Abstract
Alpha-2-antiplasmin (α2AP) undergoes both N- and C-terminal cleavages, which significantly modify its activities. Compared with other Ser protease inhibitors (serpins), α2AP contains an ∼ 50-residue–extended C-terminus, which binds plasmin(ogen). We developed 2 new ELISAs to measure the antigen levels of free total α2AP and free C-terminally intact α2AP to investigate whether α2AP antigen levels or α2AP C-terminal cleavage were associated with myocardial infarction (MI) in 320 male MI survivors and 169 age-matched controls. Patients had 15.2% reduced total α2AP antigen levels compared with controls (93.8 vs 110.6 U/dL, P < .001), with a 10.1-fold (95% confidence interval [CI]: 5.5-18.9) increased MI risk for levels in the 1st quartile compared with the 4th quartile. The percentage of C-terminal cleavage did not differ between patients and controls (38.7% and 38.1%, respectively, P = .44). In addition, all individuals were genotyped for the polymorphism Arg407Lys, which is located near the start of the extended C-terminus. Arg407Lys was not associated with α2AP C-terminal cleavage, total α2AP antigen levels, or MI risk (odds ratios compared with Arg/Arg: Arg/Lys 0.74, 95% CI: 0.50-1.10; Lys/Lys 0.77, 95% CI: 0.31-1.92). Our data show that levels of free full-length α2AP were decreased in MI, that the percentage of C-terminally cleaved α2AP was unaltered, and that Arg407Lys did not influence α2AP levels or MI risk.
Introduction
Plasma glycoprotein α2-antiplasmin (α2AP) is a member of the Ser protease inhibitor (serpin) superfamily.1 The main function of α2AP is the inhibition of plasmin-mediated fibrinolysis by the rapid inactivation of plasmin.2,3 α2AP forms a stable 1:1 stoichiometric complex with plasmin, first by the noncovalent and reversible binding of the C-terminus of α2AP to the Lys-binding sites at kringles 1-3 of plasmin(ogen), followed by the formation of a covalent bond between the reactive site of α2AP and the active-site Ser of plasmin.4 In addition, during clot formation, α2AP is cross-linked to each fibrin α chain by activated coagulation factor FXIII (FXIIIa), thereby protecting the forming fibrin clot from plasmin-mediated lysis.5
Full-length α2AP consists of 464 amino acids, contains 11%-14% carbohydrate, and has a molecular weight of ∼ 67 kDa.3,6 During circulation in the plasma, the protein undergoes both N- and C-terminal proteolytic cleavages, which significantly modify its activities. Approximately 70% of circulating α2AP is cleaved between the Pro residue on position 12 and the Asn residue on position 13 by the antiplasmin cleaving enzyme,7 resulting in a 452–amino acid residue version with an Asn at the N-terminus.8,9 It has been shown that this N-terminally cleaved Asn-α2AP form becomes cross-linked to fibrin more effectively and ∼ 13 times faster than the N-terminally intact α2AP form (also called Met-α2AP), thereby increasing the fibrinolytic resistance of the fibrin clot.7,8 Cross-linking occurs by FXIIIa via the Gln residue at position 14, which in Asn-α2AP is only 1 amino acid residue from the N-terminus at position 2. It appears that the N-terminal 12 residues in full-length α2AP have an inhibitory effect on the cross-linking reaction.
In addition to N-terminal variation, there are also C-terminal variants of the α2AP molecule. It was first shown by studies on the purification of α2AP and the kinetics of plasmin inhibition that 2 immunochemically indistinguishable but different molecular species of α2AP exist, both of which can interact with plasmin but only one of which binds to plasminogen.10-12 These 2 forms have been termed the plasminogen-binding and the nonplasminogen-binding forms of α2AP. Subsequently, Sasaki et al determined that the plasminogen-binding site of α2AP is located in the C-terminal 26 amino acids of the molecule.13 Of the total circulating α2AP, ∼ 65% is C-terminally intact, whereas the remainder is cleaved and lacks the C-terminus.13-17 This cleaved form remains an active plasmin inhibitor, but reacts more slowly with plasmin18 because it does not bind to the Lys-binding sites of plasmin(ogen).10,11
Compared with other serpins of the hemostatic system, all of which consist of ∼ 410 amino acid residues, full-length α2AP contains ∼ 55 additional amino acid residues.16,19 Alignment of these serpins shows that α2AP is extended at the C-terminus (Figure 1). A coding genetic variant in exon 10 of the α2AP gene, an Arg-to-Lys substitution in codon 407 (Arg407Lys; rs1057335), is located very near the start of this extended C-terminus.
Alignment of serpins. Shown are the C-termini with the location of polymorphism Arg407Lys in α2AP. Residues in bold show identical (*), conserved (:) or semiconserved (.) substitutions. ATIII indicates antithrombin III; α1AT, α-1-antitrypsin; α1AC, α-1-antichymotrypsin; PAI1, plasminogen activator inhibitor 1; and PCI, protein C inhibitor. The alignment was made with ClustalW2.30
Alignment of serpins. Shown are the C-termini with the location of polymorphism Arg407Lys in α2AP. Residues in bold show identical (*), conserved (:) or semiconserved (.) substitutions. ATIII indicates antithrombin III; α1AT, α-1-antitrypsin; α1AC, α-1-antichymotrypsin; PAI1, plasminogen activator inhibitor 1; and PCI, protein C inhibitor. The alignment was made with ClustalW2.30
In the present study, we developed and applied 2 new ELISAs to measure the free circulating antigen levels of total α2AP and C-terminally intact α2AP to study C-terminal variations in α2AP. ELISA measurements were performed in samples from 169 healthy control individuals and 320 male survivors of myocardial infarction (MI) who were age-matched with control individuals. In addition, plasma clot lysis times were measured in the control group and all individuals were genotyped for the α2AP polymorphism Arg407Lys.
Methods
Study population
The design of the study population has been described previously.20 In brief, male MI patients younger than 65 years were recruited from accident and emergency admissions at Leeds General Infirmary (Leeds, United Kingdom) between November 1998 and February 2003. Detailed clinical histories were taken and venous blood samples were collected at least 2 months after the acute event into 0.109M citrate (9:1 vol/vol ratio of blood to anticoagulant). As control subjects, healthy male individuals frequency-matched for age in 2-year age blocks and without any personal or family history of premature cardiovascular disease were selected from a local family health register and recruited by letter invitation. All individuals gave written informed consent in accordance with the Declaration of Helsinki and according to a protocol approved by the Leeds Research Ethics Committee. Subjects fasted and abstained from cigarette smoking overnight for at least 10 hours before blood sampling. Plasma was prepared by centrifugation for 20 minutes at 3000g and stored in 0.5-mL aliquots at −40°C.
α2AP antigen measurements
Free circulating antigen levels of total α2AP and C-terminally intact α2AP were measured by 2 newly developed ELISA assays. For both ELISAs, 96-well microtiter plates (Nunc) were coated (110 μL/well in 0.1M Na2CO3, pH 9.6) and incubated overnight at 4°C with 3 μg/mL of a custom made rabbit anti–human α2AP antibody raised against a peptide corresponding to amino acids Gln14 to Pro30 of the α2AP protein (Charles River Laboratories). After coating, plates were blocked with 1% casein in TBS (50mM Tris and 150mM NaCl, pH 7.4) for 1 hour at room temperature. Plasma samples were diluted in a high-salt buffer (50mM Tris and 1M NaCl, pH 7.4) to reduce the binding of α2AP with other plasma proteins such as plasminogen and fibrinogen. Diluted plasma sample (100 μL) was added to the wells and plates were incubated for 2 hours at room temperature. Bound total α2AP was detected with 100 μL of 1:1000 diluted HRP-conjugated rabbit anti–human α2AP (Enzyme Research Laboratories) for 1.5 hours at room temperature. Bound C-terminally intact α2AP was detected with 100 μL of a 1:1000 diluted mouse monoclonal antibody against the C-terminus of α2AP21 (3613; American Diagnostica) for 1.5 hours at room temperature, followed by 100 μL of 1:1000 diluted HRP-conjugated rabbit anti–mouse IgG (Dako) for 1.5 hours at room temperature. The specificity of the antibodies for total α2AP and C-terminally intact α2AP, respectively, was confirmed by Western blotting. For development, plates were incubated with 100 μL/well of 0.667 mg/mL o-phenylenediamine solution (Dako). After 15 minutes, the reaction was stopped by adding 1M H2SO4 (50 μL/well) and the absorbance at 490 nm was read spectrophotometrically. Between all incubation steps, wells were washed 3 times with 10mM Na2HPO4, 500mM NaCl, and 0.1% Tween-20, pH 7.4. Results of free total α2AP levels and C-terminally intact α2AP levels were expressed in arbitrary units (aU) per decaliter. The assays were calibrated using 1:500-1:32 000 dilutions of pooled normal plasma, which by definition contained 100 aU/dL of total α2AP and 65 aU/dL of C-terminally intact α2AP. The α2AP antigen level of a plasma sample was calculated as the mean result of the measurements of 2 different dilutions (1:1000 and 1:4000). The variation of the results of the 2 dilutions was on average below 15%. Intra- and interassay variation was 2.1% and 10.2%, respectively, for the total α2AP ELISA and 3.3% and 8.0%, respectively, for the C-terminally intact α2AP ELISA. For assay validation, purified human α2AP (from Calbiochem), α2AP-depleted human plasma, and purified human plasmin (both from Enzyme Research Laboratories) reagents were used.
Plasma clot lysis assay
Fibrin structure and function were previously analyzed in the plasma samples of the control individuals using a high-throughput turbidimetric lysis assay, as described previously.22 Briefly, 25 μL of citrated plasma was added to 75 μL of lysis buffer (0.05 mol/L of Tris-HCl and 0.1 mol/L of NaCl, pH 7.4, containing 12.5 ng of tissue plasminogen activator for a final concentration of 83 ng/mL). Fibrin polymerization was initiated with the addition of 50 μL of an activation mixture (final concentrations: 0.03 U/mL of thrombin and 7.5 mmol/L of CaCl2 in buffer). Plates were read at 340 nm every 12 seconds in an ELx-808 microplate reader (BioTek). Time to 50% lysis was calculated as the time from maximum absorbance to the time at which a 50% reduction in absorbance occurred.22
Genotyping
Genotyping of the Arg407Lys polymorphism was performed by PCR and restriction fragment length polymorphism analysis using the restriction enzyme NLaIV in the presence of bovine serum albumin. Fragments were run on 2% agarose gels. PCR with forward primer CGTGGGATCTCCGAGCAG and reverse primer CTGCCAAACTGGGGGTAAT resulted in a fragment of 371 bp. Digestion by NLaIV resulted in fragments of 208 and 163 bp for Arg homozygotes; 371, 208, and 163 bp for Arg/Lys heterozygotes, and 371 bp for Lys homozygotes.
Cloning, expression, and purification of recombinant α2AP variants
To determine the coating ELISA antibody specificity for Met-α2AP and Asn-α2AP, recombinant α2AP variants were expressed and purified. Plasmid cDNA of full-length α2AP from vector PCMV6-XL4 (OriGene) was subcloned into expression vector pMT/BiP/V5-His A (Invitrogen) using the BglII and XbaI restriction sites. The α2AP stop codon and 3′UTR, and later the N-terminal 12 amino acids, were removed from the full-length sequence using QuickChange II site-directed mutagenesis kits (Agilent Technologies). After mutagenesis, the inserts were subcloned into a clean vector using the NsiI and AgeI restriction sites. After subcloning, the nucleotide sequence of the inserts and the ligation sites of the variants were confirmed by DNA sequencing.
Recombinant Met-α2AP and Asn-α2AP variants were expressed by transfection into Drosophila Schneider S2 cells (Invitrogen). For stable cell lines, 19 μg of plasmid was cotransfected with 1 μg of the selection plasmid pCoHygro. For expression of the α2AP variants, transfected Drosophila Schneider S2 cells were cultured and extended in Schneider medium containing l-Glu (Invitrogen), 10% heat-inactivated fetal bovine serum (Sigma-Aldrich), 50 U/mL of penicillin, 50 μg/mL of streptomycin, 10 μg/mL of amphotericin B (Antibiotic/antimycotic solution, Invitrogen), 500μM CuSO4 (Sigma-Aldrich), 0.05% Pluronic F-68 (Invitrogen), and 250 μg/mL of hygromycin B (Invitrogen). Cells were cultured with gentle stirring at 70 rpm at 28°C for 4-5 days. The cells were harvested by centrifugation at 1000g for 5 minutes and passed in a dilution of 5 × 105 cells/mL. The cell medium containing the expressed α2AP protein was stored at −20°C until purification.
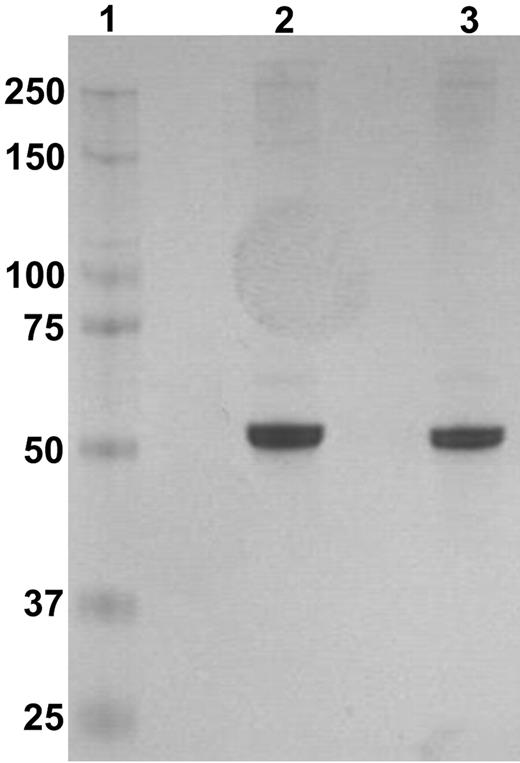
To purify the expressed recombinant α2AP variants, the cell medium was centrifuged at 2000g for 30 minutes, followed by filtration through Whatman paper. The filtered medium was spiked with 30mM imidazole (Sigma-Aldrich) and loaded onto a HisTrap HP column (GE Amersham Biosciences). After loading, the column was washed with equilibrium buffer (20mM Na2HPO4, 0.5M NaCl, and 30mM imidazole, pH 7.4), and bound α2AP was eluted with elution buffer (20mM Na2HPO4, 0.5M NaCl, and 165mM imidazole, pH 7.4). The eluted protein was subsequently dialyzed into 50mM Tris and 150mM NaCl, pH 7.4, and stored in aliquots at −80°C. The concentration of purified α2AP in solution was determined from its absorbance at 280 nm using an absorption coefficient of 0.67 for a 1 mg/mL solution.11 The purified α2AP protein was > 95% pure (Figure 2).
Purified recombinant α2AP protein. Recombinant protein was applied to electrophoresis on a 4%-12% Bis-Tris gel. Lane 1 is the marker; Lane 2, Met-α2AP; and lane 3, Asn-α2AP.
Purified recombinant α2AP protein. Recombinant protein was applied to electrophoresis on a 4%-12% Bis-Tris gel. Lane 1 is the marker; Lane 2, Met-α2AP; and lane 3, Asn-α2AP.
Statistical analysis
Normal distribution of the total α2AP antigen levels, C-terminally intact α2AP antigen levels, and the percentage of C-terminally cleaved α2AP was tested with the Kolmogorov-Smirnov test. The percentage of C-terminally cleaved α2AP was calculated as a portion of the total α2AP levels, which represents the percentage of C-terminally intact α2AP, followed by subtraction from 100%. Differences in total α2AP antigen levels and the percentage of C-terminal cleavage between patients and control individuals were tested using Student t test. The effect of total α2AP antigen levels and α2AP C-terminal cleavage on the association with MI was investigated by grouping the levels into quartiles based on the distribution among control individuals. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated as a measure of the relative risk. Adjustments for age, body mass index (BMI), smoking, diabetes, hypertension, and cholesterol levels were made using unconditional logistical regression. ANOVA analyses using tertiles of α2AP activity levels were performed to calculate the statistical significance of the trend of the correlation between α2AP antigen and activity levels. Hardy-Weinberg equilibrium of polymorphism Arg407Lys was tested by χ2 analysis. ANOVA analyses were performed to investigate the association between the polymorphism Arg407Lys and α2AP C-terminal cleavage, total α2AP antigen levels, and plasma clot lysis times. ORs and 95% CIs were calculated to investigate the association between Arg407Lys and the risk of MI.
Results
The characteristics of the study subjects have been described previously.20 The median age for the 320 MI patients was 56.3 years (25th-75th percentiles: 50.8-61.6 years) and the median age for the 169 control individuals was 54.4 years (25th-75th percentiles: 49.6-60.6 years). Risk factors for arterial disease such as smoking, hypertension, diabetes, obesity, and hypercholesterolemia were more prevalent in the patients than in the control individuals.20
ELISA validation
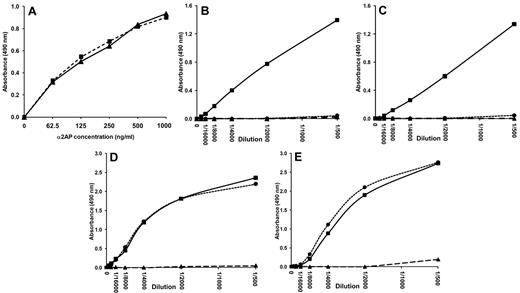
Using recombinant Met-α2AP and Asn-α2AP produced in-house, we confirmed that the coating antibody used in both ELISAs binds equally to both forms of α2AP (Figure 3A). This indicates that the antigen measurements on total α2AP and the C-terminal variation of α2AP were independent of N-terminal variation of α2AP. Using Western blot analysis on commercially available human α2AP, which contains both C-terminally intact α2AP and C-terminally cleaved α2AP, we verified that the antibody against the C-terminus of α2AP only detects the full-length form of α2AP21 (data not shown). To determine whether both ELISAs were able to detect plasmin-antiplasmin complexes, an excess of plasmin was added to our pooled normal plasma or to a purified system. Results showed that neither ELISA detected plasmin-antiplasmin complexes formed in the normal pool of plasma (Figure 3B-C) or in a purified system (data not shown). These data demonstrate that our ELISAs detect free circulating α2AP only. Recovery experiments were performed by adding known quantities of human α2AP to α2AP-depleted plasma. Both ELISAs showed good recovery of α2AP (range investigated: 70-2.2 ng/mL) in α2AP-depleted plasma with good linearity (Figure 3D-E). Recovery for the total α2AP antigen ELISA ranged from 96.0%-131.9%. Recovery for the C-terminally intact α2AP antigen ELISA ranged from 100.9%-146.9%. These results indicate no effects of the biologic sample matrix on the ELISA assays.
ELISA validation. (A) The coating antibody binds equally to Met-α2AP (▴, solid line) and Asn-α2AP (■, dashed line), as shown by detection with HRP-conjugated rabbit anti–human α2AP. In both the total α2AP antigen ELISA (B) and the C-terminally intact α2AP antigen ELISA (C), the absorbance of pooled normal plasma (■, solid line) was completely lost after the addition of a 5× (●, dotted line) or a 10× (▴, dashed line) excess of plasmin. Recovery plots for both the total α2AP antigen ELISA (D) and the C-terminally intact α2AP antigen ELISA (E) show good recovery of purified α2AP added to α2AP-depleted plasma (●, dotted line) compared with purified α2AP only (■, solid line). α2AP-depleted plasma (▴, dashed line) did not respond in either ELISA.
ELISA validation. (A) The coating antibody binds equally to Met-α2AP (▴, solid line) and Asn-α2AP (■, dashed line), as shown by detection with HRP-conjugated rabbit anti–human α2AP. In both the total α2AP antigen ELISA (B) and the C-terminally intact α2AP antigen ELISA (C), the absorbance of pooled normal plasma (■, solid line) was completely lost after the addition of a 5× (●, dotted line) or a 10× (▴, dashed line) excess of plasmin. Recovery plots for both the total α2AP antigen ELISA (D) and the C-terminally intact α2AP antigen ELISA (E) show good recovery of purified α2AP added to α2AP-depleted plasma (●, dotted line) compared with purified α2AP only (■, solid line). α2AP-depleted plasma (▴, dashed line) did not respond in either ELISA.
α2AP antigen levels and C-terminal cleavage
Histograms of the distribution of α2AP antigen levels and the percentage of C-terminally cleaved α2AP in control individuals are shown in Figure 4A. The distributions did not deviate from the normal distribution (Kolmogorov-Smirnov, P = .41 for total α2AP antigen levels, P = .94 for C-terminally intact α2AP antigen levels, and P = .63 for percentage of C-terminally cleaved α2AP). We found a 15.2% reduction in free total α2AP antigen levels in patients (93.8 aU/dL, 95% CI: 92.1-95.5) compared with control individuals (110.6 aU/dL, 95% CI: 108.0-113.1; Figure 4B). Similarly, free C-terminally intact α2AP levels were 15.0% reduced in the patient samples (57.2 aU/dL, 95% CI: 56.5-57.9) compared with control individuals (67.3 aU/dL, 95% CI: 65.8-68.8; Figure 4B). The percentage of α2AP C-terminal cleavage did not differ between patients and control individuals, because we found a similar percentage for both groups (38.1%, 95% CI: 37.2-39.0 and 38.7%, 95% CI: 37.6-39.8, respectively; Figure 4B).
Levels of α2AP in controls and MI patients. (A) Histograms of α2AP antigen levels and the distribution of the percentage of C-terminally cleaved α2AP in control individuals. (B) Error bar plots (means ± 95% CI) of total α2AP levels (I), C-terminally intact α2AP levels (II), and percentage of C-terminally cleaved α2AP (III) in patients and control individuals.
Levels of α2AP in controls and MI patients. (A) Histograms of α2AP antigen levels and the distribution of the percentage of C-terminally cleaved α2AP in control individuals. (B) Error bar plots (means ± 95% CI) of total α2AP levels (I), C-terminally intact α2AP levels (II), and percentage of C-terminally cleaved α2AP (III) in patients and control individuals.
Because most patients were using aspirin, statins, or both at the time of venipuncture, and because risk factors for arterial disease such as smoking, hypertension, diabetes, and hypercholesterolemia were more prevalent in the patients than in the control individuals, we investigated the effects of these variables on free α2AP antigen levels and C-terminal cleavage, as shown in Table 1. The levels of free α2AP in the absence or the presence of these variables did not differ significantly. Therefore, these data indicate that the various risk factors do not have major effects on free α2AP antigen levels or C-terminal cleavage.
Mean α2AP antigen level and percentage of C-terminally cleaved α2AP with 95%CI in patients and controls
| . | . | α2AP antigen level, aU/dL . | C-terminally cleaved α2AP, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients . | Controls . | Patients . | Controls . | ||||||
| n . | Mean (95% CI) . | n . | Mean (95% CI) . | n . | Mean (95% CI) . | n . | Mean (95% CI) . | ||
| Statin/aspirin use | No | 7 | 91.2 (79.2-103.2) | 165 | 110.8 (108.2-113.3) | 7 | 38.1 (32.0-44.1) | 165 | 38.5 (37.4-39.6) |
| Yes | 313 | 93.9 (92.1-95.6) | 4 | 102.7 (66.0-139.3) | 313 | 38.1 (37.2-39.0) | 4 | 46.2 (38.4-54.0) | |
| Hypertension* | No | 261 | 93.4 (91.5-95.3) | 156 | 110.5 (107.8-113.1) | 261 | 37.8 (36.8-38.7) | 156 | 38.8 (37.6-40.0) |
| Yes | 59 | 95.7 (91.7-99.8) | 13 | 111.9 (103.0-120.7) | 59 | 39.4 (37.1-41.7) | 13 | 36.7 (33.7-39.7) | |
| Smoking | No | 241 | 93.5 (91.6-95.5) | 123 | 112.0 (108.9-115.2) | 241 | 37.6 (36.6-38.7) | 123 | 38.8 (37.5-40.2) |
| Yes | 79 | 94.6 (91.1-98.2) | 46 | 106.6 (102.6-110.7) | 79 | 39.4 (37.9-41.0) | 46 | 38.2 (36.2-40.1) | |
| Diabetes mellitus | No | 251 | 93.1 (91.2-95.0) | 157 | 110.8 (108.1-113.4) | 251 | 38.3 (37.3-39.3) | 157 | 38.5 (37.4-39.7) |
| Yes | 63 | 96.0 (91.4-100.5) | 9 | 109.2 (101.2-117.2) | 63 | 37.3 (35.1-39.5) | 9 | 38.1 (33.4-42.9) | |
| Total cholesterol (quartiles) | |||||||||
| ≤ 4.6 | 177 | 92.5 (90.2-94.8) | 44 | 108.1 (102.7-113.4) | 177 | 37.4 (36.1-38.7) | 44 | 38.1 (35.8-40.3) | |
| 4.7-5.3 | 79 | 95.1 (91.7-98.6) | 39 | 109.3 (104.2-114.3) | 79 | 39.2 (37.6-40.9) | 39 | 38.6 (36.6-40.7) | |
| 5.4-5.9 | 31 | 96.1 (89.2-102.9) | 36 | 110.1 (103.8-116.3) | 31 | 38.5 (35.6-41.4) | 36 | 38.8 (36.1-41.5) | |
| ≥ 6.0 | 28 | 93.7 (88.4-99.0) | 47 | 114.6 (110.1-119.0) | 28 | 38.3 (35.3-41.2) | 47 | 38.5 (36.4-40.6) | |
| . | . | α2AP antigen level, aU/dL . | C-terminally cleaved α2AP, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients . | Controls . | Patients . | Controls . | ||||||
| n . | Mean (95% CI) . | n . | Mean (95% CI) . | n . | Mean (95% CI) . | n . | Mean (95% CI) . | ||
| Statin/aspirin use | No | 7 | 91.2 (79.2-103.2) | 165 | 110.8 (108.2-113.3) | 7 | 38.1 (32.0-44.1) | 165 | 38.5 (37.4-39.6) |
| Yes | 313 | 93.9 (92.1-95.6) | 4 | 102.7 (66.0-139.3) | 313 | 38.1 (37.2-39.0) | 4 | 46.2 (38.4-54.0) | |
| Hypertension* | No | 261 | 93.4 (91.5-95.3) | 156 | 110.5 (107.8-113.1) | 261 | 37.8 (36.8-38.7) | 156 | 38.8 (37.6-40.0) |
| Yes | 59 | 95.7 (91.7-99.8) | 13 | 111.9 (103.0-120.7) | 59 | 39.4 (37.1-41.7) | 13 | 36.7 (33.7-39.7) | |
| Smoking | No | 241 | 93.5 (91.6-95.5) | 123 | 112.0 (108.9-115.2) | 241 | 37.6 (36.6-38.7) | 123 | 38.8 (37.5-40.2) |
| Yes | 79 | 94.6 (91.1-98.2) | 46 | 106.6 (102.6-110.7) | 79 | 39.4 (37.9-41.0) | 46 | 38.2 (36.2-40.1) | |
| Diabetes mellitus | No | 251 | 93.1 (91.2-95.0) | 157 | 110.8 (108.1-113.4) | 251 | 38.3 (37.3-39.3) | 157 | 38.5 (37.4-39.7) |
| Yes | 63 | 96.0 (91.4-100.5) | 9 | 109.2 (101.2-117.2) | 63 | 37.3 (35.1-39.5) | 9 | 38.1 (33.4-42.9) | |
| Total cholesterol (quartiles) | |||||||||
| ≤ 4.6 | 177 | 92.5 (90.2-94.8) | 44 | 108.1 (102.7-113.4) | 177 | 37.4 (36.1-38.7) | 44 | 38.1 (35.8-40.3) | |
| 4.7-5.3 | 79 | 95.1 (91.7-98.6) | 39 | 109.3 (104.2-114.3) | 79 | 39.2 (37.6-40.9) | 39 | 38.6 (36.6-40.7) | |
| 5.4-5.9 | 31 | 96.1 (89.2-102.9) | 36 | 110.1 (103.8-116.3) | 31 | 38.5 (35.6-41.4) | 36 | 38.8 (36.1-41.5) | |
| ≥ 6.0 | 28 | 93.7 (88.4-99.0) | 47 | 114.6 (110.1-119.0) | 28 | 38.3 (35.3-41.2) | 47 | 38.5 (36.4-40.6) | |
CI indicates confidence interval.
Hypertension was determined from case notes and current use of antihypertensive therapy.
OR estimates for MI by α2AP levels
We investigated whether free circulating total α2AP antigen levels and C-terminally intact α2AP antigen levels were associated with MI by ranking the antigen levels into quartiles based on the distribution among control individuals. We found that decreased total α2AP antigen levels were dose-dependently associated with MI, with an OR of 10.1 for men with α2AP levels in the 1st quartile compared with the 4th quartile (Table 2). This OR did not change after adjustment for age, BMI, smoking, diabetes, hypertension, and cholesterol levels. Risk estimates for C-terminally intact α2AP antigen levels showed a similar trend (Table 2).
Association of MI with decreasing quartiles of total α2AP antigen level and C-terminally intact α2AP antigen level
| Quartile . | 1 . | 2 . | 3 . | 4 . | P for trend . |
|---|---|---|---|---|---|
| Association with total α2AP antigen level | |||||
| Cut-off point | 99.3 | 109.8 | 119.4 | ||
| Patients, n | 213 | 53 | 33 | 21 | |
| Control individuals, n | 42 | 44 | 41 | 42 | |
| Crude OR (95%CI) | 10.1 (5.5-18.9) | 2.4 (1.3-4.7) | 1.6 (0.8-3.2) | 1* | < .001 |
| Adjusted OR (95%CI)† | 9.9 (4.8-20.1) | 2.0 (0.9-4.4) | 1.4 (0.6-3.1) | 1* | < .001 |
| Association with C-terminally intact α2AP antigen level | |||||
| Patients, n | 235 | 59 | 21 | 5 | |
| Control individuals, n | 42 | 43 | 42 | 42 | |
| Crude OR (95%CI) | 47.0 (17.6-125.7) | 11.5 (4.2-31.6) | 4.2 (1.4-12.2) | 1* | < .001 |
| Adjusted OR (95%CI)† | 55.4 (18.3-167.3) | 11.2 (3.6-34.4) | 4.1 (1.2-13.5) | 1* | < .001 |
| Quartile . | 1 . | 2 . | 3 . | 4 . | P for trend . |
|---|---|---|---|---|---|
| Association with total α2AP antigen level | |||||
| Cut-off point | 99.3 | 109.8 | 119.4 | ||
| Patients, n | 213 | 53 | 33 | 21 | |
| Control individuals, n | 42 | 44 | 41 | 42 | |
| Crude OR (95%CI) | 10.1 (5.5-18.9) | 2.4 (1.3-4.7) | 1.6 (0.8-3.2) | 1* | < .001 |
| Adjusted OR (95%CI)† | 9.9 (4.8-20.1) | 2.0 (0.9-4.4) | 1.4 (0.6-3.1) | 1* | < .001 |
| Association with C-terminally intact α2AP antigen level | |||||
| Patients, n | 235 | 59 | 21 | 5 | |
| Control individuals, n | 42 | 43 | 42 | 42 | |
| Crude OR (95%CI) | 47.0 (17.6-125.7) | 11.5 (4.2-31.6) | 4.2 (1.4-12.2) | 1* | < .001 |
| Adjusted OR (95%CI)† | 55.4 (18.3-167.3) | 11.2 (3.6-34.4) | 4.1 (1.2-13.5) | 1* | < .001 |
MI indicates myocardial infarction.
Reference category.
Adjustment for age, body mass index (BMI), smoking, diabetes, hypertension, and cholesterol levels.
Comparison of α2AP antigen and activity levels
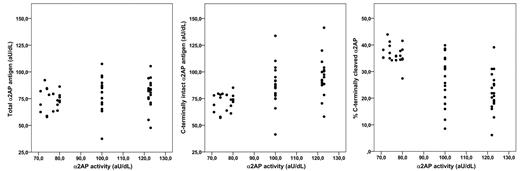
Other studies that have reported on α2AP levels have measured the α2AP activity levels. Because we measured circulating free antigen levels of α2AP, we decided to investigate the correlation between α2AP antigen levels as measured by our ELISAs and α2AP activity levels. For this, the antigen levels of total α2AP and C-terminally intact α2AP were measured in a subset of 60 plasma samples from control individuals who participated in the Multiple Environmental and Genetic Assessment (MEGA) study.23 The α2AP activity levels in these samples had been measured previously.24 The samples were selected to have low, medium, or high α2AP activity levels. There was no clear correlation between total α2AP antigen and α2AP activity levels (Figure 5, ANOVA P = .33). In contrast, there was a positive correlation between C-terminally intact α2AP antigen levels and α2AP activity levels (ANOVA P < .001) and a negative correlation between the percentage of C-terminally cleaved α2AP and α2AP activity levels (ANOVA P < .001). These results suggest that the α2AP activity assay is mainly sensitive to the levels of C-terminally intact α2AP, the more active plasmin inhibitor.
Comparison of α2AP antigen with activity levels. Levels of total free α2AP antigen, C-terminally intact α2AP antigen, and percentage of C-terminally cleaved α2AP were compared with activity α2AP levels as measured using a chromogenic substrate assay in a subset of 60 plasma samples from the MEGA study.23,24 There was no clear relationship between total α2AP antigen levels and α2AP activity levels, whereas C-terminally intact α2AP levels and percentage of C-terminally cleaved α2AP showed a positive and negative association, respectively.
Comparison of α2AP antigen with activity levels. Levels of total free α2AP antigen, C-terminally intact α2AP antigen, and percentage of C-terminally cleaved α2AP were compared with activity α2AP levels as measured using a chromogenic substrate assay in a subset of 60 plasma samples from the MEGA study.23,24 There was no clear relationship between total α2AP antigen levels and α2AP activity levels, whereas C-terminally intact α2AP levels and percentage of C-terminally cleaved α2AP showed a positive and negative association, respectively.
Polymorphism Arg407Lys
The genotype distribution of Arg407Lys was in Hardy-Weinberg equilibrium. Allele frequencies in control individuals were 0.77 for the Arg allele and 0.23 for the Lys allele. For patients, allele frequencies were 0.81 for the Arg allele and 0.19 for the Lys allele (P = .32). Because the Arg407Lys polymorphism is located near the start of the extended C-terminus of α2AP, we hypothesized that this polymorphism could influence C-terminal cleavage of α2AP. To test this hypothesis, we investigated the association between Arg407Lys and the percentage of C-terminally cleaved α2AP. Arg407Lys did not influence C-terminal cleavage of α2AP, because we found a similar percentage of C-terminal cleavage for all 3 genotypes in both control individuals and patients (Table 3). In the control individuals, we found that Lys/Lys-homozygotes had slightly reduced total α2AP levels and reduced C-terminally intact α2AP levels compared with Arg/Arg-homozygotes and Arg/Lys-heterozygotes, but this was not the case in the patients. We also investigated the effect of Arg407Lys on plasma clot lysis times in the control individuals. Overall, we found that Arg407Lys did not influence plasma clot lysis times (Table 3). However, as expected from the reduced α2AP levels in the Lys/Lys homozygotes, these individuals also had the lowest plasma clot lysis times.
Distribution of polymorphism Arg407Lys and association with α2AP levels and plasma clot lysis times
| . | Patients (n = 319) . | Control individuals (n = 169) . | ||
|---|---|---|---|---|
| C-terminally cleaved α2AP, % | n | mean (95% CI) | n | mean (95% CI) |
| 407 Arg/Arg | 209 | 37.7 (36.6-38.9) | 99 | 37.8 (36.4-39.2) |
| 407 Arg/Lys | 97 | 38.5 (37.1-40.0) | 62 | 39.9 (38.0-41.9) |
| 407 Lys/Lys | 13 | 39.6 (35.3-43.8) | 8 | 38.7 (33.3-44.0) |
| P = .575 | P = .205 | |||
| Total α2AP antigen level, aU/dL | ||||
| 407 Arg/Arg | 209 | 93.6 (91.4-95.9) | 99 | 110.0 (106.6-113.4) |
| 407 Arg/Lys | 97 | 94.0 (91.1-97.0) | 62 | 112.9 (108.8-116.9) |
| 407 Lys/Lys | 13 | 94.4 (95.9-102.9) | 8 | 99.9 (83.5-116.3) |
| P = .969 | P = .105 | |||
| C-terminally intact α2AP antigen level, aU/dL | ||||
| 407 Arg/Arg | 209 | 57.3 (56.4-58.3) | 99 | 67.8 (66.0-69.7) |
| 407 Arg/Lys | 97 | 57.0 (55.9-58.1) | 62 | 67.3 (64.8-69.8) |
| 407 Lys/Lys | 13 | 56.5 (52.6-60.3) | 8 | 60.7 (52.7-68.7) |
| P = .851 | P = .129 | |||
| Plasma clot lysis time, min | ||||
| 407 Arg/Arg | - | 87 | 10.0 (9.4-10.6) | |
| 407 Arg/Lys | - | 57 | 10.3 (9.4-11.2) | |
| 407 Lys/Lys | - | 8 | 8.7 (7.3-10.1) | |
| P = .355 | ||||
| . | Patients (n = 319) . | Control individuals (n = 169) . | ||
|---|---|---|---|---|
| C-terminally cleaved α2AP, % | n | mean (95% CI) | n | mean (95% CI) |
| 407 Arg/Arg | 209 | 37.7 (36.6-38.9) | 99 | 37.8 (36.4-39.2) |
| 407 Arg/Lys | 97 | 38.5 (37.1-40.0) | 62 | 39.9 (38.0-41.9) |
| 407 Lys/Lys | 13 | 39.6 (35.3-43.8) | 8 | 38.7 (33.3-44.0) |
| P = .575 | P = .205 | |||
| Total α2AP antigen level, aU/dL | ||||
| 407 Arg/Arg | 209 | 93.6 (91.4-95.9) | 99 | 110.0 (106.6-113.4) |
| 407 Arg/Lys | 97 | 94.0 (91.1-97.0) | 62 | 112.9 (108.8-116.9) |
| 407 Lys/Lys | 13 | 94.4 (95.9-102.9) | 8 | 99.9 (83.5-116.3) |
| P = .969 | P = .105 | |||
| C-terminally intact α2AP antigen level, aU/dL | ||||
| 407 Arg/Arg | 209 | 57.3 (56.4-58.3) | 99 | 67.8 (66.0-69.7) |
| 407 Arg/Lys | 97 | 57.0 (55.9-58.1) | 62 | 67.3 (64.8-69.8) |
| 407 Lys/Lys | 13 | 56.5 (52.6-60.3) | 8 | 60.7 (52.7-68.7) |
| P = .851 | P = .129 | |||
| Plasma clot lysis time, min | ||||
| 407 Arg/Arg | - | 87 | 10.0 (9.4-10.6) | |
| 407 Arg/Lys | - | 57 | 10.3 (9.4-11.2) | |
| 407 Lys/Lys | - | 8 | 8.7 (7.3-10.1) | |
| P = .355 | ||||
CI indicates confidence interval.
We also examined whether Arg407Lys influenced the risk of MI, and found that it did not, because the small reduction in risk for MI in Arg/Lys heterozygotes (OR: 0.74; 95% CI: 0.50-1.10) and Lys/Lys homozygotes (OR: 0.77; 95% CI: 0.31-1.92) did not reach statistical significance.
Discussion
After expression of the full, mature protein, α2AP undergoes both N- and C-terminal proteolytic cleavages, which significantly modify its activities. Whereas the effect of N-terminal cleavage of α2AP on coagulation and fibrinolysis has been studied in depth previously,7-9 the effect of C-terminal variation of α2AP on coagulation and fibrinolysis is less well known. In the present study, we developed 2 new ELISAs to measure total α2AP antigen level and the C-terminally intact α2AP antigen level to investigate the effect of total α2AP antigen levels and α2AP C-terminal cleavage on the association with MI.
We found a 15% reduction in total α2AP antigen levels in male survivors of MI compared with age-matched control individuals. After adjustment for age, BMI, smoking, diabetes, hypertension, and cholesterol levels, low total α2AP antigen levels were still associated with MI. This is in contrast to what we expected and to what has been reported previously regarding α2AP levels in thrombotic disease. The Atherosclerosis Risk in Communities (ARIC) study showed in a case-cohort design that α2AP levels were positively associated with coronary heart disease.25 However, this association disappeared after adjustment for classic coronary risk factors such as systolic blood pressure, total and HDL cholesterol, diabetes, and smoking status.25 Recent results from the Study of Myocardial Infarctions Leiden (SMILE) study showed increased α2AP levels in men who suffered a first MI, with a 1.4-fold increase in risk (4th quartile vs 1st quartile) after adjusting for cardiovascular risk factors.26 The main difference between these studies and ours is that we measured free circulating antigen levels of α2AP, whereas all other studies measured total α2AP activity toward plasmin inhibition using a chromogenic substrate assay. In this activity assay, a known excess of plasmin is incubated with the plasma sample, which results in the formation of plasmin-antiplasmin complexes. Subsequently, the residual quantity of plasmin is determined by its amidolytic activity toward the chromogenic substrate, which is inversely proportional to the activity level of α2AP contained in the plasma sample. We examined the correlation between this activity assay and our antigen measurements by measuring the total α2AP antigen levels and C-terminally intact α2AP antigen levels in 60 control plasma samples from the MEGA study23 for which α2AP activity levels had been measured previously.24 Correlation analyses showed no clear correlation between free total α2AP antigen levels and α2AP activity levels, but did show a good correlation between C-terminally intact α2AP antigen levels and α2AP activity levels. This indicates that the α2AP activity assay is mainly based on the levels of C-terminally intact α2AP, the more active and kinetically faster plasmin inhibitor.18
There are several potential explanations for our finding that α2AP is reduced in MI. One of these explanations could be that α2AP is subject to increased clearance due to the release of tissue plasminogen activator and subsequent plasmin generation. Elevated plasmin generation could cause an increased turnover of α2AP due to its complex formation with plasmin. The formation of plasmin-antiplasmin complexes could also lead to changes in the levels observed with our ELISA due to reduced immunologic recognition of the complex in our assay. Indeed, we found that our ELISA assays for α2AP did not react with the inhibitor in complex with plasmin (Figure 3). Increased generation of plasmin-antiplasmin complexes, which tend to be increased in diverse clinical conditions in which fibrinolysis is activated,27,28 would therefore reduce free antiplasmin levels as measured by our ELISA. In agreement with this, preliminary analysis of plasmin-antiplasmin levels in a subset of our patients and controls showed increased levels of this complex in the patients (data not shown). Further studies will be required to investigate this in detail and to confirm whether this hypothesis is correct.
In the present study, we were interested in investigating C-terminal cleavage of α2AP. We developed an ELISA to measure C-terminal intact α2AP antigen levels to calculate the percentage of α2AP C-terminal cleavage. The percentage of C-terminal cleavage found in our control population (around 38%) corresponds very well to the approximately 35% that has been described previously.13,15-17 However, we did not find a difference in the percentage of α2AP C-terminal cleavage between patients and control individuals. We measured total and C-terminally intact α2AP antigen levels independently of α2AP N-terminal cleavage, because our coating antibody picked up Met-α2AP and Asn-α2AP with similar affinity (Figure 3). The N-terminal 12 amino acids of α2AP are inhibitory in the cross-linking reaction of α2AP to fibrin by FXIIIa, but the effect of the presence or absence of this N-terminus on the α2AP activity assay is unknown.
In addition, we showed that the polymorphism Arg407Lys, although located very near the start of the extended C-terminus of α2AP, did not influence α2AP C-terminal cleavage, because we found the percentage of α2AP C-terminal cleavage to be similar for all 3 genotypes in both patients and control individuals. In agreement with this, the polymorphism did not have an effect on plasma clot lysis times. The allele frequencies found in our control individuals for Arg407Lys (0.77 for the Arg allele and 0.23 for the Lys allele) do not deviate from those reported by the International HapMap Project (0.74 for the Arg allele and 0.26 for the Lys allele).29 In our study population, Arg407Lys did not influence the risk of MI. To our knowledge, no other studies have investigated the effect of this polymorphism on the risk of MI or on the antigen levels of α2AP.
There are a few limitations to our study. First, to minimize the influence of the acute-phase response after an MI event, blood samples were taken at least 2 months after the acute event. This means that patients with fatal outcomes may have been excluded from the study. In addition, the antigen levels measured in this study may not reflect the levels before the event. Furthermore, the majority of the patients were taking aspirin or statins, although this did not influence α2AP antigen levels because we found similar levels for users and nonusers.
In conclusion, the present study is the first to measure antigen levels of free α2AP and C-terminally intact α2AP in relation to thrombotic disease. We found reduced free total α2AP antigen levels in male survivors of MI compared with age-matched control individuals. The percentage of α2AP C-terminal cleavage was similar in patients and control individuals. Furthermore, we found that the α2AP polymorphism Arg407Lys does not modulate total α2AP antigen levels, α2AP C-terminal cleavage, or the risk of MI. Larger studies measuring α2AP antigen levels will be needed to confirm our results.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.U.d.W. was supported by grants from the British Heart Foundation (PG/06/089/21244) and the Wellcome Trust (478574).
Authorship
Contribution: S.U.d.W. designed the present study, performed the genotyping and ELISA measurements, analyzed and interpreted the data, and wrote the manuscript; M.M. assisted with the genotyping and ELISA measurements; A.M.C. assisted with the data analyses and critically reviewed the manuscript; T.L. provided the MEGA samples for correlation analyses and critically reviewed the manuscript; F.R.R. enrolled the individuals from the MEGA study, provided the MEGA samples for correlation analyses, and critically reviewed the manuscript; P.J.G. enrolled the individuals from the Leeds MI study and critically reviewed the manuscript; H.P. assisted in developing the ELISA assays and critically reviewed the manuscript; and R.A.S.A. helped in the design of the study and participated in writing and critically reviewing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert A. S. Ariëns. Division of Cardiovascular & Diabetes Research, Section on Mechanisms of Thrombosis, Faculty of Medicine, and Health, University of Leeds, Leeds LS2 9JT, United Kingdom; e-mail: r.a.s.ariens@leeds.ac.uk.
References
Author notes
H.P. and R.A.S.A. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal