively studied. Clinical trials have demonstrated a survival benefit in patients with acute myeloid leukemia receiving alloreactive KIR ligand–mismatched NK cells.5–6 Likewise, studies in HLA-matched HSCT have demonstrated improved outcome in patients lacking MHC class I ligands for donor-inhibitory KIR. In this setting, in vitro studies have shown the emergence of human NK-cell populations expressing a single inhibitory KIR for MHC class I ligands missing in the recipient that are functionally competent against tumor cells early after transplantation.7 These clinical observations taken in the context of the murine data presented by Barao et al further support the concept that a subset of unlicensed NK cells emanating from hematopoietic progenitors may be natural born killers capable of mediating clinically meaningful cytolytic effects against tumors after both autologous and allogeneic HSCT.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal