Abstract
Before the introduction of new drugs, we designed a trial where treatment of newly diagnosed myeloma patients was based on the presence or absence of HLA-identical siblings. First-line treatments included a cytoreductive autograft followed by a nonmyeloablative allograft or a second melphalan-based autograft. Here, we report long-term clinical outcomes and discuss them in the light of the recent remarkable advancements in the treatment of myeloma. After a median follow-up of 7 years, median overall survival (OS) was not reached (P = .001) and event-free survival (EFS) was 2.8 years (P = .005) for 80 patients with HLA-identical siblings and 4.25 and 2.4 years for 82 without, respectively. Median OS was not reached (P = .02) and EFS was 39 months (P = .02) in the 58 patients who received a nonmyeloablative allograft whereas OS was 5.3 years and EFS 33 months in the 46 who received 2 high-dose melphalan autografts. Among patients who reached complete remission in these 2 cohorts, 53% and 19% are in continuous complete remission. Among relapsed patients rescued with “new drugs,” median OS from the start of salvage therapy was not reached and was 1.7 (P = .01) years, respectively. Allografting conferred a long-term survival and disease-free advantage over standard autografting in this comparative study.
Introduction
Autologous transplantation has been regarded as the standard of care for young myeloma patients.1 Recently, “new drugs” such as lenalidomide and bortezomib have prolonged survival.2,3 Allografting has been considered the only potential cure.4 However, the high transplant-related mortality (TRM) has limited its use.5,6 After the observation that donor engraftment could be obtained after reduced-intensity purine analog-based or nonmyeloablative low-dose total body irradiation (TBI)–based conditionings, allografting has become more feasible with acceptable toxicity.7-9 Combining an autograft with a nonmyeloablative conditioning and an allograft has lowered TRM to approximately 15% in myeloma.10,11 However, role and timing of allografting remain to be determined and convincing evidence that an allograft should routinely be performed is lacking.
We report the long-term results of a trial, designed before the introduction of “new drugs,” where the treatment assignment of newly diagnosed patients under the age of 66 was based on the presence or absence of an HLA-identical sibling (ClinicalTrials. gov number, NCT00415987).12
Methods
Patients and treatments
A total of 245 patients were consecutively diagnosed with stage IIA-IIIB myeloma from September 1998 to July 2004 at 5 Italian centers: San Giovanni Battista Hospital, Tosine; University of Udine, Udine; Santa Croce e Carle Hospital, Cuneo; Sant Antonio e Biagio Hospital, Alessandrio; IRCC, Candiole. Of 199 patients, 166 with at least 1 sibling were HLA-typed to search for a potential sibling donor. Written informed consent was obtained from all patients. The study was approved by the 5 Institutional Review Boards according to the Declaration of Helsinki. A first report of this trial was previously published.12
Patients eligible for chemotherapy received 2-3 courses of vincristine, adriamycin, and dexamethasone (VAD)–based regimens. G-CSF–mobilized peripheral blood stem cells (PBSCs) were collected after cyclophosphamide. Patients with HLA-identical siblings were offered the preceding induction followed by a standard autograft after melphalan, 200 mg/m2, and, 2-4 months later, by an allograft with PBSCs after nonmyeloablative TBI (200 cGy). No maintenance/consolidation therapies were allowed. Patients without HLA-identical siblings were assigned to double autologous transplantation after intermediate-dose (100 mg/m2) or high-dose (140-200 mg/m2) melphalan (Figure 1).
Clinical outcomes were compared between patients with and without HLA-identical siblings and between the 2 patient cohorts who received the allograft and the 2 high-dose melphalan autografts.12
Response criteria
Complete remission (CR) required undetectable serum monoclonal Igs or urine light chains by electrophoresis and no monoclonal bands on immunofixation; < 1% marrow plasma cells, and no increase in size or number of osteolytic lesions. Partial remission (PR) was defined as > 75% reduction of the serum monoclonal Ig, at least 90% reduction in 24-hour urinary light chain excretion, no increase in size or number of osteolytic lesions or increase in marrow plasma cells. Patients with less than a PR after induction or autografting were considered refractory; the disease was considered stable if neither CR or PR was observed after allografting. Progressive disease was defined as a > 25% increase in serum monoclonal Ig or urine light chains in patients with refractory or stable disease; relapse as the reappearance of marrow plasma cells, serum monoclonal Igs, urinary light chains or new bone lesions in patients in CR, or a 25% increase in any disease marker for patients in PR.
Statistical analysis
Primary endpoints were overall survival (OS) and event-free survival (EFS) from diagnosis by the intention-to-treat principle. Secondary endpoints included OS and EFS, disease response and TRM in patients who completed the assigned procedures. Survival was calculated by the Kaplan-Meier method from diagnosis until death from any cause for OS, and from diagnosis until progression, relapse, or death from any cause for EFS. OS was also evaluated from the start of salvage therapy after the allograft or the second high-dose autograft. Incidences of TRM, acute GVHD, and chronic limited or extensive GVHD, of being on immunosuppression (IS) after developing chronic GVHD, and of dying while on IS were calculated by the cumulative incidence method by Gooley et al.13 Death without chronic GVHD was considered a competing risk for chronic GVHD; getting off of IS was considered a competing risk for dying while on IS. Dying while on IS was considered a competing risk for getting off of IS. Deaths because of nonrelapse causes, except for nonhematologic malignancies, were regarded as TRM. Proportions between groups were compared with the Fisher exact test. Differences in OS and EFS were estimated with the Cox proportional hazards model. All P values from regression models were derived from the log-rank test. After the allograft or the second autograft, the probability that a patient was alive in the original CR and PR, or in a subsequent remission after salvage treatment, was estimated via an extension of the method described by Couper and Pepe, allowing for multiple transitions between remission and relapse states.14 Multivariate models included presence or absence of an HLA-identical, sibling age, sex, myeloma protein isotype, Durie & Salmon stage, disease response at the first autograft. SAS 8.2 statistical software (SAS Institute) and R 2.1.0, package “cmprsk” were used.
Results
Treatment assignment
Patient characteristics are reported in Table 1. Prognostic factors were evenly distributed in all subgroups.12 Of the 162 HLA-typed patients, 80 had at least 1 potential HLA-identical donor and 60 of them were enrolled in a nonmyeloablative allograft program. Fifteen (18%) who refused because of concerns about TRM and 5 (6%) who had ineligible donors were included in the intention-to-treat analysis. Fifty-eight (97%) of the 60 completed their assigned treatment. Of the 82 without donors, 59 were enrolled in the double high-dose melphalan autograft and 46 (78%) completed the planned treatment. The remaining patients were either ineligible for chemotherapy or received reduced doses of melphalan.
Patient Characteristics
| Characteristic . | No. (%) . |
|---|---|
| Patients | 245 |
| Patients with siblings | 199 (81) |
| HLA-typed patients | 162 (81) |
| Patients with/without donors | 80 (49)/82 (51) |
| Male | 140 (57) |
| Mean age, y (range) | 55 (30-65) |
| Durie & Salmon stage I-II | 73 (30) |
| Durie & Salmon stage III | 172 (70) |
| Ig-G myeloma | 133 (54) |
| Ig-A myeloma | 50 (20) |
| Ig-M myeloma | 1 (< 1%) |
| Bence Jones myeloma | 49 (20) |
| Non-secretory myeloma | 12 (5) |
| β-2-microglobulin ≥ 3.5 mg/dL | 76/220 (35) |
| Albumin < 3.5 g/dL | 42/204 (21) |
| Creatinine ≥ 2 mg/dL | 30 (12) |
| LDH above normal level | 38/210 (18) |
| Presence of Ch 13 deletion | 31/85 (36) |
| Characteristic . | No. (%) . |
|---|---|
| Patients | 245 |
| Patients with siblings | 199 (81) |
| HLA-typed patients | 162 (81) |
| Patients with/without donors | 80 (49)/82 (51) |
| Male | 140 (57) |
| Mean age, y (range) | 55 (30-65) |
| Durie & Salmon stage I-II | 73 (30) |
| Durie & Salmon stage III | 172 (70) |
| Ig-G myeloma | 133 (54) |
| Ig-A myeloma | 50 (20) |
| Ig-M myeloma | 1 (< 1%) |
| Bence Jones myeloma | 49 (20) |
| Non-secretory myeloma | 12 (5) |
| β-2-microglobulin ≥ 3.5 mg/dL | 76/220 (35) |
| Albumin < 3.5 g/dL | 42/204 (21) |
| Creatinine ≥ 2 mg/dL | 30 (12) |
| LDH above normal level | 38/210 (18) |
| Presence of Ch 13 deletion | 31/85 (36) |
Long-term transplant-related toxicity and mortality
Nonmyeloablative allografts.
Grade II-IV acute GVHD developed in 40% (23 of 58) of the patients.12 Of 55 patients, limited and extensive chronic GVHD developed in 9 (16%) and in 32 at a median of 199 (range 100-441) and 199 (range 84-1192) days.
After a median follow-up of 7.3 (range 5.4-10.4+) years from diagnosis and 6.5 (range 4.2-9.4+) years from the nonmyeloablative allograft, 24 of 58 (41%) patients died: 13 (22%) from disease progression, 9 (16%) from TRM and 2 (3%) from lung cancer. TRM was mainly because of complications associated with acute GVHD and chronic GVHD. Four patients in CR died of TRM.
High-dose melphalan autografts.
Overall, 13 (22%) of the 59 patients did not complete the assigned treatment because of disease progression (no. 4), disease-related renal failure (no. 3), TRM (no. 1), consent withdrawal (no. 3), and poor PBSC mobilization (no. 2).
After a median follow-up of 7.4 (range 5.6-10.7+) years from diagnosis and 6.2 (range 4.7-9.1+) years from the second autograft, 30 (65%) of the 46 patients died: 26 (56%) from disease progression, 1 (2%) from TRM (invasive aspergillosis), 1 (2%) from gall bladder cancer, and 2 (4%) of complications during salvage treatments.
Disease response
Nonmyeloablative allografts.
At the time of allografting, 8 (14%) of the 58 patients were in CR and 36 (62%) in PR giving an overall response (CR + PR) of 76%. Thirty-two (55%) of the 58 achieved CR at a median time of 5 months after the nonmyeloablative allograft (range 0-35) and 18 (31%) PR, for an overall response rate of 86%. One additional patient, after obtaining an initial PR achieved CR after subsequent salvage therapy. The achievement of CR was not associated with the development of chronic GVHD (P = 1). Twelve (37%) of the 32 patients in CR and 9 (50%) of the 18 in PR subsequently relapsed.
High-dose melphalan autografts.
At the time of the second autograft, 4 (9%) of the 46 patients were in CR and 31 (67%) in PR for an overall “chemosensitive disease” of 76% (35/46). After the second autograft, 12 (26%) patients entered CR and 30 (65%) PR for an overall response rate of 91%. Overall, 35 (83%) patients relapsed from a previous CR or PR.
Overall response rates (CR + PR) at the time and after the nonmyeloablative allograft and at the time and after the second autograft did not differ between the 2 cohorts (P = 1 and P = 0.54, respectively). However, the CR rate was significantly higher after the nonmyeloablative allograft than after the second autograft (P = .0026).
Salvage therapy
Nonmyeloablative allografts.
Overall, 30 of the 58 patients were treated for disease relapse/progression after the nonmyeloablative allograft. First-line salvage therapy consisted of bortezomib- or thalidomide-containing regimens in 19, standard chemotherapy and/or radiotherapy in 7, and 1 patient was treated with a rapid taper of the IS. Furthermore, 9 of these patients also received DLI. Three patients received DLI alone, and of them only 1 achieved a transient response.
After 1-3 lines of salvage therapy, 22 of 30 (73%) had a response, including 5 CR and 17 PR; 12 (54%) of 22 experienced a second relapse and 3 (25%) of 12 still showed responsive disease.
High-dose melphalan autografts.
Thirty-nine patients experienced relapse/progression after the second autograft. First-line salvage therapy consisted of bortezomib- (no. 8) or thalidomide/lenalidomide-containing regimens (no. 19) in 27 patients, and standard chemotherapy and/or radiotherapy in 10. One patient had not been treated and another patient was lost to follow-up. Overall, 4 (11%) of the treated patients obtained a CR and 16 (43%) a PR for an overall response rate of 54%. Fourteen (70%) of these 20 patients experienced a second relapse.
Long-term clinical outcomes
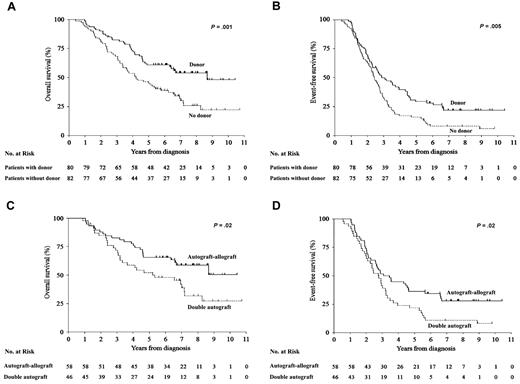
At a median follow-up of 7.1 (range 2.5-10.7+) years, median OS of all 245 patients was 5.2 years; however, by intention-to-treat analysis, median OS and EFS were significantly longer in patients with HLA-identical siblings compared with those without: not reached vs 4.25 years (hazard ratio [HR] 0.51, confidence interval [CI] 95% 0.34-0.76, P = .001) and 2.8 vs 2.4 years (HR 0.62, CI 95% 0.44-0.87, P = .005; Figure 2A-B). By multivariate analysis, independent of age, sex, myeloma protein isotype, Durie & Salmon stage, and disease status at the first autograft, the presence of an HLA-identical sibling and, therefore, the possibility of an allograft, was significantly associated with longer OS (HR 0.5, CI 95% 0.3-0.8, P = .001) and EFS (HR 0.63, CI 95% 0.4-0.9, P = .01; Table 2). Disease status at the first autograft and myeloma protein isotype showed a significant impact on EFS.
OS and EFS. (A) Overall survival (OS) and (B) event-free survival (EFS) from the time of diagnosis of patients with an HLA-identical sibling (n = 80, solid line) and those without (n = 82, dotted line). (C) OS and (D) EFS of patients who received a nonmyeloablative allograft (n = 58, solid line) and those who received a second high-dose melphalan autograft (n = 46, dotted line).
OS and EFS. (A) Overall survival (OS) and (B) event-free survival (EFS) from the time of diagnosis of patients with an HLA-identical sibling (n = 80, solid line) and those without (n = 82, dotted line). (C) OS and (D) EFS of patients who received a nonmyeloablative allograft (n = 58, solid line) and those who received a second high-dose melphalan autograft (n = 46, dotted line).
Univariate and multivariate analyses (Cox models)* for OS and EFS in 2 cohorts: patients HLA-typed (n = 162) and patients who completed their assigned treatments (n = 104)
| Variable . | OS . | EFS . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analyses . | Multivariate analyses . | Univariate analyses . | Multivariate analyses . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| 162 patients HLA typed | ||||||||
| HLA-identical sibling | ||||||||
| No† | 1 | .001 | 1 | .001 | 1 | .005 | 1 | .01 |
| Yes† | 0.50 (0.3-0.8) | .001 | 0.50 (0.3-0.8) | .001 | 0.62 (0.4-0.9) | .005 | 0.63 (0.4-0.9) | .01 |
| Male | 0.98 (0.7-1.5) | .92 | 0.99 (0.7-1.5) | .97 | 1.01 (0.7-1.4) | .97 | 0.94 (0.7-1.3) | .71 |
| Age (< 55y vs > 55y) | 1.04 (0.7-1.6) | .84 | 1.02 (0.7-1.6) | .93 | 1.23 (0.9-1.7) | .24 | 1.23 (0.9-1.7) | .25 |
| Ig-G myeloma | 0.85 (0.6-1.3) | .43 | 0.81 (0.5-1.2) | .33 | 0.68 (0.5-0.9) | .02 | 0.58 (0.4-0.8) | .004 |
| Durie & Salmon stage III | 0.97 (0.6-1.5) | .90 | 1.04 (0.7-1.6) | .87 | 1.13 (0.8-1.6) | .49 | 1.14 (0.8-1.6) | .49 |
| Disease status at first autograft | 0.83 (0.6-1.3) | .38 | 0.80 (0.5-1.2) | .30 | 0.66 (0.5-0.9) | .02 | 0.57 (0.4-0.8) | .003 |
| 104 patients completed assigned treatment | ||||||||
| High-dose melphalan autograft | 1 | .03 | 1 | .04 | 1 | .03 | 1 | 0.02 |
| Nonmyeloablative allograft | 0.55 (0.3-0.9) | .03 | 0.56 (0.3-1.0) | .04 | 0.61 (0.4-0.9) | .03 | 0.60 (0.4-0.9) | 0.02 |
| Variable . | OS . | EFS . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analyses . | Multivariate analyses . | Univariate analyses . | Multivariate analyses . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| 162 patients HLA typed | ||||||||
| HLA-identical sibling | ||||||||
| No† | 1 | .001 | 1 | .001 | 1 | .005 | 1 | .01 |
| Yes† | 0.50 (0.3-0.8) | .001 | 0.50 (0.3-0.8) | .001 | 0.62 (0.4-0.9) | .005 | 0.63 (0.4-0.9) | .01 |
| Male | 0.98 (0.7-1.5) | .92 | 0.99 (0.7-1.5) | .97 | 1.01 (0.7-1.4) | .97 | 0.94 (0.7-1.3) | .71 |
| Age (< 55y vs > 55y) | 1.04 (0.7-1.6) | .84 | 1.02 (0.7-1.6) | .93 | 1.23 (0.9-1.7) | .24 | 1.23 (0.9-1.7) | .25 |
| Ig-G myeloma | 0.85 (0.6-1.3) | .43 | 0.81 (0.5-1.2) | .33 | 0.68 (0.5-0.9) | .02 | 0.58 (0.4-0.8) | .004 |
| Durie & Salmon stage III | 0.97 (0.6-1.5) | .90 | 1.04 (0.7-1.6) | .87 | 1.13 (0.8-1.6) | .49 | 1.14 (0.8-1.6) | .49 |
| Disease status at first autograft | 0.83 (0.6-1.3) | .38 | 0.80 (0.5-1.2) | .30 | 0.66 (0.5-0.9) | .02 | 0.57 (0.4-0.8) | .003 |
| 104 patients completed assigned treatment | ||||||||
| High-dose melphalan autograft | 1 | .03 | 1 | .04 | 1 | .03 | 1 | 0.02 |
| Nonmyeloablative allograft | 0.55 (0.3-0.9) | .03 | 0.56 (0.3-1.0) | .04 | 0.61 (0.4-0.9) | .03 | 0.60 (0.4-0.9) | 0.02 |
OS indicates overall survival; EFS, event-free survival; HR, hazard ratio; and CI, confidence interval.
In both cohorts, multivariable HRs were adjusted for sex (male vs female), age (< 55 years vs > 55), Ig-G myeloma (Ig-G vs others), Durie & Salmon stage (III vs I or II), disease status pre-first autograft (partial and complete remission vs less than a partial remission).
At a median follow-up of 7.3 (range 5.4-10.7+) years, median OS was not reached in the 58 patients who received a nonmyeloablative allograft and 5.3 years (range 0.9-10.7+) in the 46 who received a second high-dose melphalan autograft (HR 0.55, CI 95% 0.32-0.94, P = .02), whereas EFS was 39 months and 33 months (HR 0.62, CI 95% 0.40-0.96, P = .02), respectively (Figure 2C-D). By multivariate analysis, patients who received the nonmyeloablative allograft showed significantly improved OS (HR 0.56, CI 95% 0.3-1.0, P = .04) and EFS (HR 0.60, 95% CI 0.4-0.9, P = .02) compared with those who received a second high-dose melphalan (Table 2).
The probability of a patient being alive in the original or in a subsequent CR or PR after salvage treatments after the nonmyeloablative allograft and the second autograft is illustrated in Figure 3. At a median follow-up of 3.9 years from relapse, OS was not reached and 1.7 years in patients who had relapsed after the nonmyeloablative allograft and the second high-dose melphalan (HR 0.44, CI 95% 0.24-0.82, P = .01; Figure 3).
Survival after salvage treatment. Probabilities of a patient being alive in the original complete (CR) or partial remission (PR) or in a subsequent remission because of salvage therapy after the nonmyeloablative allograft (A) or the second autograft (B) calculated by the Couper method (dotted line). Black and gray solid lines represent overall (OS) and event-free survival (EFS) by the Kaplan-Meier methods (see “Long-term clinical outcomes”). (C) OS, calculated from first relapse, of patients who relapsed after the nonmyeloablative allograft (solid line) and after the second high-dose melphalan autograft (dotted line).
Survival after salvage treatment. Probabilities of a patient being alive in the original complete (CR) or partial remission (PR) or in a subsequent remission because of salvage therapy after the nonmyeloablative allograft (A) or the second autograft (B) calculated by the Couper method (dotted line). Black and gray solid lines represent overall (OS) and event-free survival (EFS) by the Kaplan-Meier methods (see “Long-term clinical outcomes”). (C) OS, calculated from first relapse, of patients who relapsed after the nonmyeloablative allograft (solid line) and after the second high-dose melphalan autograft (dotted line).
Long-term immunosuppression
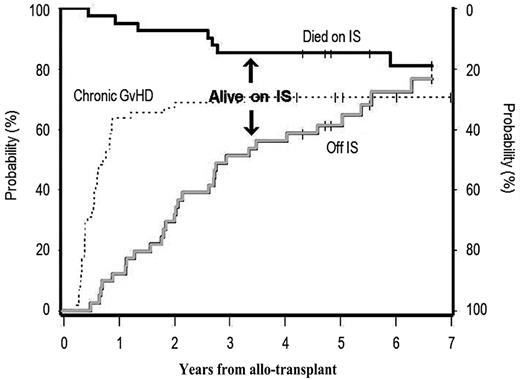
Forty-one (74%) of 55 patients developed limited or extensive chronic GVHD. At 3 years from the allograft, 34 were alive, 15 were on IS, and 19 were not. At the time of this report, 31 had reached a follow-up of at least 5 years: 5 died while on IS whereas 23 were alive off IS and only 3 were still on IS (Figure 4). Overall, 19 (66%) of 29 discontinued IS because of resolution of chronic GVHD while 10 (34%) of 29 underwent a rapid IS taper after relapse.
Probability of being alive on or off immunosuppression (IS) medications among patients who developed limited or extensive chronic GVHD. Overall incidence of chronic GVHD (dotted line); patients who died while on IS medications (black solid line); and patients alive who discontinued all IS medications (gray solid line) after developing chronic GVHD. The distance between the black and solid lines represents patients who are alive and still on IS medications (see also “Long-term immunosuppression”).
Probability of being alive on or off immunosuppression (IS) medications among patients who developed limited or extensive chronic GVHD. Overall incidence of chronic GVHD (dotted line); patients who died while on IS medications (black solid line); and patients alive who discontinued all IS medications (gray solid line) after developing chronic GVHD. The distance between the black and solid lines represents patients who are alive and still on IS medications (see also “Long-term immunosuppression”).
Discussion
Allografting in myeloma has hotly been debated since its clinical introduction.15 Lower relapses following allografts rather than autografts were already reported in the late 1990s, though this did not translate into better OS given the high TRM.16 Recently, reduced-intensity or truly nonmyeloablative conditionings have been investigated.10,11,17,18
Before the era of “new drugs,” whether nonmyeloablative allografting could improve OS and EFS compared with autografting was a matter of debate.19 In our study, treatment assignment was based only on the presence or absence of HLA-identical siblings. Neither induction treatments with “new drugs” nor currently used maintenance/consolidation therapies were included in the study. Previously reported results were encouraging.12 Here, we have extended follow-up to a median of 7 years.
Median OS of our entire study population was 5.2 years, consistent with the expected OS for patients diagnosed during the study period.20 At a median follow-up of 7.1 year, both OS and EFS were significantly longer in patients with HLA-identical siblings than those without. Median OS was not reached in the allograft patients while EFS was 39 months. Both OS and EFS remained significantly longer compared with those patients treated with 2 autografts who showed OS of 5.3 years and EFS of 33 months. Cavo et al reported median EFS of 23 and 35 months after single and double autologous transplantation, respectively.21 However, it is important to point out that more recent reports on the use of maintenance therapy after autografting have shown remarkable improvements. McCarthy et al reported an estimated median time to progression of 42 months in patients treated with lenalidomide after a single autograft, while Attal et al reported a progression-free survival of 42 months from the time of randomization in the arm with lenalidomide as maintenance compared with 24 months in the placebo arm.22,23 These trials may define a new standard of treatment which includes maintenance after autografting. No definitive data are currently available on the use of new drugs after allografting.
Other studies comparing allografting with autografting have been reported.24-29 All included an autograft before a reduced-intensity allograft. The first published study by the Intergroupe Farmaphene du Myelome (IFM) enrolled high-risk patients. A recent update showed no significant differences in EFS and OS with a trend for poorer survival in the allograft patients.24 One study by the PETHEMA group randomized 25 patients to receive the allograft and 85 a second autograft.25 All patients had failed to achieve at least near-CR after a first autograft. The median time for progression free survival and OS had not been reached in the allograft cohort, whereas they were 31 months and 58 months in the autograft group, respectively.
Three other studies used the “Seattle regimen” with low-dose TBI after a cytoreductive autograft.26-29 In the HOVON study, the control cohort was treated with one autograft followed by maintenance with thalidomide. An interim analysis showed no significant differences in EFS and OS between the 2 cohorts of 124 patients each: 39% vs 34% and 56% vs 63% at 4 years in the allograft group and the autograft-thalidomide group, respectively.26 The recently closed EBMT study enrolled 107 patients with an HLA-identical sibling and 251 without from 26 European Centers. Eighty-eight and 104 patients completed the assigned treatments, respectively.27 By intention-to-treat analysis and in those patients who completed the assigned treatment, the risk of relapse was significantly lower in the allograft cohort. Despite a significantly higher TRM, 13% versus 5%, a trend for better OS was seen in the allograft cohort in both poor and good prognosis subgroups. Clinical findings of a large US multicenter trial from the Blood and Marrow Transplant Clinical Trials Network were recently reported.28,29 A total of 710 patients were randomized at 43 US transplant centers. Induction treatments were not standardized and patients could be enrolled after HLA typing between 3 and 9 months from the start of systemic induction therapy. Patients were defined as standard or high risk in the light of high β-2-microglobulin levels and chromosomal abnormalities by standard cytogenetics. Both intention-to-treat and as-treated analyses showed equivalent 3-year progression-free survival and OS in standard-risk patients whereas there were trends in late progression-free survival and time to progression in high-risk patients who underwent allografting.28,29
All these studies used a Mendelian randomization as a surrogate for a more formal randomization.30 However, given substantial differences in the study inclusion criteria and treatment schemas, results are inevitably conflicting.
We reported the clinical outcomes of the entire young population of patients consecutively diagnosed at our centers, regardless of the treatment protocol. This is rarely reported in phase 2-3 studies preventing the readers from knowing the proportion of patients actually eligible and eventually enrolled in a given protocol. We initially defined 2 patient cohorts with and without potential donors. This did not imply that all patients were eligible for investigational phase 2-3 studies either because of comorbidities, patient will, or donor eligibility. Of the patients enrolled in the nonmyeloablative allograft arm and in the tandem autograft arm, 96% and 78%, respectively, completed the assigned treatment. The inclusion of all eligible patients in the allograft cohort, regardless of disease stage and prognostic factors, gave a good chance to capture the subset who most benefited from graft-vs-myeloma that resulted in long-term disease-free survival. Unfortunately, our study was not designed to detect any myeloma-specific marker that could possibly predict plasma cell sensitivity to graft-vs-myeloma.
Chronic GVHD may be cause of long-term morbidity and poor quality of life. However, IS discontinuation because of its resolution greatly improves quality of life and may be used as a surrogate of the achievement of immunotolerance. Importantly, most of our surviving patients, at 5 years after the allograft, had discontinued IS because of continued resolution of GVHD.
Molecular remissions, prerequisite for cure, have more frequently been observed after myeloablative allografting rather than autografting with/without consolidation therapy.31-33 The cumulative risk of relapse at 5 years was 0% for patients with durable PCR negativity after the allograft. We previously reported molecular remission after low-dose TBI allografting.34 Some remissions were reached months after transplantation suggesting a gradual graft-versus-myeloma effect that may be less effective in bulky and aggressive diseases. A currently in-progress large retrospective study on molecular responses will help define whether the intensity of the conditioning or graft versus myeloma is more important to obtain molecular remissions.
After the introduction of “new drugs,” allografting has become a far less attractive option because of its toxicity. The role of allografting, however, may prospectively be evaluated in selected high-risk patients where life expectancy remains very poor despite the use of bortezomib and lenalidomide.35 Although genetic abnormalities such as del(17p) were shown to have a negative prognostic factor in a retrospective analysis on patients transplanted from both related and unrelated donors, the combination of allografting with “new drugs” has not yet been thoroughly explored.36 The efficacy of “new drugs” in patients relapsed after allografting has already been reported.37,38 Interestingly, we observed higher response rates to salvage therapies in the allograft patients and OS from relapse was significantly longer after the allograft than the second autograft (P = .01). Although not part of a prospective trial, this finding may partly be explained by the hypotheses that the high percentage of donor T cells, usually seen at relapse after a nonmyeloablative allograft, may synergize with immunomodulatory drugs and help restore graft versus myeloma or that donor cells may favor the antimyeloma effects of these drugs in the marrow milieu.
In summary, a subset of patients may have been cured with an allograft given the persistent disease free status extending up to longer than 10 years. However, given the risk of transplantation-related toxicity and the recent remarkable advancements in newly diagnosed myeloma patients, the combination of allografting with new drugs should most preferably be explored in high-risk patients, where life expectancy is poor, in prospective clinical trials.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the nurses and medical staff for caring for the patients, and the data managers who collected the study and follow-up information.
This research was supported in part by Regione Piemonte: Ricerca Finalizzata 2006, 2007; Compagnia di San Paolo and Comitato Regionale Piemontese Gigi Ghirotti; Fondazione Neoplasie Sangue Onlus; and grant CA78902 from the National Institutes of Health, Department of Health and Human Services (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: M.B. and B.B. contributed to the initial conception and designed the study; L.G., F.P., M.R., R. Sorasio, B.A., F.C.-S., M.F., L.B., S.B., M.A., A.L., N.M., A.G., R.F., M.M., A.P., R. Storb, M.B., and B.B. provided the study materials or patients; M.R., R. Sorasio, M.F., L.B., P.O., and B.B. collected and assembled the data; L.G., B.S., M.R., R. Sorasio, M.F., G.C., R. Storb, T.A.G., and B.B. analyzed and interpreted the data; and L.G., M.F., and B.B. wrote the manuscript. All authors gave final approval to the manuscript.
Conflict-of-interest disclosure: M.B. has received research support from, served as a consultant for, and is on the scientific advisory board for Celgene and Janssen-Cilag. A.P. has received honoraria from Celgene, Janssen-Cilag, Merck, and Amgen, and is on the advisory committees for Celgene and Janssen-Cilag. S.B. has received honoraria from Celgene, Janssen-Cilag, Novartis, and Merck. The remaining authors declare no competing financial interests.
Correspondence: Benedetto Bruno, MD, PhD, Divisione Universitaria di Ematologia, Azienda Ospedaliera San Giovanni Battista, Via Genova 3, 10126, Torino, Italy; e-mail: benedetto.bruno@unito.it.