Abstract
The most common form of neutrophil death is apoptosis. In the present study, we report surprising differences in the molecular mechanisms used for caspase activation between FAS/CD95-stimulated and TNF receptor 1 (TNFR1)–stimulated neutrophils. Whereas FAS-induced apoptosis was followed by caspase-8 activation and required Bid to initiate the mitochondrial amplification loop, TNF-α–induced apoptosis involved class IA PI3Ks, which were activated by MAPK p38. TNF-α–induced PI3K activation resulted in the generation of reactive oxygen species, which activated caspase-3, a mechanism that did not operate in neutrophils without active NADPH oxidase. We conclude that in neutrophils, proapoptotic pathways after TNFR1 stimulation are initiated by p38 and PI3K, but not by caspase-8, a finding that should be considered in anti-inflammatory drug-development strategies.
Introduction
Neutrophils are the most abundant leukocytes in human blood and are essential in innate immune responses against pathogens.1 Both in vitro and in vivo, apoptosis is the most common physiologic cause of neutrophil death, preventing the release of histotoxic contents from the dying cell and therefore limiting tissue damage.2 Moreover, neutrophil apoptosis contributes to the regulation of the duration and intensity of inflammatory responses.3,4 Activation of neutrophils with members of the TNF cytokine family such as FAS ligand or TNF-α can result in apoptosis and might therefore be relevant in controlling inflammation. Whereas apoptosis induction has consistently been reported in neutrophils after stimulation of FAS/CD95,5 the consequences of TNF-α stimulation on the life span of neutrophils is less clear because apoptosis, survival, and no effect have all been described.5 The diverse outcomes of TNF-α stimulation of neutrophils seem to depend on culture conditions, including the concentration of TNF-α.6 Whereas exposure to TNF-α concentrations below 1 ng/mL results in neutrophil survival, stimulation with concentrations above 10 ng/mL leads to neutrophil apoptosis.7 Under inflammatory conditions, TNF-α concentrations between 1 and 10 ng/mL have been measured in broncho-alveolar fluids of patients suffering from acute respiratory distress syndrome8 and in the serum and joint fluids of subjects with rheumatoid arthritis,9 suggesting that local tissue concentrations may even exceed these numbers.
The proapoptotic signaling cascades induced by FAS ligand and TNF-α have been intensively studied in multiple cellular systems. Whereas at least 2 TNF-α receptors exist, proapoptotic TNF-α signaling is mediated through TNF receptor 1 (TNFR1).10 Both FAS and TNFR1 proapoptotic signaling results in caspase-8 activation.11 Active caspase-8 can process its own pro-caspase and effector pro-caspases such as pro-caspase-3.12 Caspase-8 can also process the BCL-2 family member BID, which is involved in mitochondrial outer membrane permeabilization characterized by cytochrome c release and subsequent caspase-9 and caspase-3 activation.13
Cells that undergo FAS-induced apoptosis without the requirement for truncated BID and its cross-talk with the intrinsic apoptotic pathway have been termed type I cells, whereas type II cells require the mitochondrial amplification loop for apoptosis induction after FAS stimulation.14 Evidence is accumulating that neutrophils represent type II cells. For example, FAS-mediated neutrophil apoptosis accelerates BID cleavage,15 calpain-mediated BAX cleavage,16 and mitochondrial release of cytochrome c and SMAC17 compared with spontaneous apoptosis. In contrast to FAS, a distinction between types I and II TNFR1-induced apoptosis has not been proposed in any cell type until now.
In the present study, we describe a novel, mitochondria-independent apoptosis pathway in TNF-α–stimulated neutrophils. Strikingly, neither caspase-8 nor BID activation was essential in TNF-α–mediated neutrophil apoptosis. Instead, class IA PI3Ks, which are usually involved in survival pathways, were identified as proximal players. PI3K activation was dependent on MAPK p38, but not on caspase activation. We also show that reactive oxygen species (ROS) are generated in a PI3K-dependent manner and subsequently activate caspase-3. The newly identified apoptotic pathway might contribute to cell number regulation under inflammatory conditions and may be influenced by drugs targeting TNF-α, p38, or PI3Ks.
Methods
Reagents
Antibodies to FAS (CH11) were from MBL International Corporation and antibodies to TNFR1 (mAb225) and TNFR2 (mAb226) were from R&D Systems. Other reagents were: human recombinant TNF-α (210-TA; R&D Systems), diphenylene iodonium (DPI, 300260; Merck), dihydro-rhodamine-123 (37370; Sigma-Aldrich), z-VAD-fmk (VAD) (550377; BD Biosciences), cell culture reagents and protein standards (Invitrogen), other buffer components (Sigma-Aldrich), small-molecule inhibitors of PI3Ks (wortmannin, LY294002, and TGX221 [Merck]; PI103 and AS604850 [Enzo Life Sciences]), JAK/STAT pathway (AG490; Merck), MAPKs (PD169316, PD98059, SB203580, and SP600125; Merck), RIPK1 (necrostatin-1; Enzo Life Sciences), Src (SU6656; Merck), Syk (piceatannol; Merck), and farnesyltransferase (FTI227; Sigma-Aldrich). The p110δ-specific inhibitor IC87114 was a kind gift of Prof Peter Shepherd (University of Auckland, New Zealand).
Isolation and culture of human neutrophils
Peripheral blood neutrophils were purified from healthy individuals or chronic granulomatous disease (CGD) patients by Ficoll-Hypaque centrifugation, as described previously.18 The resulting cell population contained > 95% neutrophils, assessed by staining of cells with Diff-Quik (Medion Diagnostics) and light microscopy. Peripheral blood samples from patients with CGD were kindly provided by Dr Janine Reichenbach (University Children's Hospital, Zurich, Switzerland). Joint fluid neutrophils from patients with rheumatoid arthritis were isolated by Ficoll-Hypaque centrifugation, as described previously.18 Joint fluid samples were kindly provided by Dr Peter Villiger (University Hospital, Bern, Switzerland). Written consent was obtained from all healthy normal donors in accordance with the Declaration of Helsinki, and the study was approved by the Medical Ethics Committee of the State of Bern. Human neutrophils were cultured at 2 × 106 cells/mL in RPMI 1640 medium plus GlutaMAX (Invitrogen) supplemented with 5% FCS (endotoxin < 1 EU/mL) and antibiotics. Cells were kept in a humidified 37°C incubator at 5% CO2.
Determination of cell death and apoptosis
Cell death was assessed by uptake of 25μM ethidium bromide and flow cytometric analysis (FACSCalibur; BD Biosciences).3,17,18 Apoptosis was analyzed by redistribution of phosphatidylserine in the presence of propidium iodide, measured by flow cytometry (FACSCalibur).3,17,18 For morphologic analysis, an Axiovert 35 microscope equipped with a 63×/1.4 oil objective lens was used (Carl Zeiss). Images were processed with Adobe Photoshop 5.0 software (Adobe).
Isolation of murine neutrophils and death assay
Murine neutrophils were isolated from bone marrow of C57BL/6 wild-type and bid−/− mice.19 Bone marrow cells were purified by magnetic cell separation using anti-Gr1 antibody (RB6-8C5; BioLegend) following the manufacturer's instructions (IMagnet; BD Biosciences). The purity of the obtained neutrophils was assessed by Diff-Quik staining and light microscopy and was routinely > 95%. For death assays, neutrophils were resuspended in RPMI 1640 plus GlutaMAX medium (Invitrogen) supplemented with 10% FCS (endotoxin < 1 EU/mL), antibiotics, and 50μM 2-mercaptoethanol, and stresses were added as follows: recombinant mouse TNF-α (ALX-522-009; Enzo Life Sciences) and recombinant human FAS ligand (ALX-522-001; Enzo Life Sciences) cross-linked with 2 μg/mL of anti-FLAG antibody (M2; Sigma-Aldrich). Cells were stained with green fluorescent protein–Annexin V20 and propidium iodide, and apoptosis was quantified by flow cytometry. Mice were maintained under pathogen-free conditions. All animal experiments were reviewed and approved by the Animal Experimentation Review Board of the State of Bern.
Cytochrome c release
Mitochondrial cytochrome c release was analyzed as described previously.21 Briefly, human neutrophils were permeabilized in buffer D (75mM KCl, 250mM sucrose, 1mM NaH2PO4, and 8mM Na2HPO4) containing 125 μg/mL of digitonin. Subsequently, the cells were fixed with paraformaldehyde. After incubation in blocking buffer (0.05% saponin, 10% FCS, 10% polyvalent human IgG, 1% human IgG [009-000-003; Jackson ImmunoResearch Laboratories]), anti–cytochrome c antibody (556432; BD Biosciences) was added overnight and the Alexa Fluor 488–coupled secondary antibody was added subsequently (558869; BD Biosciences). The fluorescence of cells was analyzed by flow cytometry.
Mitochondrial transmembrane potential measurement
The mitochondrial transmembrane potential (ΔΨm) was assessed by staining neutrophils with 40nM 3,3′-dihexyloxacarbocyanine iodide for 20 minutes at 37°C—a positively charged fluorescent dye binding to the negatively charged mitochondrial matrix. After disruption of the mitochondrial transmembrane potential, less dye will accumulate in the mitochondria because of the distortion of the charge distribution within the mitochondria and a weaker fluorescence signal will be detected by the flow cytometer.
In vitro caspase activity assays
Caspase-8 or caspase-3 activities were assessed by in vitro caspase-8 (IETDase) or caspase-3 (DEVDase) colorimetric (AK715 or AK703; Biomol) and fluorometric assays (ALX-260-110-M005 or ALX-260-032-M005; Enzo Life Sciences). For colorimetric analysis, caspase activity assays were performed according to the manufacturer's instructions (Biomol). For fluorometric analysis, human neutrophils were lysed in caspase assay lysis buffer (250mM sucrose, 70mM KCl, 0.25 mg/mL of digitonin, and 10 μL/mL of protease inhibitor cocktail [P8340; Sigma-Aldrich]), and the caspase activity assay was performed with 30 μg of cell lysate in assay buffer (10mM piperazine diethanesulfonic acid, pH 7.2, 100mM NaCl, 10% sucrose, 1mM EDTA, 10mM DTT, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate). Before measurement, 0.2mM substrate (Ac-IETD-AFC or Ac-DEVD-AFC) was added. Caspase activities were measured in the kinetic mode (excitation 405 nm, emission 500 nm). The vmax for each condition was calculated relative to that of caspase activity in freshly isolated neutrophils.
Immunoblotting
Human neutrophils were lysed in either modified radioimmunoprecipitation assay lysis buffer for total cell lysate analysis (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1% NP-40, 1mM EGTA, and 0.25% Na-deoxycholate) or Triton X-100 lysis buffer for analysis of phosphorylated proteins or immunoprecipitations (50mM Tris-HCl, pH 7.4, 150mM NaCl, 1mM EDTA, and 1% Triton X-100) both containing protease (protease inhibitor cocktail P8340; Sigma-Aldrich) and phosphatase inhibitors (5mM NaF, 1mM Na3VO4, and 1μM okadaic acid). For analysis of BID cleavage, boiling H8 lysis buffer (20mM Tris, pH 7.5, 2mM EGTA, 2mM EDTA, and 1% SDS) containing protease inhibitors was added to cells and the lysate was boiled at 95°C for 5 minutes. Proteins were separated by SDS-PAGE and electroblotted onto PVDF membranes (Immobilon P; Millipore). Membranes were routinely blocked in PBS/0.1% Tween-20/5% nonfat milk, and incubated overnight with the following antibodies: anti-AKT (9272), anti–phospho-Ser473-AKT (9271), anti–phospho-Thr308-AKT (9275), anti–caspase-3 (9662), anti–phospho-Thr334-MK2 (3007), anti-p38 (9212), and anti–phospho-Thr180/Tyr182-p38 (9211) (all from Cell Signaling Technology); anti-BID (sc-6538), anti–lamin B (sc-6217), anti-p110β (sc-602), anti-p110δ (sc-7176), and anti-TRADD (sc-46653) (all from Santa Cruz Biotechnology); anti-GAPDH (6C5) and anti-p85α/β (p85pan 06-195) (from Millipore); and anti–caspase-8 (12F5) (from Enzo Life Sciences). The membranes were subsequently incubated with the HRP-coupled secondary antibody (anti–mouse IgG, NA9310 [GE Healthcare]; anti–rabbit IgG, NA9340 [GE Healthcare]; and anti-goat IgG, P0449 [Dako]) and the filters were developed according to the manufacturer's instructions using an ECL kit (GE Healthcare). ImageJ software was used to quantify protein expression levels. Unless stated otherwise, protein expression levels are shown relative to the control condition, which was set as 1.
Immunoprecipitation
TNFR1 was immunoprecipitated from primary human neutrophils as follows: cells were lysed in Triton X-100 lysis buffer and cell lysates were cleared by centrifugation, preincubated at 4°C with 20 μL of packed protein agarose G beads (sc-2003; Santa Cruz Biotechnology), incubated at 4°C tumbling with anti-TNFR1 antibody (H398; Enzo Life Sciences) or isotype IgG2a control antibody (MAB003; R&D Systems) and with packed protein agarose G beads. The immune complexes were washed with lysis buffer and stored at −20°C in 2× SDS sample buffer (Laemmli).
ROS measurement
Human neutrophils were incubated with 1μM dihydro-rhodamine-123 for 30 minutes and cells analyzed by flow cytometry. To block ROS generation by NADPH oxidase, 20μM of the NADPH oxidase inhibitor DPI was added to neutrophils for 10 minutes, and the cells were washed and then stimulated.
Statistical analysis
Statistical analysis was performed using the Student t test. The figures show means ± SD. P < .05 was considered statistically significant.
Results
TNF-α induces neutrophil apoptosis independently of Bid
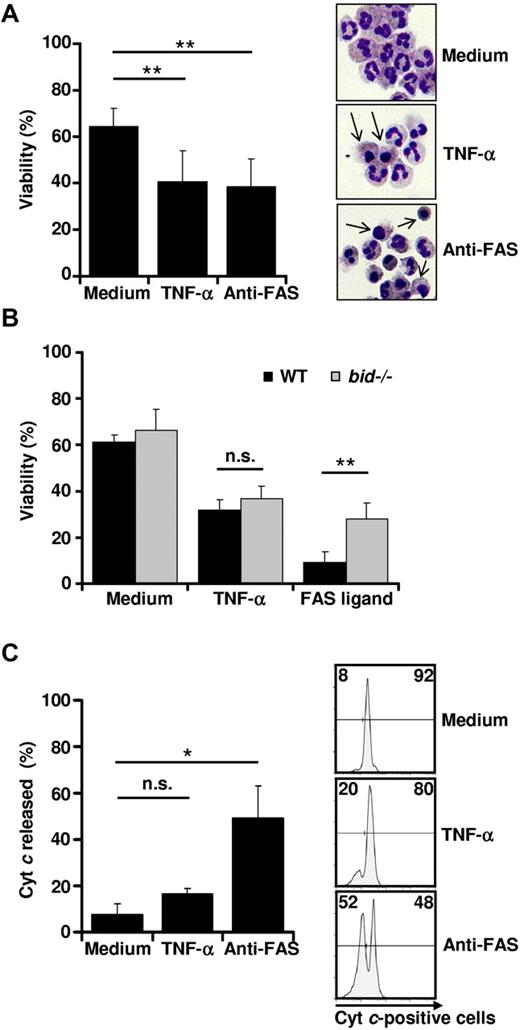
As described previously,5 stimulation of freshly isolated human blood neutrophils with high concentrations of TNF-α (10-100 ng/mL) resulted in increased cell death compared with control neutrophils, which are known to undergo spontaneous apoptosis (Figure 1A left panel). The type of cell death induced by TNF-α and anti-FAS antibody was apoptosis, as assessed by morphology (Figure 1A right panel) and the phosphatidyl-serine redistribution assay (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Although the numbers of dead cells were similar at 24 hours of culture (Figure 1A left panel), anti-FAS antibody–induced apoptosis proceeded slightly more quickly than TNF-α–induced apoptosis at the early time points of stimulation (supplemental Figure 1A). Moreover, TNF-α–induced death was mediated by TNFR1 and not by TNFR2 (supplemental Figure 1B).
TNF-α induces neutrophil apoptosis independently of Bid. (A) TNF-α stimulation results in neutrophil cell death. Primary human neutrophils were stimulated with TNF-α (50 ng/mL) or anti-FAS antibody (1 μg/mL). Twenty-four hours after neutrophil isolation, viability was assessed by ethidium bromide staining and flow cytometry (n > 10) (left panel). Eight hours after neutrophil isolation, neutrophils were harvested on glass plates, fixed, and stained. Neutrophils with apoptotic features are marked with arrows. A representative section in 1 of 4 independent experiments is shown (right panel). (B) TNF-α–induced murine neutrophil death is independent of Bid. After isolation of neutrophils from wild-type or bid−/− mice, cells were left untreated or stimulated with TNF-α (50 ng/mL) or FAS ligand (100 ng/mL). After 24 hours of culture, neutrophil apoptosis was assessed by annexin V–propidium iodide staining and flow cytometry (n = 5). (C) Cytochrome c is not released in TNF-α–stimulated neutrophils. Primary human neutrophils were stimulated with TNF-α or anti-FAS antibody for 4 hours. Cytochrome (Cyt) c release from mitochondria was analyzed by flow cytometry. Shown are a quantification of cytochrome c release (n = 3) (left panel) and representative flow cytometry diagrams (right panel).
TNF-α induces neutrophil apoptosis independently of Bid. (A) TNF-α stimulation results in neutrophil cell death. Primary human neutrophils were stimulated with TNF-α (50 ng/mL) or anti-FAS antibody (1 μg/mL). Twenty-four hours after neutrophil isolation, viability was assessed by ethidium bromide staining and flow cytometry (n > 10) (left panel). Eight hours after neutrophil isolation, neutrophils were harvested on glass plates, fixed, and stained. Neutrophils with apoptotic features are marked with arrows. A representative section in 1 of 4 independent experiments is shown (right panel). (B) TNF-α–induced murine neutrophil death is independent of Bid. After isolation of neutrophils from wild-type or bid−/− mice, cells were left untreated or stimulated with TNF-α (50 ng/mL) or FAS ligand (100 ng/mL). After 24 hours of culture, neutrophil apoptosis was assessed by annexin V–propidium iodide staining and flow cytometry (n = 5). (C) Cytochrome c is not released in TNF-α–stimulated neutrophils. Primary human neutrophils were stimulated with TNF-α or anti-FAS antibody for 4 hours. Cytochrome (Cyt) c release from mitochondria was analyzed by flow cytometry. Shown are a quantification of cytochrome c release (n = 3) (left panel) and representative flow cytometry diagrams (right panel).
Stimulation of Gr1-positive murine bone marrow neutrophils with TNF-α or FAS ligand also resulted in significant death induction (Figure 1B). Neutrophils derived from bid−/− mice were partially protected from FAS ligand–induced death (Figure 1B), suggesting that neutrophils indeed undergo type II FAS-mediated apoptosis. In contrast, bid-deficient neutrophils showed the same susceptibility toward TNF-α–mediated death as wild-type neutrophils (Figure 1B). Because BID was shown to be critical for the type II FAS-mediated mitochondrial death pathway,22 we monitored Δψm and analyzed the release of mitochondrial cytochrome c on TNF-α and anti-FAS antibody stimulation in human neutrophils using flow cytometry. In contrast to neutrophils treated with anti-FAS antibody, which lost their Δψm within 6 hours, we observed a decrease in Δψm in TNF-α–stimulated neutrophils that was similar to that of control neutrophils (supplemental Figure 2). Moreover, very little cytochrome c was released after 4 hours (Figure 1C) and 8 hours (data not shown) of TNF-α stimulation compared with anti-FAS antibody–treated cells, suggesting that the mechanism of effector caspase activation is different between FAS- and TNFR1-mediated apoptosis in these cells. Whereas TNF-α induces neutrophil apoptosis independently of the mitochondrial death pathway, FAS ligation depends on Bid and results in the activation of the mitochondrial amplification loop.
TNF-α–induced neutrophil apoptosis is dependent on caspases
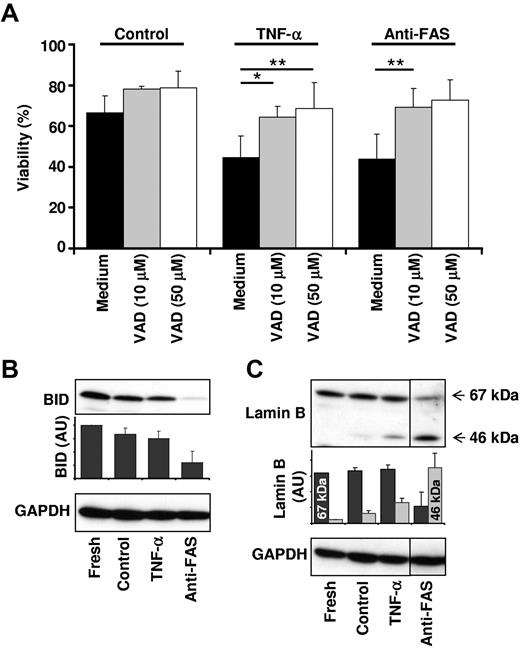
We next investigated the role of caspases in TNF-α–mediated neutrophil apoptosis, about which controversial data have been published. Whereas some studies have shown that blocking caspases by low23,24 and high24 concentrations of the pan-caspase inhibitor VAD rescues neutrophils from TNF-α–mediated apoptosis, others have reported that TNF-α–induced death is accentuated by extremely high VAD concentrations.23,25 However, this effect appeared to be compound specific and not necessarily due to caspase inhibition.23 We observed that TNF-α–induced human (Figure 2A) and murine (data not shown) neutrophil death was inhibited by both low (10μM) and high (50μM) concentrations of VAD, supporting the view that the type of neutrophil death as a consequence of TNF-α stimulation is apoptosis. Moreover, we used necrostatin-1 to block the enzymatic activity of receptor-interacting protein 1 (RIP1)26 and observed no effect on neutrophil viability (data not shown), excluding a programmed necrotic cell death under these conditions.
TNF-α–induced neutrophil apoptosis is dependent on effector caspase activity. (A) Caspase inhibition before TNF-α stimulation results in neutrophil survival. Primary human neutrophils were pretreated with vehicle (Medium) or 10 or 50μM pan-caspase inhibitor VAD in control, TNF-α-treated, and anti-FAS antibody–treated neutrophils. Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours of stimulation (n = 3 for 50μM VAD; n = 7 for 10μM VAD). (B) Caspase-8 is not activated after TNF-α stimulation. Caspase-8 activity was assessed by immunoblot analysis of BID processing. Neutrophils were harvested immediately (Fresh) or left untreated (Control) and stimulated with TNF-α (20 ng/mL) or anti-FAS antibody (1 μg/mL) for 4 hours. A representative immunoblot is shown and BID protein expression levels were quantified relative to the fresh sample (n = 3). (C) Effector caspases are activated after TNF-α stimulation. Total effector caspase activity was assessed by immunoblot analysis of lamin B processing. Neutrophils were treated as in panel B. A representative immunoblot is shown. Lamin B protein expression levels were quantified relative to the 67-kDa lamin B band in the fresh sample (n = 2).
TNF-α–induced neutrophil apoptosis is dependent on effector caspase activity. (A) Caspase inhibition before TNF-α stimulation results in neutrophil survival. Primary human neutrophils were pretreated with vehicle (Medium) or 10 or 50μM pan-caspase inhibitor VAD in control, TNF-α-treated, and anti-FAS antibody–treated neutrophils. Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours of stimulation (n = 3 for 50μM VAD; n = 7 for 10μM VAD). (B) Caspase-8 is not activated after TNF-α stimulation. Caspase-8 activity was assessed by immunoblot analysis of BID processing. Neutrophils were harvested immediately (Fresh) or left untreated (Control) and stimulated with TNF-α (20 ng/mL) or anti-FAS antibody (1 μg/mL) for 4 hours. A representative immunoblot is shown and BID protein expression levels were quantified relative to the fresh sample (n = 3). (C) Effector caspases are activated after TNF-α stimulation. Total effector caspase activity was assessed by immunoblot analysis of lamin B processing. Neutrophils were treated as in panel B. A representative immunoblot is shown. Lamin B protein expression levels were quantified relative to the 67-kDa lamin B band in the fresh sample (n = 2).
Specific caspase activation was investigated by immunoblotting of caspase targets. BID is a known target of caspase-8, and lamin B is cleaved by effector caspases. Activation of FAS on neutrophils resulted in degradation of BID and cleavage of lamin B, indicating that both caspase-8 and caspase-3 activation occurred under these conditions (Figure 2B-C). This was associated with increased enzymatic activity of both caspases (see “Essential role of ROS for increased caspase-3 activity after TNF-α stimulation”). In contrast, TNF-α stimulation of neutrophils did not result in detectable BID processing, but resulted in lamin B cleavage (Figure 2B-C), suggesting increased caspase-3 but not caspase-8 activity in these cells. Direct measurements of caspase-8 enzymatic activity (see “Essential role of ROS for increased caspase-3 activity after TNF-α stimulation”) supported this assumption. The apparent lack of BID processing after TNF-α stimulation of human neutrophils may also explain why bid-deficient murine neutrophils died with the same efficacy and kinetics as wild-type neutrophils (Figure 1B). These data suggest that the differences between the proapoptotic signaling events initiated by FAS and TNFR1 in neutrophils are located proximal to effector caspase activation.
TNF-α–induced neutrophil apoptosis is dependent on MAPK p38 and class IA PI3K
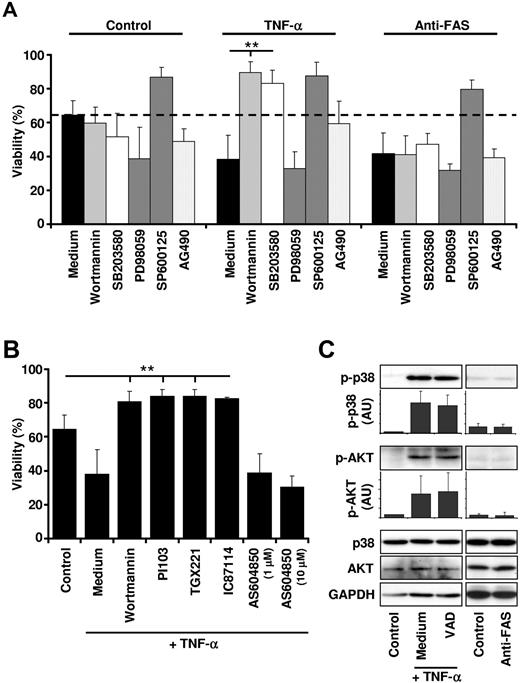
To gain insight into the signaling events leading to caspase activation downstream of TNFR1, we next investigated whether neutrophil death induced by TNF-α stimulation is dependent on PI3K, MAPK, and/or Janus kinase (JAK)/signal transduction and activator of transcription (STAT) signaling pathways using small-molecule inhibitors. Inhibition of MAPK p38 by SB203580 and PI3Ks by wortmannin not only prevented neutrophil apoptosis induced by TNF-α, but also induced prolonged survival (Figure 3A and supplemental Figure 3). Pharmacologic inhibition of p38 and PI3Ks also blocked TNF-α–mediated death in inflammatory neutrophils obtained from the joint fluids of patients suffering from rheumatoid arthritis (supplemental Figure 4). This is in contrast to FAS-induced death, which was not influenced by SB203580 or wortmannin (Figure 3A and supplemental Figure 3). Inhibition of c-Jun N-terminal kinase (JNK) by SP600125 resulted in decreased neutrophil death in the presence and absence of both TNF-α and anti-FAS antibody (Figure 3A and supplemental Figure 3). Pharmacologic inhibition of the JAK/STAT pathway (AG490) or MAPK/ERK kinase 1 (MEK-1) (PD98059) had no effect on neutrophil viability in the presence or absence of TNF-α or anti-FAS antibody (Figure 3A).
TNF-α–induced neutrophil apoptosis is dependent on MAPK p38 and class IA PI3K activity. (A) Inhibition of p38 and PI3K before TNF-α stimulation results in neutrophil survival. Before TNF-α (50 ng/mL) or anti-FAS antibody (1 μg/mL) stimulation, primary human neutrophils were incubated for 30-60 minutes with vehicle (Medium) or with small-molecule inhibitors against PI3K (100nM wortmannin), p38 (1μM SB203580), MEK (50μM PD98059), SAPK/JNK (10μM SP600125), or JAK/STAT (25μM AG490). Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours (n ≥ 3). (B) Class IA PI3Ks are death molecules after TNF-α stimulation. Neutrophils were incubated with vehicle (Control, Medium) or different PI3K inhibitors before TNF-α (50 ng/mL) stimulation. Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours. The following inhibitors were used: the broad-spectrum PI3K inhibitor wortmannin (100nM), the class IA PI3K-selective inhibitor PI103 (100nM), the p110β isoform-selective inhibitor TGX221 (100nM), the p110δ isoform-selective inhibitor IC87114 (1μM), and the p110γ isoform-selective inhibitor AS604850 (1 and 10μM) (n ≥ 4). (C) Activation of p38 and PI3Ks is independent of caspase activity. Neutrophils were pretreated with or without VAD for 60 minutes and subsequently stimulated for 15 minutes with 50 ng/mL of TNF-α or 1 μg/mL of anti-FAS antibody. Cell lysates were analyzed by immunoblotting for phosphorylated Ser473 AKT (an indirect readout for PI3K activity) or phosphorylated Thr180/Tyr182 p38. p38, AKT, and GAPDH protein levels were analyzed as loading controls. A representative immunoblot is shown, and protein expression levels were quantified relative to the control condition (n = 3).
TNF-α–induced neutrophil apoptosis is dependent on MAPK p38 and class IA PI3K activity. (A) Inhibition of p38 and PI3K before TNF-α stimulation results in neutrophil survival. Before TNF-α (50 ng/mL) or anti-FAS antibody (1 μg/mL) stimulation, primary human neutrophils were incubated for 30-60 minutes with vehicle (Medium) or with small-molecule inhibitors against PI3K (100nM wortmannin), p38 (1μM SB203580), MEK (50μM PD98059), SAPK/JNK (10μM SP600125), or JAK/STAT (25μM AG490). Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours (n ≥ 3). (B) Class IA PI3Ks are death molecules after TNF-α stimulation. Neutrophils were incubated with vehicle (Control, Medium) or different PI3K inhibitors before TNF-α (50 ng/mL) stimulation. Viability was assessed by ethidium bromide staining and flow cytometry after 24 hours. The following inhibitors were used: the broad-spectrum PI3K inhibitor wortmannin (100nM), the class IA PI3K-selective inhibitor PI103 (100nM), the p110β isoform-selective inhibitor TGX221 (100nM), the p110δ isoform-selective inhibitor IC87114 (1μM), and the p110γ isoform-selective inhibitor AS604850 (1 and 10μM) (n ≥ 4). (C) Activation of p38 and PI3Ks is independent of caspase activity. Neutrophils were pretreated with or without VAD for 60 minutes and subsequently stimulated for 15 minutes with 50 ng/mL of TNF-α or 1 μg/mL of anti-FAS antibody. Cell lysates were analyzed by immunoblotting for phosphorylated Ser473 AKT (an indirect readout for PI3K activity) or phosphorylated Thr180/Tyr182 p38. p38, AKT, and GAPDH protein levels were analyzed as loading controls. A representative immunoblot is shown, and protein expression levels were quantified relative to the control condition (n = 3).
The survival effect of TNF-α in the presence of wortmannin was surprising, because PI3Ks are known to be involved in multiple survival pathways.27 To verify this finding and to investigate which PI3K isoform is responsible for the death-inducing effect downstream of TNFR1 in neutrophils, we tested several PI3K inhibitors in our system. These experiments revealed that inhibition of class IA PI3Ks (p110α, p110β, and p110δ) by pan-class IA PI3K inhibitor PI103, but not of class IB PI3K p110γ by AS604850, increased neutrophil survival on stimulation with TNF-α to a similar extent as wortmannin (Figure 3B). Furthermore, the p110β-specific inhibitor TGX221 and the p110δ-specific inhibitor IC87114 showed the same efficacy in this system, suggesting that several class IA PI3K isoforms might be involved in the death machinery distal to TNFR1 (Figure 3B). None of the PI3K inhibitors had any effect on FAS-mediated neutrophil death (supplemental Figure 5).
We next analyzed activation of p38 and PI3Ks after TNF-α and anti-FAS antibody stimulation in neutrophils. Phosphorylation of p38 or AKT (a commonly used indirect readout for PI3K activity) was increased after TNF-α but not anti-FAS antibody stimulation of neutrophils (Figure 3C). Peak phosphorylation for both p38 and AKT was seen at 15 minutes (data not shown) and was not influenced by the presence of VAD (Figure 3C), suggesting that these early signaling events are caspase independent. We also compared the activation of p38 and PI3K after stimulation of neutrophils with low (0.1 ng/mL) and high (10 ng/mL) concentrations of TNF-α (supplemental Figure 6). Whereas stimulation of neutrophils with 0.1 ng/mL of TNF-α resulted in moderate phosphorylation of p38 and AKT (supplemental Figure 6C) and neutrophil survival (supplemental Figure 6A-B), the addition of TNF-α concentrations above 10 ng/mL led to high phosphorylation of p38 and AKT and to neutrophil apoptosis. These data suggested that the levels of p38 and AKT phosphorylation were positively correlated with the concentrations of TNF-α used to stimulate neutrophils.
PI3Ks are activated by p38 after TNF-α stimulation
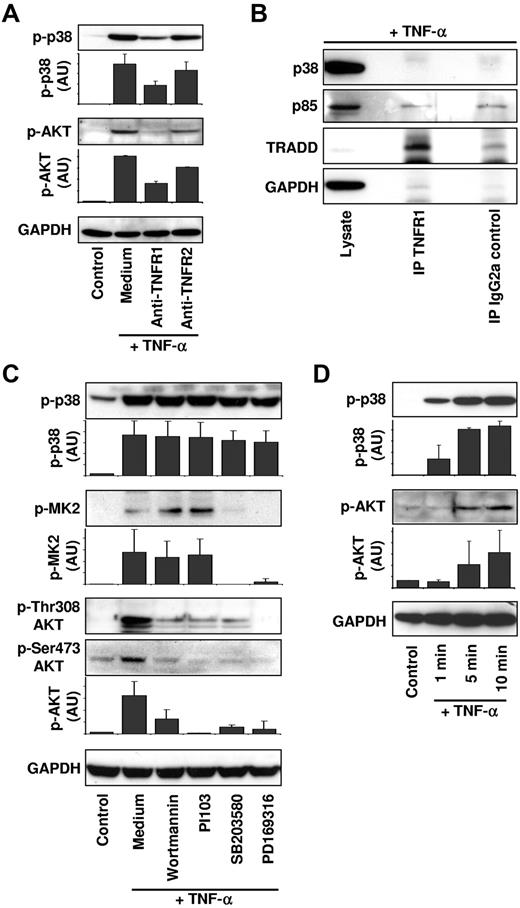
To understand how p38 and class IA PI3Ks are activated after TNF-α stimulation of neutrophils, we investigated whether p38 and AKT phosphorylation occurred as a consequence of TNFR1 or TNFR2 stimulation. Neutrophils were treated with blocking antibodies against these receptors before TNF-α stimulation. Phosphorylation of both p38 and AKT were significantly reduced when TNFR1, but not TNFR2, was blocked (Figure 4A), suggesting that p38 and PI3K signaling are mainly activated as a consequence of TNFR1 ligation.
PI3Ks are activated by p38 downstream of TNFR1. (A) p38 and PI3Ks are activated downstream of TNFR1. Neutrophils were kept untreated (Control, Medium) or incubated with 10 μg/mL of anti-TNFR1 or anti-TNFR2 antibodies for 90 minutes. Cells were subsequently stimulated with 50 ng/mL of TNF-α for 15 minutes and cell lysates were analyzed as in Figure 3C. A representative immunoblot is shown, and protein expression levels were quantified relative to the control condition (n = 3). (B) Neither class IA PI3Ks nor p38 is associated with activated TNFR1. Human neutrophils were stimulated with 20 ng/mL of TNF-α for 5 minutes. The lysates were incubated with anti-TNFR1 antibody (H398) or IgG2a control antibody. Immunoblots were probed for p38, the PI3K class IA regulatory subunits p85α/p85β (p85), TRADD, and GAPDH (n = 3). IP indicates immunoprecipitation. (C) p38 is upstream of PI3K activation. Neutrophils were treated for 90 minutes with vehicle (Control, Medium), with the PI3K inhibitors wortmannin (100nM) and PI103 (100nM), or with the p38 inhibitors SB203580 (1μM) and PD169316 (10μM) before TNF-α (50 ng/mL) stimulation. Immunoblots were probed for phosphorylated Thr308 and Ser473 AKT, phosphorylated Thr180 and Tyr182 p38, phosphorylated Thr334 MK2, and GAPDH. A representative immunoblot is shown. Protein expression levels were quantified relative to the control condition (n ≥ 3). (D) Phosphorylation of p38 precedes phosphorylation of AKT. Neutrophils were stimulated for the indicated times with 50 ng/mL of TNF-α. Cell lysates were analyzed by immunoblotting for phosphorylated Ser473 AKT or phosphorylated Thr180/Tyr182 p38. GAPDH protein levels were analyzed as loading controls. A representative immunoblot is shown. Protein expression levels were quantified relative to the control condition (n = 3).
PI3Ks are activated by p38 downstream of TNFR1. (A) p38 and PI3Ks are activated downstream of TNFR1. Neutrophils were kept untreated (Control, Medium) or incubated with 10 μg/mL of anti-TNFR1 or anti-TNFR2 antibodies for 90 minutes. Cells were subsequently stimulated with 50 ng/mL of TNF-α for 15 minutes and cell lysates were analyzed as in Figure 3C. A representative immunoblot is shown, and protein expression levels were quantified relative to the control condition (n = 3). (B) Neither class IA PI3Ks nor p38 is associated with activated TNFR1. Human neutrophils were stimulated with 20 ng/mL of TNF-α for 5 minutes. The lysates were incubated with anti-TNFR1 antibody (H398) or IgG2a control antibody. Immunoblots were probed for p38, the PI3K class IA regulatory subunits p85α/p85β (p85), TRADD, and GAPDH (n = 3). IP indicates immunoprecipitation. (C) p38 is upstream of PI3K activation. Neutrophils were treated for 90 minutes with vehicle (Control, Medium), with the PI3K inhibitors wortmannin (100nM) and PI103 (100nM), or with the p38 inhibitors SB203580 (1μM) and PD169316 (10μM) before TNF-α (50 ng/mL) stimulation. Immunoblots were probed for phosphorylated Thr308 and Ser473 AKT, phosphorylated Thr180 and Tyr182 p38, phosphorylated Thr334 MK2, and GAPDH. A representative immunoblot is shown. Protein expression levels were quantified relative to the control condition (n ≥ 3). (D) Phosphorylation of p38 precedes phosphorylation of AKT. Neutrophils were stimulated for the indicated times with 50 ng/mL of TNF-α. Cell lysates were analyzed by immunoblotting for phosphorylated Ser473 AKT or phosphorylated Thr180/Tyr182 p38. GAPDH protein levels were analyzed as loading controls. A representative immunoblot is shown. Protein expression levels were quantified relative to the control condition (n = 3).
Because the activation of class IA PI3Ks frequently occurs through recruitment of the proteins to the plasma membrane,27 we next investigated whether class IA PI3Ks are recruited to TNFR1 after TNF-α stimulation of neutrophils. The TNFR1 complex was immunoprecipitated with an anti-TNFR1 antibody from neutrophils stimulated for 5 minutes (Figure 4B) or for 10, 15, and 20 minutes (data not shown) with TNF-α. Separation of the complex by SDS-PAGE and analysis of the protein content by immunoblotting revealed that neither class IA PI3Ks (detected by the common p85 regulatory subunit) nor p38 was recruited to activated TNFR1. The TNFR1 complex protein TNFR1-associated death domain protein (TRADD) was used as a positive control and GAPDH as a negative control in these experiments (Figure 4B). To control for nonspecific antibody binding of proteins, the same amount of cell lysate was immunoprecipitated using an IgG2a control antibody.
Class IA PI3Ks may be further activated by interaction with Ras28 or by changes in posttranslational modifications of p85 or p110.29,30 However, blocking Ras using the farnesyltransferase inhibitor FTI227 did not change TNF-α–induced neutrophil viability or Akt phosphorylation (data not shown), and inhibition of tyrosine kinases Syk or Src by piceatannol and SU6656, respectively, showed no impact on phosphorylation of AKT downstream of TNFR1 stimulation (data not shown).
To examine the possibility of a potential sequential activation of p38 and class IA PI3K after TNF-α stimulation of neutrophils, we determined the effect of pharmacologic inhibition of each of the kinases after activation of the other kinase. Phosphorylation of p38 was not affected by inhibition of PI3Ks (using wortmannin and PI103) or p38 (using SB203580 and PD169316) (Figure 4C). To verify that the 2 p38 inhibitors actually blocked p38 activity, we analyzed the phosphorylation status of MAPKAP kinase-2 (MK2), which is a direct target of p3831 (Figure 4C). In contrast to p38 phosphorylation, the TNF-α–induced phosphorylation of AKT on both Thr308 and Ser473 was reduced by PI3K and p38 inhibitors (Figure 4C). Moreover, after TNF-α stimulation of neutrophils, phosphorylation of p38 was detected more quickly than phosphorylation of AKT (Figure 4D), suggesting that p38 activation preceded AKT phosphorylation. These data suggest that p38 is proximal to and required for PI3K activation in the TNFR1-induced proapoptotic pathway in neutrophils.
Class IA PI3Ks mediate neutrophil apoptosis by controlling ROS generation
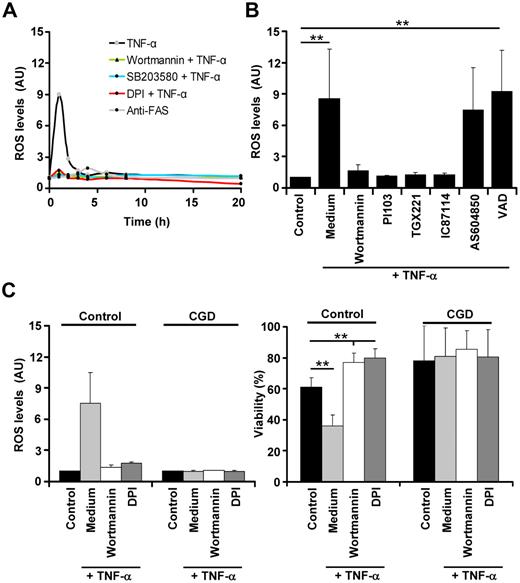
p3832 and PI3K33 directly or indirectly activate Rac and mediate phosphorylation of p47phox, which are both subunits of the NADPH oxidase complex. Therefore, we measured intracellular ROS generation after TNF-α stimulation of neutrophils. TNF-α induced a sharp peak in intracellular ROS production within 1 hour in neutrophils (Figure 5A), and the level of ROS generation was positively correlated with the TNF-α concentrations used to stimulate the cells (supplemental Figure 6D). Generation of ROS was likely due to activation of TNFR1, because it was abolished in neutrophils pretreated with anti-TNFR1 antibodies (data not shown). In contrast, FAS stimulation was not associated with increased ROS levels (Figure 5A). Moreover, inhibition of PI3K by wortmannin and p38 by SB203580 completely abolished TNF-α–induced ROS generation, suggesting that TNFR1 utilizes both kinases for this purpose (Figure 5A). Because inhibition of the NADPH oxidase by DPI did also not result in increased ROS levels after TNF-α stimulation (Figure 5A), ROS were likely generated by the NADPH oxidase in neutrophils. To obtain additional insights into the PI3K isoforms required for activation of the NADPH oxidase, we used specific class IA PI3Ks inhibitors and a class IB PI3K p110γ inhibitor. Similar to the results obtained by viability assays (Figure 3B), we observed that the p110β-specific inhibitor TGX221 and the p110δ-specific inhibitor IC87114 completely blocked TNF-α–induced ROS production in neutrophils (Figure 5B), suggesting that class IA, but not class IB, PI3Ks are involved in ROS generation and cell death induction in these cells.
p38 and class IA PI3K mediate neutrophil apoptosis by controlling ROS generation. (A) Inhibition of p38 or PI3Ks before TNF-α stimulation abolishes ROS generation. Time course of ROS generation after TNF-α and FAS antibody stimulation. Neutrophils were pretreated with vehicle or 100nM wortmannin, 10μM SB203580, or 20μM of the NADPH oxidase inhibitor DPI before TNF-α stimulation (50 ng/mL). Cells were also treated with anti-FAS antibody (n > 5). (B) Class IA PI3Ks control ROS generation after TNF-α stimulation. Neutrophils were treated with vehicle (Control, Medium), diverse class IA PI3K inhibitors (for concentrations, see Figure 3B), or 10μM of the pan-caspase inhibitor VAD before TNF-α stimulation (50 ng/mL) (n > 5, except pretreatment with AS604850, where n = 2). (C) Inhibition of PI3Ks does not affect the viability of neutrophils derived from CGD patients. Neutrophils from 3 different CGD patients (who lack a functional NADPH oxidase) were analyzed for ROS generation (left panel) and for viability (right panel; ethidium bromide staining and flow cytometry) after PI3K or NADPH oxidase inhibition and TNF-α stimulation.
p38 and class IA PI3K mediate neutrophil apoptosis by controlling ROS generation. (A) Inhibition of p38 or PI3Ks before TNF-α stimulation abolishes ROS generation. Time course of ROS generation after TNF-α and FAS antibody stimulation. Neutrophils were pretreated with vehicle or 100nM wortmannin, 10μM SB203580, or 20μM of the NADPH oxidase inhibitor DPI before TNF-α stimulation (50 ng/mL). Cells were also treated with anti-FAS antibody (n > 5). (B) Class IA PI3Ks control ROS generation after TNF-α stimulation. Neutrophils were treated with vehicle (Control, Medium), diverse class IA PI3K inhibitors (for concentrations, see Figure 3B), or 10μM of the pan-caspase inhibitor VAD before TNF-α stimulation (50 ng/mL) (n > 5, except pretreatment with AS604850, where n = 2). (C) Inhibition of PI3Ks does not affect the viability of neutrophils derived from CGD patients. Neutrophils from 3 different CGD patients (who lack a functional NADPH oxidase) were analyzed for ROS generation (left panel) and for viability (right panel; ethidium bromide staining and flow cytometry) after PI3K or NADPH oxidase inhibition and TNF-α stimulation.
To verify the requirement for ROS generation in TNF-α–mediated neutrophil death, we analyzed neutrophils derived from patients with CGD that lack a functional NADPH oxidase due to a genetic defect. CGD neutrophils were unable to generate ROS or to undergo cell death after TNF-α stimulation (Figure 5C left panel). Because blocking of PI3Ks using wortmannin did not influence the viability of CGD neutrophils (Figure 5C right panel), it seems likely that PI3Ks exert their death potential primarily through the control of the NADPH oxidase.
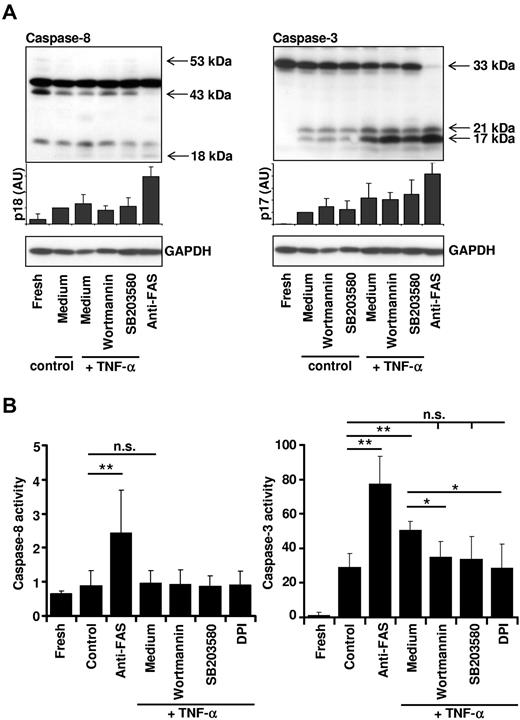
Essential role of ROS for increased caspase-3 activity after TNF-α stimulation
We analyzed specific caspase activation by immunoblotting and enzymatic activity assays to address the question of how ROS mediate increased apoptosis in TNF-α–stimulated neutrophils. In agreement with the indirect measurements of caspase activity (Figure 2B-C), activation of FAS resulted in cleavage of both pro–caspase-8 and pro–caspase 3 in neutrophils (Figure 6A) that was associated with increased enzymatic activity of caspase-8 (IETDase activity) and caspase-3 (DEVDase activity) (Figure 6B). In contrast, TNF-α stimulation of neutrophils did not result in detectable caspase-8 cleavage and activation, whereas it did induce caspase-3 activity (Figure 6A-B). TNF-α–induced caspase-3 processing was not blocked by inhibitors of p38 and PI3Ks (Figure 6A); however, blocking of p38 or PI3Ks resulted in reduced caspase-3 activity (Figure 6B) and lamin B cleavage (supplemental Figure 7). These data suggest that ROS are required for full caspase-3 activation. Indeed, enzymatic activation of caspase-3 in TNF-α–stimulated neutrophils was completely blocked by DPI (Figure 6B). Furthermore, the addition of physiologic concentrations of hydrogen peroxide34 to neutrophils, mimicking ROS generation after TNF-α stimulation, resulted in increased apoptotic cell death (supplemental Figure 8A) and caspase-3 activity (supplemental Figure 8B), further supporting the view that ROS contribute either directly or indirectly to caspase-3 activation. It should be noted that hydrogen peroxide did not activate caspase-8 (supplemental Figure 8B) and was not associated with mitochondrial cytochrome c release (supplemental Figure 8C), a finding that is in agreement with our model that the mitochondrial amplification loop is not active in TNF-α–stimulated neutrophils.
Essential role of ROS for caspase-3 activity after TNF-α stimulation. (A) Inhibition of p38 or PI3Ks does not affect caspase-8 or caspase-3 cleavage. Primary human neutrophils were incubated in the presence or absence of the p38 inhibitor SB203580 (1μM) or the PI3K inhibitor wortmannin (100nM) for 1 hour, and then stimulated with TNF-α (50 ng/mL) or anti-FAS antibody. Cells were harvested after 4 hours for analysis of caspase-8 processing (left panel) or 8 hours for analysis of caspase-3 processing (right panel) of culture. A representative immunoblot is shown, and protein expression levels of the active fragment p18 and p17, respectively, were quantified relative to the fresh sample (n = 4). (B) Caspase-3, but not caspase-8, activity is controlled by ROS. Caspase-8 (IETDase) and caspase-3 (DEVDase) activities were assessed by a colorimetric or fluorometric in vitro caspase activity assay. Neutrophils were harvested immediately after isolation (Fresh), left untreated (Control), or incubated with 20μM DPI, 100nM wortmannin, or 1μM SB203580 before stimulation with TNF-α (50 ng/mL). Neutrophils were also stimulated with 1 μg/mL of anti-FAS antibody. Cells were harvested and lysed at 4 hours for analysis of caspase-8 activity or at 8 hours for analysis of caspase-3 activity. Caspase-8 and caspase-3 activities are shown relative to the caspase activity detected in fresh neutrophil lysates (set as 1) (n ≥ 3).
Essential role of ROS for caspase-3 activity after TNF-α stimulation. (A) Inhibition of p38 or PI3Ks does not affect caspase-8 or caspase-3 cleavage. Primary human neutrophils were incubated in the presence or absence of the p38 inhibitor SB203580 (1μM) or the PI3K inhibitor wortmannin (100nM) for 1 hour, and then stimulated with TNF-α (50 ng/mL) or anti-FAS antibody. Cells were harvested after 4 hours for analysis of caspase-8 processing (left panel) or 8 hours for analysis of caspase-3 processing (right panel) of culture. A representative immunoblot is shown, and protein expression levels of the active fragment p18 and p17, respectively, were quantified relative to the fresh sample (n = 4). (B) Caspase-3, but not caspase-8, activity is controlled by ROS. Caspase-8 (IETDase) and caspase-3 (DEVDase) activities were assessed by a colorimetric or fluorometric in vitro caspase activity assay. Neutrophils were harvested immediately after isolation (Fresh), left untreated (Control), or incubated with 20μM DPI, 100nM wortmannin, or 1μM SB203580 before stimulation with TNF-α (50 ng/mL). Neutrophils were also stimulated with 1 μg/mL of anti-FAS antibody. Cells were harvested and lysed at 4 hours for analysis of caspase-8 activity or at 8 hours for analysis of caspase-3 activity. Caspase-8 and caspase-3 activities are shown relative to the caspase activity detected in fresh neutrophil lysates (set as 1) (n ≥ 3).
Discussion
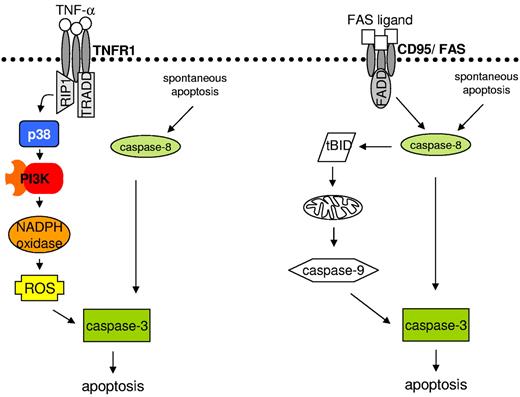
We investigated the proapoptotic signaling pathway initiated by TNF-α stimulation of neutrophils and report the following new findings. First, TNFR1 ligation is not followed by detectable caspase-8 activation, but still leads to caspase-mediated apoptosis. Second, TNF-α–mediated neutrophil apoptosis is independent of Bid and does not involve the mitochondrial death pathway. Third, the newly described death pathway is initiated by sequential activation of p38 and class IA PI3Ks. Fourth, class IA PI3Ks but not caspases are required for the generation of ROS, which is another essential step in TNF-α–mediated neutrophil apoptosis. Fifth, ROS activate either directly or indirectly effector caspases. The proximal events leading to activation of the caspase cascade differ largely between TNFR1 and FAS stimulation within neutrophils (Figure 7). Moreover, our data reveal major differences in TNFR1 signaling in neutrophils compared with published results from cells with less active NADPH oxidase.35
Working model of TNF-α- and FAS ligand–induced neutrophil apoptosis. Left panel shows that TNFR1 ligation results in p38 activation. p38 further activates the PI3K signaling pathway and ROS generation. ROS increase caspase-3 activity, leading to apoptosis. Right panel shows that ligation of FAS results in caspase-8 processing, which further activates caspase-3 and BID. BID cleavage launches the mitochondrial amplification loop, leading to mitochondrial outer membrane permeabilization, cytochrome c release, apoptosome formation, and caspase-3 activation, resulting in apoptosis.
Working model of TNF-α- and FAS ligand–induced neutrophil apoptosis. Left panel shows that TNFR1 ligation results in p38 activation. p38 further activates the PI3K signaling pathway and ROS generation. ROS increase caspase-3 activity, leading to apoptosis. Right panel shows that ligation of FAS results in caspase-8 processing, which further activates caspase-3 and BID. BID cleavage launches the mitochondrial amplification loop, leading to mitochondrial outer membrane permeabilization, cytochrome c release, apoptosome formation, and caspase-3 activation, resulting in apoptosis.
There are multiple models of TNFR signaling. The generally accepted view is that TNFR1 proapoptotic signaling is initiated by a death-inducing signaling complex containing at least TRADD, FADD, and caspase-8.36,37 Therefore, the lack of caspase-8 processing and activation after TNF-α stimulation of neutrophils was initially surprising to us. However, in neutrophils, caspase-8 processing and activation after TNFR1 stimulation have been reported to occur23,25 or to be absent.38 Our data suggest that the lack of caspase-8 activity after TNF-α stimulation may be substituted by generation of ROS, which increase caspase-3 activity. It is currently unclear which protease could process caspase-3 after TNF-α stimulation in the absence of caspase-8 activity.
The essential role of class IA PI3Ks downstream of TNFR1 was a striking finding of our study. Class IA PI3Ks are typically prosurvival proteins in different cell types,27 and have also been implicated in TNF-α–induced neutrophil survival.39 We analyzed activation of PI3K in neutrophils stimulated with low and high concentrations of TNF-α that result in survival and death, respectively. These experiments revealed that PI3Ks are indeed activated in both settings, but to different degrees. AKT phosphorylation and neutrophil apoptosis were positively correlated with the TNF-α concentrations used in this study. Therefore, we propose that TNF-α concentrations determine the level of PI3K activation that is crucial for the switch between survival and death in neutrophils.
The activation of class IA PI3Ks is known to occur by recruitment of the regulatory subunit p85 to tyrosine phosphorylated proteins located in the plasma membrane.27 Although TNFR1 and its most prominent adaptor proteins do not contain YxxM motifs that could serve as docking sites for p85-p110, class IA PI3Ks were shown to be recruited to TNFR1 after receptor stimulation in HEK293 and H1299 cells.40 Moreover, TNF-α stimulation of adherent neutrophils resulted in PI3K recruitment to TNFR1 and prolonged survival.41,42 In contrast to these reports, we did not observe recruitment of class IA PI3K p85 to TNFR1 at various times after TNF-α stimulation. Because p85 showed high affinity for the IgG control antibody, some of the published data suggesting an interaction of p85 with TNFR1 may have to be reevaluated using the appropriate controls.
Ras, Src, and Syk inhibition did not affect PI3K activity, but p38 was activated after TNFR1 stimulation and pharmacologic blocking of p38 prevented TNF-α–induced apoptosis, and therefore p38 was likely responsible for either direct or indirect PI3K activation. Previously published work suggested that the p38 substrate MK2 is a 3-phosphoinositide-dependent kinase-2 for Ser473 of AKT in neutrophils.43 Our data demonstrate that p38 inhibition reduced phosphorylation of both Ser473 and Thr308 of AKT (the latter is known to be phosphorylated by 3-phosphoinositide-dependent kinase-1). In addition, phosphorylation of p38 preceded phosphorylation of AKT, suggesting that p38 is activated before the PI3K signaling pathway. Therefore, this is the first report demonstrating the positive regulation of PI3K activity by p38. Whether p38 phosphorylates and activates class IA PI3Ks directly or through activation of intermediate proteins remains to be investigated.
The link between TNFR1 stimulation and p38 activation has been established in multiple cell types. The current model44 is a role for RIP1 interaction with the MAPK kinase kinase (MEKK3), which further activates MAP kinase kinases (MKK3/6). MKK3 and MKK6 are both known to phosphorylate p38. Therefore, ligation of TNFR1 in neutrophils results in the phosphorylation and activation of MKK3/MKK6 and subsequently p38,45,46 and p38 activation after TNF-α stimulation induces neutrophil apoptosis.47 Indirect activation of this signaling cascade by TNF-α via adhesion molecules was unlikely in our experiments, because no significant adhesion of neutrophils occurred under the culture conditions used (ie, the presence of 5% FCS).
Both p38 and PI3Ks control ROS generation in neutrophils.32,33 Whereas ROS can be generated by various sources, including the electron transport chain in mitochondria, xanthine oxydase, lipoxygenases, myeloperoxidases, and cyclooxygenases, professional phagocytes such as neutrophils and macrophages exhibit highly efficient ROS production by activation of their NADPH oxidase. We established a functional role of ROS for TNF-α–mediated neutrophil apoptosis by both genetic and pharmacologic means. Although the exact molecular mechanisms responsible for ROS generation remain to be investigated, we show that p38 and PI3K activity but not caspases are critical in this process. Neutrophils from approximately 10% of control individuals behaved like CGD neutrophils and did not generate significant ROS levels nor undergo apoptosis after TNF-α stimulation (data not shown). Furthermore, a positive correlation between the amount of ROS produced and the TNF-α concentration used to stimulate neutrophils was established. Therefore, it appears that PI3K-mediated ROS production represents the molecular key event in neutrophils that abolishes possible PI3K-mediated survival signaling events and triggers apoptosis.
ROS have previously been implicated in driving apoptosis and necrosis in different cell types. In neutrophils, 2 recent reports placed ROS proximal to caspase activity in both pathogen-induced apoptosis48 and spontaneous apoptosis.3 Under these conditions, ROS may help to permeabilize the membrane of azurophilic granules, allowing the release of cathepsin D and subsequent caspase-8 activation.3 In the present study, we provide evidence that ROS generated after TNFR1 stimulation activate caspase-3. Subcellular and kinetic differences in ROS generation after stimulation may explain why ROS can be involved both proximally and distally of caspases dependent on the death trigger.
Although ROS are usually associated with inflammation, increasing evidence suggests that enhanced inflammation occurs in the absence of ROS generated by NADPH oxidase. For example, patients with CGD suffer from a variety of inflammatory conditions.49 To reconcile these conflicting observations, the concept of ROShigh and ROSlow inflammatory responses was recently suggested.50 ROSlow responses are histologically more severe, lead to more tissue damage, and are associated with autoimmunity. Therefore, the possibility exists that high ROS generation in TNF-α–stimulated neutrophils, in addition to clearing pathogenic material, initiates a counter-regulatory mechanism that results in reduced neutrophil numbers and thereby keeps inflammation limited. In this scenario, membrane TNF-α on macrophages might be responsible for neutrophil apoptosis induction.51 The uptake of increased numbers of apoptotic neutrophils may then release additional anti-inflammatory factors from macrophages,52 further contributing to inflammation control.
Because increased ROS generation within neutrophils may also control neutrophil numbers at the site of inflammation, inhibition of the NADPH oxidase by blocking p38 or class IA PI3Ks with small-molecule inhibitors could be detrimental to the resolution of inflammation. Therefore, our data raise awareness for the need to critically assess any effect of these inhibitors on neutrophil life span and numbers in vivo, and suggest caution in the use of p38 and/or PI3K inhibitors in clinical trials targeted against inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grants 310030_129640/1 to H.-U.S. and PP00A-119203 to T.K. and U.G.). B.G. is supported by the Roche Research Foundation (Basel, Switzerland) and by L'Oréal-UNESCO For Women in Science (Bern, Switzerland).
Authorship
Contribution: B.G., T.K., and H.-U.S. designed research; B.G., U.G., and E.F. performed experiments and analyzed results; B.G. prepared the figures; and B.G. and H.-U.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hans-Uwe Simon, MD, PhD, Institute of Pharmacology, University of Bern, Friedbuehlstrasse 49, CH-3010 Bern, Switzerland; e-mail: hus@pki.unibe.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal