Abstract
We studied leukemic stem cells (LSCs) in a Smad4−/− mouse model of acute myelogenous leukemia (AML) induced either by the HOXA9 gene or by the fusion oncogene NUP98-HOXA9. Although Hoxa9-Smad4 complexes accumulate in the cytoplasm of normal hematopoietic stem cells and progenitor cells (HSPCs) transduced with these oncogenes, there is no cytoplasmic stabilization of HOXA9 in Smad4−/− HSPCs, and as a consequence increased levels of Hoxa9 is observed in the nucleus leading to increased immortalization in vitro. Loss of Smad4 accelerates the development of leukemia in vivo because of an increase in transformation of HSPCs. Therefore, the cytoplasmic binding of Hoxa9 by Smad4 is a mechanism to protect Hoxa9-induced transformation of normal HSPCs. Because Smad4 is a potent tumor suppressor involved in growth control, we developed a strategy to modify the subcellular distribution of Smad4. We successfully disrupted the interaction between Hoxa9 and Smad4 to activate the TGF-β pathway and apoptosis, leading to a loss of LSCs. Together, these findings reveal a major role for Smad4 in the negative regulation of leukemia initiation and maintenance induced by HOXA9/NUP98-HOXA9 and provide strong evidence that antagonizing Smad4 stabilization by these oncoproteins might be a promising novel therapeutic approach in leukemia.
Introduction
HOXA9 is the most highly expressed homeobox (HOX) gene in the hematopoietic stem cell (HSC) compartment of bone marrow (BM) and is down-regulated on differentiation.1,2 Retrovirally enforced overexpression of HOXA9 gene in BM cells enhances expansion of hematopoietic stem cells in vitro.3 HOXA9 is also the most commonly deregulated HOX gene in leukemia and constitutive expression of the HOXA9 gene might be a common mechanism that unifies diverse initiating events in acute myeloid leukemia (AML) and is associated with a poor clinical prognosis.4 The leukemogenic potential of HOXA9 was directly demonstrated by the development of AML in mouse BM transplantation chimeras that received a graft of primitive HSCs engineered by retroviral gene transfer to overexpress HOXA9. Murine transplantation studies showed that forced expression of HOXA9 in hematopoietic precursors results in a significant transformation of primitive hematopoietic cells and leukemia.5 HOXA9 is also directly involved in human leukemia caused by the translocation t(7;11)(p15;p15) of HOXA9 with the gene encoding NUP98, a nucleoporin protein.6,7 Expression of this fusion protein enforced expression of HOXA9 target genes8 and immortalized hematopoietic myeloid progenitors.9 Similarly, overexpression of the NUP98-HOXA9 fusion protein immortalizes murine hematopoietic progenitor cells and results in a myeloproliferative disorder (MPD) in BM transplantation experiments with development of lethal AML in a fraction of the animals.10
Transforming growth factor β (TGF-β) generates an antiproliferative effect on cells by modulating expression of genes involved in cell-cycle regulation and apoptosis.11 TGF-β–induced activation of p15 and the Cip/Kip family of CDKIs (p21, p27, p57) has been observed in hematopoietic progenitors.12-14 The mechanisms behind TGF-β induced growth arrest in normal hematopoiesis are more complex, because TGF-β potently induces apoptosis of primitive murine hematopoietic progenitor cells,15 while reduced differentiation capacity without affecting viability of early hematopoietic progenitors has also been observed after treatment with TGF-β.16,17 It has been recently described that TGF-β signaling shows differential regulation in vivo between repopulating HSCs subtypes that can be prospectively isolated.18
Members of the TGF-β family are potent tumor suppressors and malignant cells can acquire resistance to the antiproliferative effect of TGF-β by different mechanisms, including defects in TGF-β receptors and mutational inactivation of downstream effectors of the TGF-β signaling pathway, as reported in several malignancies.19,20 Deregulation of TGF-β signaling is also well recognized in leukemogenesis.21,22 Loss-of-function mutations that disrupt the TGF-β pathway involving the SMAD4 gene23,24 or the TGFBR2 gene25,26 have also been reported in patients with AML. Furthermore, using ultra-dense array comparative genomic hybridization on AML genomes, the SMAD4 gene was frequently found to be deleted in AML.27 Finally, through in vitro studies, it has been demonstrated that Smad4 physically binds to and affects the function of Hoxa9.28 All of these findings suggest that SMAD4 might be an AML-associated gene.
Although the study cited above suggests a negative regulatory role for Smad4 in the leukemogenesis mediated by Hoxa9, it has not been demonstrated whether Smad4 can prevent or block the initiation and/or maintenance of leukemia in vivo. Using an inducible Smad4 knockout (Smad4−/−) mouse model where the Smad4 gene is deleted in hematopoietic stem cells,29 we asked whether regulatory circuits controlled by Smad4 provide a protective mechanism in primitive hematopoietic cells (HSPCs; hematopoietic stem and progenitor cells) to prevent the initiation of leukemia.
Methods
Isolation of BM cells from Smad4−/− and Wt mice
Animal experiments, isolation of BM cells, and purification (Lin− depletion) of hematopoietic stem and progenitors cells (HSPCs) were performed after previously described protocols,29 approved by the Lund University Animal Ethical Committee.
Retroviral transduction
HOXA9 and NUP98-HOXA9 (MSCV-IRES-EGFP) retroviral vectors were transfected into Phoenix Ampho cells (Nolan laboratory) and supernatants were harvested for transduction of HSPCs.
Colony-forming unit assay
HSPC-transduced cells were plated into methylcellulose (MethoCult; StemCell Technologies). M3434 containing 15% of FBS was used with or without addition of 10 ng/mL recombinant TGF-β (Peprotech). M3236 was used as a TGF-β–free medium and was supplemented with 50 ng/mL mSCF, 10 ng/mL mIL-3, 50 ng/mL hIL-6, and where appropriate, 20nM of the TGF-β receptor kinase inhibitor II (Calbiochem). Ten days later, colony-forming units were counted and replated for 3 rounds of plating.
Limiting dilution assay
Limiting dilution assay was done by plating transduced cells in 96-well culture plates, in SFEM medium supplemented with mSCF, mIL-3, and hIL-6. Percentage of positive wells where expansion of cells was observed was counted after 10 days of culture.
BM transplantation
To induce leukemia, 2 × 105 transduced Lin− HSPCs (Ly5.2) were mixed with 2 × 105 support BM cells (Ly5.1) and transplanted into the tail vein of lethally irradiated (9 Gy) C57BL/6 × B6SJL recipient mice (Ly5.1/Ly5.2). For quantification of leukemia-initiating cells (LICs), 1 × 104, 1 × 103, 1 × 102 leukemic cells from primary recipients were mixed with 2 × 105 support BM cells and transplanted into the tail vein of lethally irradiated secondary recipient mice.
Cloning of MH1, transduction, and BM transplantation of MH1-expressing cells
Four portions of the MH1 part of the Smad4 cDNA were amplified by PCR and cloned into a retroviral vector expressing RFP (pRetroX-IRES-DsRedExpress Vector; Clontech). HSPCs were transduced with NUP98-HOXA9 (GFP+) and 5 days later with MH1 (RFP+), cotransduced cells (GFP+/RFP+) were sorted (Diva; Becton Dickinson) and analyzed by colony-forming unit assay or transplanted into the tail vein of lethally irradiated recipient mice.
Hematology of peripheral blood
Peripheral blood from the tail vein was collected 7 weeks after transplantation. Erythrocytes were lysed with NH4Cl and remaining cells were stained with conjugated antibodies for FACS analysis. Hematologic parameters were monitored to control the development of leukemia (KX-21N; Sysmex).
Flow cytometry
For lineage depletion BM cells were stained with a cocktail of antibodies; rat anti–mouse B220, Gr1, Mac1, CD4, CD5, CD8, Ter119 (Becton Dickinson), and goat anti–rat Tricolor antibody (Caltag Lab). For the lineage staining analysis, Sca-1 PE-Cy5.5, c-Kit APC, CD34 PE-Cy7, Flt3-PE antibodies (Becton Dickinson) were used. 7-amino-actinomycin D (7-AAD) was used for detection of nonviable cells (Sigma-Aldrich). BrdU Flow Kit was used for cell cycle analysis (Becton Dickinson). Stained cells were run through a FACS Calibur or a FACS Canto II (Becton Dickinson) and the subsequent data were analyzed with FlowJo Version 9 software (TreeStar).
Patient samples
BM samples were used from AML patients containing the chromosomal translocation t(7;11)(p15;p15), resulting in the generation of NUP98-HOXA9 fusion genes. The presence of a NUP98-HOXA9 rearrangement was verified by either FISH or RT-PCR. In addition, BM samples from AML patients showing either high or low expression in HOXA9 and normal human BM samples were analyzed. Samples were obtained from patients after informed consent in accordance with the Declaration of Helsinki.
Western blot
Cytoplasm was dissociated from nucleoplasm following CellLytic-NuCLEAR extraction kit (Sigma-Aldrich). Laemmli buffer (BioRad) containing 1% Protease Inhibitor Cocktail (BioRad) was added (1:1) on total cells, nucleoplasm or cytoplasm fractions. When necessary, DNA was sonicated. Antibodies used for Western blot were mouse anti-Smad4 (clone Sc-7966; Santa Cruz Biotechnology), rabbit anti-Smad4 (1:300, clone EP618Y; Abcam), anti-Hoxa9 (clone 07-178; Upstate Biotechnology), anti-NUP98 antibody (2H10, Sigma-Aldrich), anti-Casp3 (clone CPP32; Becton Dickinson), anti-Actin (clone Ab-5; Becton Dickinson) and anti–Lamin-A (clone L1293; Sigma-Aldrich). After blotting with an HRP-conjugated secondary antibody, chemiluminescence was detected using the enhanced chemiluminescence (ECL) Advance Western blot detection kit (Amersham Biosciences). Gel images were analyzed using ImageJ Version 1.43 (NIH) for quantification.
Immunostaining
After binding on poly-L-Lysine treated slides (Menzel-Gläser), cells were air-dried, fixed with PFA 4% and permeabilized with 1× Triton X-100. Mouse anti-Smad4 (1:300, clone Sc-7966; Santa Cruz Biotechnology) and rabbit anti-Hoxa9 (1:300, clone 07-178; Upstate Biotechnology) antibodies were applied with 5% donkey serum for 12 hours at 4°C. After washes in PBS, secondary donkey anti–rabbit Alexa Fluor 555 and donkey anti–mouse Alexa Fluor 488 antibodies (1:500; Jackson ImmunoResearch Laboratories) were applied with 5% donkey serum for 2 hours. For Smad4 nuclear translocation cells were treated 12 hours with the recombinant TGF-β (10 ng/mL). Mouse anti-Smad4 and secondary donkey anti–mouse Alexa Fluor 647 antibodies (1:500; Jackson ImmunoResearch Laboratories) were used. For all experiments, immunofluorescence was documented with an Axiovert 200M microscope (Zeiss) or a TE 2000 microscope (Nikon). Colocalization was assessed using ImageJ Version 1.43 software (NIH).
Immunoprecipitation
For analysis of Smad4-Hoxa9 complexes, 107 cells were lysed and precleared with protein G (Life Sciences). The lysate was immunoprecipitated with the Smad4 antibody and loaded onto a PAGE without SDS to analyze proteins in the native condition. The immunoblot was incubated with the anti-Smad4 or the anti-Hoxa9 antibodies. To analyze the binding of the different portions of MH1 with Hoxa9, FLAGs (5-DYKDDDDK-MH1) were introduced, 2 × 106 sorted cells expressing NUP98-HOXA9 and MH1 were lysed. The lysate was immunoprecipitated with the anti-FLAG M2 antibody (Stratagene) and the immunoblot analyzed on PAGE-SDS with an anti-Hoxa9 antibody.
TUNEL assay
TdT-mediated dUTP nick end labeling (TUNEL) assay was performed using an Apoptosis Detection Kit (GenScript).
ChIP
Proteins were crosslinked with 1% formaldehyde and chromatin was sonicated to an average length of DNA fragment sizes of 0.5-1.5 kb. After cell lysate preclearing with protein G, the lysate was immunoprecipitated with the Smad4 antibody or mouse control IgG antibody. Immune complexes were collected with protein G beads for 1 hour. Beads were then washed and incubated with RNase and Proteinase K. DNA was pelleted and immunoprecipitated chromatin was assayed by semiquantitative PCR.
RNA extraction, reverse transcription, and real-time PCR
RNA extraction, cDNA synthesis, and real-time PCR were performed following a previously described protocol,30 using the ABI7900HT system (Applied Biosystems).
Statistical analysis
All data are expressed as the mean ± SEM. We assessed differences between the groups by Student unpaired t test. Statistical analysis of survival curves were performed using the Mantel Haenszel logrank test.
Results
The immortalizing function of HOXA9 and NUP98-HOXA9 is increased in Smad4−/− hematopoietic primitive cells
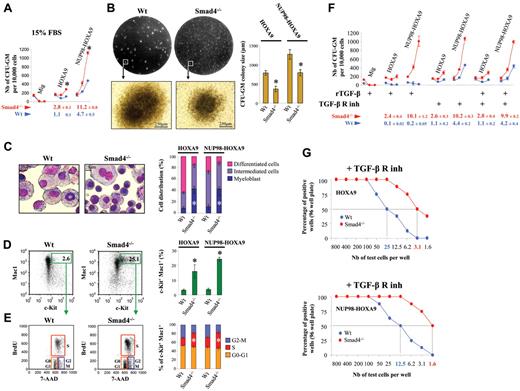
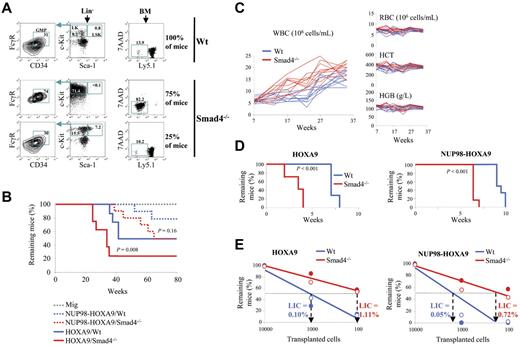
To ask whether there is a definite functional role for Smad4 in immortalization of primary hematopoietic cells by HOXA9 and NUP98-HOXA9, we serially replated hematopoietic colonies (colony-forming unit; CFU) from BM cells transduced with these oncogenes. We purified Lin− cells, which are enriched for hematopoietic stem cells and more differentiated committed myeloid progenitor cells (HSPCs) and transduced this population with HOXA9 or NUP98-HOXA9 retroviral constructs. To assess the efficiency in the immortalization of wild-type (Wt) and Smad4−/− HSPCs, we used a semisolid medium that contains 15% of FBS supplemented with IL-3, IL-6, and SCF. The cloning efficiency was markedly increased in Smad4−/− cells (Figure 1A). Only CFU-GM colonies were immortalized by HOXA9 and NUP98-HOXA9 oncogenes while other hematopoietic colonies (CFU-GEMM, CFU-E, CFU-M, and CFU-G) were not maintained through multiple passages (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). When we transduced hematopoietic cells with 2 completely different oncogenes (MOZ-TIF2, AML1-EVI1), no rapid expansion of immortalized progenitors was seen in Smad4−/− marrow compared with Wt (supplemental Figure 2), indicating that the Smad4−/− effect is HOX-dependent and probably related to previously described interaction between Smad4 and Hoxa9.28 A significant decrease in size of the CFU-GM colonies formed by Smad4−/− cells was observed in the semisolid medium plates, suggesting a difference in composition (Figure 1B). Morphology analysis confirmed that Smad4−/− cells were more immature, as Giemsa staining showed an increase in the number of myeloblasts in the Smad4−/− colonies (Figure 1C). In addition, FACS analysis demonstrated an increase in the proportion of the c-Kit+ primitive subpopulation in the Smad4−/− colonies (Figure 1D), and increased cell cycling capacity of c-Kit+Smad4−/− transduced cells (Figure 1E). It is thus clear that Smad4 deficiency induces primitive fate options in HOXA9- and NUP98-HOXA9–transduced HSPCs.
The immortalizing function of HOXA9 and NUP98-HOXA9 is increased in Smad4−/− primitive hematopoietic cells. (A) Lin− cells transduced with HOXA9, NUP98-HOXA9, or the empty vector (Mig) were assessed for immortalization by counting the number of CFU-GM colonies per 10 000 cells. Results shown are from 3 rounds of plating using Wt cells (blue dots) or Smad4−/− cells (red dots), with medium containing 15% of FBS (data show mean ± SEM, n = 4, *P < .01 is measured by Student unpaired t test). The cloning efficiency (number of cells that make colonies) was calculated for the third round of plating (below graphs). (B) Decrease in size of the CFU-GM colonies observed for the third round of plating, by visualization of the plate on a UV-transilluminator and microscopy. Representative pictures of 4 experiments observed under forced expression of NUP98-HOXA9 (left panel). Statistical analysis for HOXA9 and NUP98-HOXA9 (data show mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (C) Increase in the myeloblastic cell population in the Smad4−/− samples detected by May-Grünwald Giemsa staining of cytospun cells that constitute the CFU-GM colonies. Representative picture of the staining observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (D) Increase of the c-Kit+ primitive subpopulation in Smad4−/− bone marrow (BM) by FACS analysis. Representative FACS data observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (E) BrdU and 7-AAD staining showing increased percentage of cells in S phase among c-Kit+ primitive subpopulation in Smad4−/− BM. Representative FACS data observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison (right panel) between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). (F) CFU assay with medium supplemented with 10 ng/mL of the recombinant TGF-β (rTGF-β), with a TGF-β–free medium (M3236) supplemented with TGF-β receptor kinase inhibitor (TGF-β R inh) or both rTGF-β and TGF-β R inh (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). The cloning efficiency (number of cells that make colonies) was calculated for the third round of plating (below graphs). (G) The growth capacity of HOXA9- and NUP98-HOXA9–transduced Smad4−/− cells is increased compared with Wt cells. Limiting dilution assay was done by plating HOXA9 or NUP98-HOXA9–transduced cells in 96-well culture plates, cells were maintained in SFEM medium, and supplemented with the TGF-β receptor kinase inhibitor (+ TGF-β R inh).
The immortalizing function of HOXA9 and NUP98-HOXA9 is increased in Smad4−/− primitive hematopoietic cells. (A) Lin− cells transduced with HOXA9, NUP98-HOXA9, or the empty vector (Mig) were assessed for immortalization by counting the number of CFU-GM colonies per 10 000 cells. Results shown are from 3 rounds of plating using Wt cells (blue dots) or Smad4−/− cells (red dots), with medium containing 15% of FBS (data show mean ± SEM, n = 4, *P < .01 is measured by Student unpaired t test). The cloning efficiency (number of cells that make colonies) was calculated for the third round of plating (below graphs). (B) Decrease in size of the CFU-GM colonies observed for the third round of plating, by visualization of the plate on a UV-transilluminator and microscopy. Representative pictures of 4 experiments observed under forced expression of NUP98-HOXA9 (left panel). Statistical analysis for HOXA9 and NUP98-HOXA9 (data show mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (C) Increase in the myeloblastic cell population in the Smad4−/− samples detected by May-Grünwald Giemsa staining of cytospun cells that constitute the CFU-GM colonies. Representative picture of the staining observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (D) Increase of the c-Kit+ primitive subpopulation in Smad4−/− bone marrow (BM) by FACS analysis. Representative FACS data observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test; right panel). (E) BrdU and 7-AAD staining showing increased percentage of cells in S phase among c-Kit+ primitive subpopulation in Smad4−/− BM. Representative FACS data observed under forced expression of NUP98-HOXA9 (left panel). Statistical comparison (right panel) between HOXA9 and NUP98-HOXA9 samples (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). (F) CFU assay with medium supplemented with 10 ng/mL of the recombinant TGF-β (rTGF-β), with a TGF-β–free medium (M3236) supplemented with TGF-β receptor kinase inhibitor (TGF-β R inh) or both rTGF-β and TGF-β R inh (mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). The cloning efficiency (number of cells that make colonies) was calculated for the third round of plating (below graphs). (G) The growth capacity of HOXA9- and NUP98-HOXA9–transduced Smad4−/− cells is increased compared with Wt cells. Limiting dilution assay was done by plating HOXA9 or NUP98-HOXA9–transduced cells in 96-well culture plates, cells were maintained in SFEM medium, and supplemented with the TGF-β receptor kinase inhibitor (+ TGF-β R inh).
Reduced immortalization of Wt primitive hematopoietic cells by HOXA9 or NUP98-HOXA9 does not require the canonical function of Smad4
Administration of recombinant TGF-β (10 ng/mL) to the medium induced the nuclear translocation of Smad4 (supplemental Figure 3), which led to a reduction/block in the immortalization of Wt primitive hematopoietic cells expressing the oncogenes (Figure 1F). This clearly shows that TGF-β signaling is not impaired in Wt transduced cells overexpressing HOXA9 or NUP98-HOXA9. Therefore, administration of the recombinant TGF-β increased nuclear activity of Smad4 mediated through the canonical Smad pathway and can thereby reduce immortalization of primitive hematopoietic cells; the TGF-β/Smad4 signaling pathway is not defective in HOXA9 or NUP98-HOXA9 overexpressing HSPCs. Because high concentrations of the recombinant TGF-β failed to inhibit CFU-GM growth of Smad4−/− HSPCs, the enhanced immortalization of Smad4 deficient cells in vitro should be related to their incapacity to activate TGF-β signaling.
Next, we assessed the serial replating of hematopoietic colonies using a semisolid medium free of FBS (MethoCult M3236) supplemented with a TGF-β receptor kinase inhibitor to prevent activation of hematopoietic cells from the autocrine TGF-β production. We asked whether the immortalization of Wt transduced HSPCs would reach the immortalization frequency observed for Smad4−/− HSPCs by blocking TGF-β signaling. Interestingly, we observed that the immortalization of TGF-β receptor-blocked Wt cells was still reduced, compared with Smad4−/− cells (Figure 1F). By limiting dilution assay in 96-well plates, in DMEM medium (free of TGF-β) supplemented with the TGF-β receptor kinase inhibitor, the growth capacity of HOXA9- and NUP98-HOXA9–transduced Smad4−/− cells was increased ∼ 8-fold compared with Wt cells (Figure 1G).
The Smad4 signaling pathway functions to transduce signals downstream of the TGF-β family of ligands, including the activation of the TGF-β receptor, and the receptors for Activins and bone morphogenetic proteins (BMPs).31 We could exclude implication of the BMP pathway because administration of recombinant BMPs (mix of BMP 2, 4, and 7 at 10 ng/mL each) did not block growth of Wt transduced cells overexpressing HOXA9 or NUP98-HOXA9. However, administration of recombinant Activin A (10 ng/mL) blocked growth of Wt transduced cells (supplemental Figure 4A). To avoid involvement of other activators of Smad4 signaling, we also tested an inhibitor of endogenous Activin and TGF-β signaling (SB-431542) to inhibit both these pathways that can activate Smad4 signaling. Here again, we observed that immortalization of Wt transduced HSPCs never reached the immortalization frequency observed for Smad4−/− HSPCs (supplemental Figure 4B). We therefore concluded that the activation of the canonical Smad4-signaling pathway is not required for the growth-reducing effect of HOXA9/NUP98-HOXA9–transduced progenitors mediated by Smad4.
Increased SMAD4 protein levels in human AML samples with high levels of the HOXA9 protein
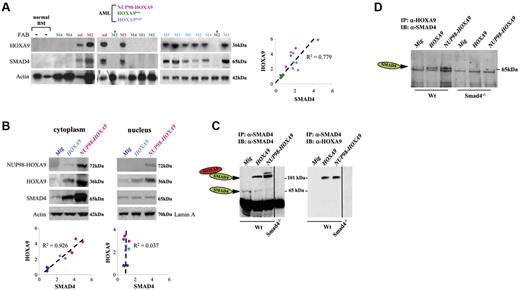
Our findings that Smad4 deficiency leads to enhanced immortalization of Smad4−/− cells in vitro urged us to gain insight into the molecular mechanisms by which aberrant TGF-β signaling may participate in leukemogenesis in patients of NUP98-HOXA9 induced AML. Western blot analysis of BM cells derived from patients with NUP98-HOXA9–related AML had highly activated HOXA9 expression, because NUP98-HOXA9 enforces strong expression of the cellular HOXA9 gene.9 Intriguingly, we discovered that SMAD4 protein levels were strongly up-regulated and that this increased expression unexpectedly correlated with the endogenous HOXA9 protein expression (Figure 2A). Because several AML leukemias exhibit increased expression of a subset of HOX genes, including HOXA9,4 we examined additional AML patient samples by Western blot analysis and observed that increased SMAD4 protein levels were restricted to AML samples with increased levels of the endogenous HOXA9 protein, and there was a correlation between the levels of the 2 proteins (Figure 2A). SMAD4 expression could not be correlated to FAB subtypes of AML samples used in the study and consequently cannot reflect different SMAD4 levels at differing stages of myeloid maturation. We could demonstrate the colocalization of HOXA9 and SMAD4 in the cytoplasm of primary human AML blasts showing high levels of HOXA9 (supplemental Figure 5).
HOXA9 and SMAD4 are coexpressed in human AMLs with NUP98-HOXA9 chromosomal translocation or with high levels in expression of HOXA9 as well as in murine primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9. (A) Western blot analysis of BM cells derived from patients with AML (left panel), using BM from AML patients with low-level expression of HOXA9 (HOXA9low, n = 5), with high-level expression of HOXA9 (HOXA9high, n = 6), or BM samples from AML patients with chromosomal translocation t(7;11)(p15;p15), resulting in NUP98-HOXA9 cytogenetic translocations (NUP98-HOXA9, n = 4). Normal human BM (n = 2) was used as control. Quantification of gel (right panel) reveals a correlation between SMAD4 and HOXA9 intensity. FAB classification is shown on the top (nd indicates not determined). (B) Representative Western blot showing the coregulation of Smad4 and Hoxa9 proteins in murine Wt HSPCs immortalized by HOXA9 (light blue dots) or NUP98-HOXA9 (pink dots) and presentation of the relative increase compared with control samples (Mig vector, blue dark dots) as seen in the right panel. (C) Representative immunoblots showing stabilization of Smad4 and Hoxa9 proteins in Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9. After immunoprecipitation (IP) with the anti-Smad4 antibody and PAGE in the absence of SDS (native condition), the Smad4-Hoxa9 complexes were analyzed by immunoblot (IB) with anti-Smad4 (left) and anti-Hoxa9 (right) antibodies (1 representative experiment of 2 independent experiments). (D) The immunoprecipitation with anti-Hoxa9 antibody and immunoblot (SDS-PAGE) with the anti-Smad4 antibody confirms the presence of Smad4-Hoxa9 complexes in Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9. (B-D) HSPCs (Lin− cells) were isolated from hematopoietic cells generated at serial passage 2 or 3 using CFU assay (HSPCs immortalized by HOXA9 or NUP98-HOXA9). Control HSPCs (Lin− cells) were isolated from hematopoietic cells generated from first passage (HSPCs not immortalized, transduced with Mig).
HOXA9 and SMAD4 are coexpressed in human AMLs with NUP98-HOXA9 chromosomal translocation or with high levels in expression of HOXA9 as well as in murine primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9. (A) Western blot analysis of BM cells derived from patients with AML (left panel), using BM from AML patients with low-level expression of HOXA9 (HOXA9low, n = 5), with high-level expression of HOXA9 (HOXA9high, n = 6), or BM samples from AML patients with chromosomal translocation t(7;11)(p15;p15), resulting in NUP98-HOXA9 cytogenetic translocations (NUP98-HOXA9, n = 4). Normal human BM (n = 2) was used as control. Quantification of gel (right panel) reveals a correlation between SMAD4 and HOXA9 intensity. FAB classification is shown on the top (nd indicates not determined). (B) Representative Western blot showing the coregulation of Smad4 and Hoxa9 proteins in murine Wt HSPCs immortalized by HOXA9 (light blue dots) or NUP98-HOXA9 (pink dots) and presentation of the relative increase compared with control samples (Mig vector, blue dark dots) as seen in the right panel. (C) Representative immunoblots showing stabilization of Smad4 and Hoxa9 proteins in Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9. After immunoprecipitation (IP) with the anti-Smad4 antibody and PAGE in the absence of SDS (native condition), the Smad4-Hoxa9 complexes were analyzed by immunoblot (IB) with anti-Smad4 (left) and anti-Hoxa9 (right) antibodies (1 representative experiment of 2 independent experiments). (D) The immunoprecipitation with anti-Hoxa9 antibody and immunoblot (SDS-PAGE) with the anti-Smad4 antibody confirms the presence of Smad4-Hoxa9 complexes in Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9. (B-D) HSPCs (Lin− cells) were isolated from hematopoietic cells generated at serial passage 2 or 3 using CFU assay (HSPCs immortalized by HOXA9 or NUP98-HOXA9). Control HSPCs (Lin− cells) were isolated from hematopoietic cells generated from first passage (HSPCs not immortalized, transduced with Mig).
Smad4/Hoxa9 stabilization in the cytoplasm of primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9
Using Western blot analysis, we analyzed the intracellular level of Smad4 and Hoxa9 in mouse HSPCs immortalized by HOXA9 or NUP98-HOXA9 and importantly observed that both proteins were increased and present at correlated levels in the cytoplasmic fraction, whereas no correlation was detectable in the nucleus (Figure 2B). Increased expression of the Smad4 protein cannot be explained by an increase in the transcriptional activation of the Smad4 mouse gene, because there was no up-regulation of the Smad4 transcripts in cells expressing HOXA9 or NUP98-HOXA9 (supplemental Figure 6). Importantly, the Smad4 protein expression was not found increased in HSPCs purified from Wt cells transduced with 2 control oncogenes (MOZ-TIF2, AML1-EVI1), suggesting that increased presence of Smad4 is HOX-dependent (supplemental Figure 7). Furthermore, we observed an increased amount of Smad4 when Cos7 cells were transduced with HOXA9 (supplemental Figure 8), which suggests that Hoxa9 can stabilize Smad4 in a cell line and not only in progenitors that are in differentiation flux.
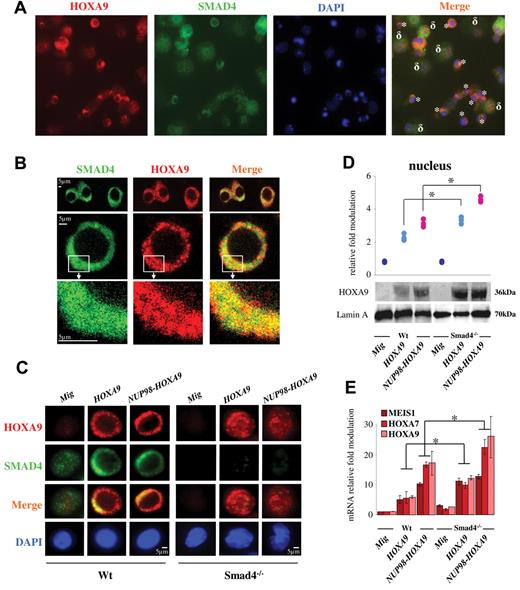
Because Hoxa9 can physically bind to Smad4,28 we asked whether Smad4 is accumulated in cells. Indeed, immunoprecipitation of Smad4 confirmed that the protein was mainly (> 80%) complexed to Hoxa9 in HSPCs either immortalized by HOXA9 or NUP98-HOXA9 (Figure 2C). The reverse immunoprecipitation also confirmed the Smad4-Hoxa9 complexes in Wt HSPCs immortalized by forced expression of HOXA9 or NUP98-HOXA9 (Figure 2D). Next, we investigated intracellular immunostaining and importantly observed that only primitive hematopoietic cells (Lin− purified) showed predominant cytoplasmic expression of Smad4 and Hoxa9, while more differentiated cells (Lin+ purified) showed nuclear localization of Hoxa9 (Figure 3A and supplemental Figure 9). We confirmed by confocal microscopy the colocalization of Smad4 and Hoxa9 in the cytoplasm of Wt primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9 (Figure 3B).
Hoxa9 colocalizes with Smad4 in the cytoplasm of primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9. (A) Transmission microscopy showing the colocalization of Smad4 (green) and Hoxa9 (red) in the cytoplasmic fraction of Wt primitive hematopoietic cells immortalized with HOXA9 (*), while the colocalization is not observed in more differentiated cells (δ). Experiment was performed using total hematopoietic cells immortalized at serial passage 2 or 3 using CFU assay. (B) Confocal microscopy in sequential scanning mode, using a 63× oil-immersion objective showing the colocalization of Smad4 (green) and Hoxa9 (red) in the cytoplasmic fraction of Wt HSPCs immortalized by HOXA9. (C) Immunostaining showing accumulation of Smad4 and Hoxa9 in the cytoplasmic fraction of Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9 (left panel) and cytoplasmic/nuclear localization of Hoxa9 in Smad4−/− HSPCs immortalized by HOXA9 or NUP98-HOXA9 (right panel; representative of 3 individual experiments). (D) Representative Western blot showing increased expression of the Hoxa9 protein in the nucleus of Smad4−/− HSPCs immortalized by HOXA9 (light blue dots) or NUP98-HOXA9 (pink dots) and presentation of the relative increase compared with control samples (Mig vector, blue dark dots), representative of 3 individual experiments. (E) Activation of specific Hoxa9 targets in Smad4−/− HSPCs immortalized by HOXA9 or NUP98-HOXA9 (relative fold change in mRNA expression compared with Wt Mig HSPCs, data show mean ± SEM, n = 4, *P < .01 measured by Student unpaired t test). (B-E) HSPCs (Lin− cells) were isolated from hematopoietic cells generated at serial passage 2 or 3 using CFU assay (HSPCs immortalized by HOXA9 or NUP98-HOXA9). Control HSPCs (Lin− cells) were isolated from hematopoietic cells generated from first passage (HSPCs not immortalized, transduced with Mig).
Hoxa9 colocalizes with Smad4 in the cytoplasm of primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9. (A) Transmission microscopy showing the colocalization of Smad4 (green) and Hoxa9 (red) in the cytoplasmic fraction of Wt primitive hematopoietic cells immortalized with HOXA9 (*), while the colocalization is not observed in more differentiated cells (δ). Experiment was performed using total hematopoietic cells immortalized at serial passage 2 or 3 using CFU assay. (B) Confocal microscopy in sequential scanning mode, using a 63× oil-immersion objective showing the colocalization of Smad4 (green) and Hoxa9 (red) in the cytoplasmic fraction of Wt HSPCs immortalized by HOXA9. (C) Immunostaining showing accumulation of Smad4 and Hoxa9 in the cytoplasmic fraction of Wt HSPCs immortalized by HOXA9 or NUP98-HOXA9 (left panel) and cytoplasmic/nuclear localization of Hoxa9 in Smad4−/− HSPCs immortalized by HOXA9 or NUP98-HOXA9 (right panel; representative of 3 individual experiments). (D) Representative Western blot showing increased expression of the Hoxa9 protein in the nucleus of Smad4−/− HSPCs immortalized by HOXA9 (light blue dots) or NUP98-HOXA9 (pink dots) and presentation of the relative increase compared with control samples (Mig vector, blue dark dots), representative of 3 individual experiments. (E) Activation of specific Hoxa9 targets in Smad4−/− HSPCs immortalized by HOXA9 or NUP98-HOXA9 (relative fold change in mRNA expression compared with Wt Mig HSPCs, data show mean ± SEM, n = 4, *P < .01 measured by Student unpaired t test). (B-E) HSPCs (Lin− cells) were isolated from hematopoietic cells generated at serial passage 2 or 3 using CFU assay (HSPCs immortalized by HOXA9 or NUP98-HOXA9). Control HSPCs (Lin− cells) were isolated from hematopoietic cells generated from first passage (HSPCs not immortalized, transduced with Mig).
Taken together, our data are consistent with a mechanism that involves Hoxa9/Smad4 stabilization/accumulation in the cytoplasm of primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9.
Increased concentration of Hoxa9 was observed in the nucleus of Smad4−/− primitive hematopoietic cells immortalized by HOXA9 or NUP98-HOXA9
Next, we analyzed whether this stabilization/accumulation might be a mechanism where Smad4 binds Hoxa9 to the cytoplasm to protect against accumulation of Hoxa9 protein in the nucleus. The Smad4−/− model represents a versatile tool because the Hoxa9 stabilization is not possible without endogenous Smad4. Using immunostaining we clearly observed that Hoxa9 was largely increased in the nucleus of immortalized Smad4−/− HSPCs (Figure 3C). The concentration of Hoxa9 found by Western blot in the nucleoplasm of immortalized Smad4−/− HSPCs (Figure 3D) clearly increases the transcriptional activation of several down-stream Hoxa9 genes (Hoxa7, Hoxa9, and Meis1) involved in immortalization (Figure 3E).
We conclude that in Wt HSPCs, Smad4 stabilizes Hoxa9 to the cytoplasm to protect against increased exposure of Hoxa9 in the nucleoplasm. This mechanism explains why immortalization of transduced hematopoietic progenitors was markedly increased in Smad4−/− cells (Figure 1).
Smad4 deficiency induces in vivo expansion of primitive hematopoietic cells expressing HOXA9 or NUP98-HOXA9
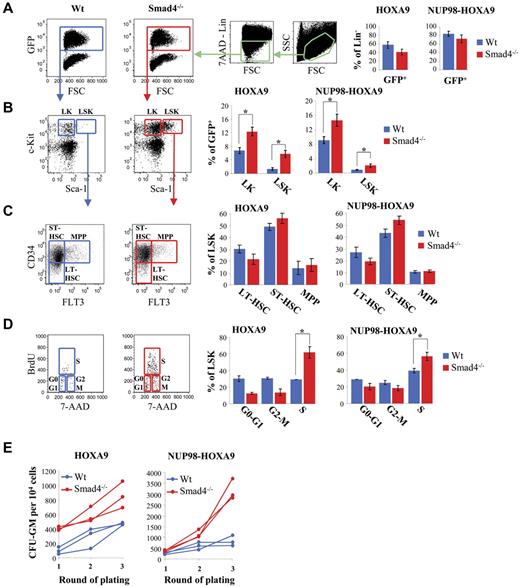
Next, we investigated whether Smad4−/− HSPCs might exhibit an increased frequency of BM transformation to induce leukemia in mice. HSPCs from Wt or Smad4−/− mice were transduced with vectors containing HOXA9 or NUP98-HOXA9 and transplanted into lethally irradiated recipient mice. Smad4−/− HSPCs exhibit impaired engraftment which leads to reduced repopulation capacity by GFP+ cells expressing HOXA9 or NUP98-HOXA9 in BM, 7 weeks after transplantation (Figure 4A). However, increased expansion of primitive hematopoietic cells was observed among the Smad4−/− lineage negative (Lin−) cells transduced with HOXA9 or NUP98-HOXA9, with, respectively, 1.6 ± 0.2 (n = 8, P < .01) and 1.8 ± 0.3 fold (n = 8, P < .01) induction of Lin−Sca1−c-Kit+ (LK) progenitors and with 2.3 ± 0.6 (n = 8, P < .01) and 4.2 ± 1.4 (n = 8, P < .01) fold increase of Lin−Sca1+c-Kit+ (LSK) fraction compared with Wt cells (Figure 4B). Furthermore, using immunophenotypic analysis, we observed a slight expansion of short-term hematopoietic stem cells (ST-HSCs) and a slight decrease of long-term hematopoietic stem cells (LT-HSCs) among Smad4−/− LSK, compared with Wt LSK (Figure 4C), while no difference was observed in the distribution of multipotent progenitors (MPP). We could not observe any variation in the distribution of more committed progenitors, including CMP, GMP, and MEP (supplemental Figure 10). Increased expansion of Smad4−/− LSK was corroborated by an increased percentage of Smad4−/− LSK cells in S phase (Figure 4D) and by an increased capacity of BM cells from mice transplanted with Smad4−/− cells to form CFU ex vivo (Figure 4E).
Increased expansion of Smad4−/− primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9 in mice after transplantation. (A-D) FACS analysis of BM cells, 7 weeks after transplantation. Representative FACS data (left panel). The right panel displays a bar diagram, for Smad4−/− (red bars) or Wt (blue bars) cells transduced with HOXA9 or NUP98-HOXA9 (data show mean ± SEM, n = 8 for each group, *P < .01 measured by Student unpaired t test). (A) FACS analysis showing engraftment of Wt (blue) and Smad4−/− (red) cells transduced with HOXA9 or NUP98-HOXA9 (% of GFP+). (B) FACS analysis showing increased expansion of LK (c-Kit+ Sca-1−) and LSK cells (c-Kit+Sca-1+) in the Lin− cell subset of BM from mice transplanted with Smad4−/− cells transduced with HOXA9 or NUP98-HOXA9. (C) Percentage of LT-HSC, ST-HSC, and MPP among the population of LSK subset of cells from BM of mice transplanted with Wt (blue) or Smad4−/− (red) HSPCs transduced with HOXA9 or NUP98-HOXA9. (D) FACS analysis showing increased percentage of cells in S phase among the population of LSK subset of cells from BM of mice transplanted with Smad4−/− primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9. (E) Ex vivo culture of BM cells showing that the frequency of cells that make a colony was markedly increased in BM from mice transplanted with Smad4−/− HSPCs, compared with mice transplanted with Wt HSPCs. Samples were analyzed 7 weeks after transplantation.
Increased expansion of Smad4−/− primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9 in mice after transplantation. (A-D) FACS analysis of BM cells, 7 weeks after transplantation. Representative FACS data (left panel). The right panel displays a bar diagram, for Smad4−/− (red bars) or Wt (blue bars) cells transduced with HOXA9 or NUP98-HOXA9 (data show mean ± SEM, n = 8 for each group, *P < .01 measured by Student unpaired t test). (A) FACS analysis showing engraftment of Wt (blue) and Smad4−/− (red) cells transduced with HOXA9 or NUP98-HOXA9 (% of GFP+). (B) FACS analysis showing increased expansion of LK (c-Kit+ Sca-1−) and LSK cells (c-Kit+Sca-1+) in the Lin− cell subset of BM from mice transplanted with Smad4−/− cells transduced with HOXA9 or NUP98-HOXA9. (C) Percentage of LT-HSC, ST-HSC, and MPP among the population of LSK subset of cells from BM of mice transplanted with Wt (blue) or Smad4−/− (red) HSPCs transduced with HOXA9 or NUP98-HOXA9. (D) FACS analysis showing increased percentage of cells in S phase among the population of LSK subset of cells from BM of mice transplanted with Smad4−/− primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9. (E) Ex vivo culture of BM cells showing that the frequency of cells that make a colony was markedly increased in BM from mice transplanted with Smad4−/− HSPCs, compared with mice transplanted with Wt HSPCs. Samples were analyzed 7 weeks after transplantation.
Loss of Smad4 increases the frequency of leukemia stem cells and increases BM transformation in mice
Mice transplanted with Smad4−/− HSPCs show an accelerated HOXA9-induced transformation of BM, illustrated by a substantial accumulation of donor-derived LK population in BM already 22 weeks after transplantation (Figure 5A). Accordingly, a larger fraction of mice transplanted with Smad4−/− transduced-cells succumbed more rapidly to AML (Figure 5B). In addition, the preleukemic myeloproliferative disease that NUP98-HOXA9 induces in mice10 was more pronounced in the Smad4−/− background (Figure 5C). Only the total WBC level was affected, whereas other constituents of peripheral blood, red blood cells, hematocrit, and hemoglobin remain constant overtime.
Fast progression to AML in mice transplanted with Smad4−/− primitive hematopoietic cells. (A) Twenty-two weeks after the transplantation with HOXA9-transduced HSPCs, a total reconstitution of LK (c-Kit+ Sca-1−) progenitors, which largely consists of granulocyte-monocyte progenitors (GMP; CD34+ FcγR+) indicates transformation of myeloid progenitors in the BM of 75% of mice in the Smad4−/− group (n = 8 mice each). The transformation is moreover accompanied by a dominant reconstitution of donor Ly5.1− cells in the BM. (B) Kaplan-Meier plot of recipient survival more than time (Mig, n = 6 mice; HOXA9, n = 10 mice; and NUP98-HOXA9, n = 10 mice; P value measured by Mantel Haenszel logrank test). (C) Development of the WBC count more than time in peripheral blood after transplantation with Wt (blue) or Smad4−/− (red) HSPCs, transduced with NUP98-HOXA9 (n = 10 mice). During the chronic phase, only the total WBC level is affected, whereas other constituents of peripheral blood, red blood cells (RBCs), hematocrit (HCT), and hemoglobin (HGB) remain constant more than time (n = 10 mice). (D) Loss of Smad4 increases the LIC frequency in mice. The Kaplan-Meier plot displays survival over time of secondary recipient mice transplanted with HOXA9- or NUP98-HOXA9–leukemic Smad4−/− and Wt cells (n = 12 mice for each group; P value measured by Mantel Haenszel logrank test; leukemic cells were isolated from moribund mice to analyze LIC frequencies at the same stage of the disease). (E) Quantification of transplanted leukemic cells needed to generate leukemia in 50% of the secondary recipients. The percentage of LICs in the population of leukemic cells is calculated as 1 LIC per total number of cells injected to induce lethality in 50% of the recipients (n = 7 mice for each group; full and empty circles represent 2 independent clones tested).
Fast progression to AML in mice transplanted with Smad4−/− primitive hematopoietic cells. (A) Twenty-two weeks after the transplantation with HOXA9-transduced HSPCs, a total reconstitution of LK (c-Kit+ Sca-1−) progenitors, which largely consists of granulocyte-monocyte progenitors (GMP; CD34+ FcγR+) indicates transformation of myeloid progenitors in the BM of 75% of mice in the Smad4−/− group (n = 8 mice each). The transformation is moreover accompanied by a dominant reconstitution of donor Ly5.1− cells in the BM. (B) Kaplan-Meier plot of recipient survival more than time (Mig, n = 6 mice; HOXA9, n = 10 mice; and NUP98-HOXA9, n = 10 mice; P value measured by Mantel Haenszel logrank test). (C) Development of the WBC count more than time in peripheral blood after transplantation with Wt (blue) or Smad4−/− (red) HSPCs, transduced with NUP98-HOXA9 (n = 10 mice). During the chronic phase, only the total WBC level is affected, whereas other constituents of peripheral blood, red blood cells (RBCs), hematocrit (HCT), and hemoglobin (HGB) remain constant more than time (n = 10 mice). (D) Loss of Smad4 increases the LIC frequency in mice. The Kaplan-Meier plot displays survival over time of secondary recipient mice transplanted with HOXA9- or NUP98-HOXA9–leukemic Smad4−/− and Wt cells (n = 12 mice for each group; P value measured by Mantel Haenszel logrank test; leukemic cells were isolated from moribund mice to analyze LIC frequencies at the same stage of the disease). (E) Quantification of transplanted leukemic cells needed to generate leukemia in 50% of the secondary recipients. The percentage of LICs in the population of leukemic cells is calculated as 1 LIC per total number of cells injected to induce lethality in 50% of the recipients (n = 7 mice for each group; full and empty circles represent 2 independent clones tested).
Next, we asked whether the frequency of leukemia stem cells (LSCs) capable of enhanced self-renewal and responsible for the maintenance of leukemia was affected in the Smad4 null background. Therefore, 10 000 leukemic cells generated in primary recipient mice were transplanted into secondary recipients, and we observed that the time to onset of leukemia was dramatically reduced for Smad4−/− leukemias (Figure 5D). Therefore, we tested the repopulative capacity of LSCs using the limiting dilution transplantation assay to analyze the proportion of secondary transplanted mice that developed leukemia. We observed that the HOXA9- and NUP98-HOXA9–transformed Smad4−/− leukemic cell population was 11-fold and 14-fold enriched for LICs respectively, compared with Wt leukemic cells (Figure 5E). Hence, we conclude that the fraction of LSCs was increased in the Smad4−/− background. Compared with normal BM, we also observed increased Smad4 protein levels in murine BM Wt leukemic cells generated under forced expression of HOXA9, with a predominant expression of Smad4 and Hoxa9 in the cytoplasm of Wt leukemic cells, while Hoxa9 was found increased in the nucleus of Smad4−/− leukemic cells (supplemental Figure 11).
The induced AML features were identical in Wt and Smad4−/− hematopoietic cells, and no difference was observed in the pathology of the disease. Myeloblastic cells were predominantly c-Kit+Mac1+ and the mice exhibited similar splenomegaly and equivalent levels of white blood cells in peripheral blood (supplemental Figure 12). Infiltration of leukemic cells in extrahematopoietic tissues was also found to be comparable (supplemental Figure 13).
The coexpression of a truncated Smad4 blocks immortalization of primitive hematopoietic cells
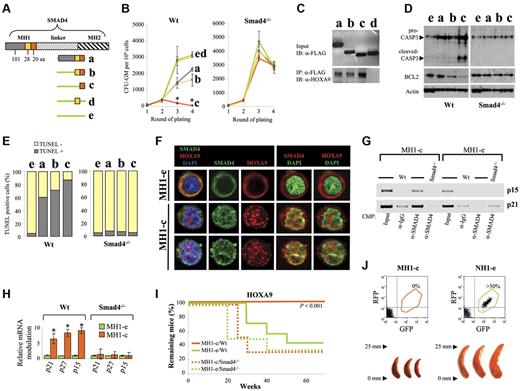
Next, we developed an approach to activate the TGF-β pathway by changing the subcellular distribution of the endogenous Smad4 protein accumulated in the cytoplasm of HSPCs transduced with HOXA9 or NUP98-HOXA9. To this end, we first identified the best competitor by generating different constructs (Figure 6A and supplemental Figure 14) that enable expression of diverse portions of the MH1 domain of Smad4, binding Hoxa9.28 All the retroviral vectors (RFP+) were tested by coexpression together with the NUP98-HOXA9 oncogene (GFP+). Cotransduced GFP+ RFP+ cells were sorted by FACS (supplemental Figure 15) to assess the effect of MH1 overexpression by CFU assay. Interestingly, 1 specific portion of Smad4 (MH1-c encoding a peptide of 20aa) dramatically reduced the immortalization capacity of NUP98-HOXA9 (Figure 6B). The insensitivity of Smad4−/− cells notably shows that the specificity of this effect is Smad4-dependent.
The coexpression of a truncated Smad4 blocks immortalization of primitive hematopoietic cells. (A) Depiction of the different portions of MH1 used to construct the retroviral plasmids. (B) Findings from the colony-forming unit assay obtained after 4 rounds of plating, with Wt or Smad4−/− HSPCs transduced with the NUP98-HOXA9 oncogene and different portions of MH1. (Data show mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). (C) Representative immunoblots showing binding of different portions of MH1 with the Hoxa9 protein in Wt HSPCs transduced with NUP98-HOXA9. Input with the anti-FLAG antibody (top panel). After immunoprecipitation with the anti-FLAG antibody, MH1/Hoxa9 complexes were analyzed by immunoblot with the anti-Hoxa9 antibody (bottom panel, 1 representative experiment of 2 independent experiments). (D) Analysis of cells from the second round of plating by Western blot showing an important activation of apoptosis in primitive hematopoietic cells transduced with MH1-c, assessed by an up-regulation of the pro- and cleaved-Caspase 3 (CASP3) and a down regulation of BCL2. (E) TUNEL assay of Wt and Smad4−/− HSPCs transduced with the NUP98-HOXA9 oncogene and different portions of MH1. (F) Confocal microscopy in sequential scanning mode, using a 63× oil-immersion objective showing that the expression of MH1-c changes the subcellular distribution of the Smad4 protein, while the cytoplasmic stabilization is still observed under the expression of MH1-e. Only Smad4 translocates to the nucleus, while Hoxa9 stays in the cytoplasm (experiments on Lin− cells). (G) Increased presence of Smad4 was confirmed on the chromatin of cells transduced with MH1-c, compared with cells transduced with the MH1-e control. Chromatin was immunoprecipitated (ChIP) using the anti-Smad4 antibody. IgG antibody was used as control. The relative amount of precipitated p21 and p15 promoter DNA was determined by PCR amplification. (H) Increase in transcription of specific targets of the Smad4 pathway (p15, p21, and p27) in cells transduced with MH1-c. Relative fold change in mRNA expression between the empty (green) and MH1-c (orange) is shown (data show mean ± SEM, n = 4, *P < .01 measured by Student unpaired t test). (I) Kaplan-Meier plot of primary recipient mice showing that mice transplanted with primitive hematopoietic cells coexpressing HOXA9 and MH1-e developed AML, while all mice transplanted with MH1-c– and HOXA9-transduced primitive hematopoietic cells stayed healthy over 1 year (n = 7 for Wt; n = 6 for Smad4−/−; P value measured by Mantel Haenszel logrank test). (J) Primary recipient mice transplanted with HSPCs transduced with NUP98-HOXA9 and MH1-c (left panel) stayed healthy and no detectable GFP+ RFP+ cells in BM and no splenomegaly were found of killed mice. In contrast, mice transplanted with primitive hematopoietic cells cotransduced with NUP98-HOXA9 and the empty control vector MH1-e (right panel) developed myeloproliferative disease with splenomegaly (analysis representative of 6 mice).
The coexpression of a truncated Smad4 blocks immortalization of primitive hematopoietic cells. (A) Depiction of the different portions of MH1 used to construct the retroviral plasmids. (B) Findings from the colony-forming unit assay obtained after 4 rounds of plating, with Wt or Smad4−/− HSPCs transduced with the NUP98-HOXA9 oncogene and different portions of MH1. (Data show mean ± SEM, n = 3, *P < .01 measured by Student unpaired t test). (C) Representative immunoblots showing binding of different portions of MH1 with the Hoxa9 protein in Wt HSPCs transduced with NUP98-HOXA9. Input with the anti-FLAG antibody (top panel). After immunoprecipitation with the anti-FLAG antibody, MH1/Hoxa9 complexes were analyzed by immunoblot with the anti-Hoxa9 antibody (bottom panel, 1 representative experiment of 2 independent experiments). (D) Analysis of cells from the second round of plating by Western blot showing an important activation of apoptosis in primitive hematopoietic cells transduced with MH1-c, assessed by an up-regulation of the pro- and cleaved-Caspase 3 (CASP3) and a down regulation of BCL2. (E) TUNEL assay of Wt and Smad4−/− HSPCs transduced with the NUP98-HOXA9 oncogene and different portions of MH1. (F) Confocal microscopy in sequential scanning mode, using a 63× oil-immersion objective showing that the expression of MH1-c changes the subcellular distribution of the Smad4 protein, while the cytoplasmic stabilization is still observed under the expression of MH1-e. Only Smad4 translocates to the nucleus, while Hoxa9 stays in the cytoplasm (experiments on Lin− cells). (G) Increased presence of Smad4 was confirmed on the chromatin of cells transduced with MH1-c, compared with cells transduced with the MH1-e control. Chromatin was immunoprecipitated (ChIP) using the anti-Smad4 antibody. IgG antibody was used as control. The relative amount of precipitated p21 and p15 promoter DNA was determined by PCR amplification. (H) Increase in transcription of specific targets of the Smad4 pathway (p15, p21, and p27) in cells transduced with MH1-c. Relative fold change in mRNA expression between the empty (green) and MH1-c (orange) is shown (data show mean ± SEM, n = 4, *P < .01 measured by Student unpaired t test). (I) Kaplan-Meier plot of primary recipient mice showing that mice transplanted with primitive hematopoietic cells coexpressing HOXA9 and MH1-e developed AML, while all mice transplanted with MH1-c– and HOXA9-transduced primitive hematopoietic cells stayed healthy over 1 year (n = 7 for Wt; n = 6 for Smad4−/−; P value measured by Mantel Haenszel logrank test). (J) Primary recipient mice transplanted with HSPCs transduced with NUP98-HOXA9 and MH1-c (left panel) stayed healthy and no detectable GFP+ RFP+ cells in BM and no splenomegaly were found of killed mice. In contrast, mice transplanted with primitive hematopoietic cells cotransduced with NUP98-HOXA9 and the empty control vector MH1-e (right panel) developed myeloproliferative disease with splenomegaly (analysis representative of 6 mice).
The coexpression of a truncated Smad4 modifies the subcellular distribution of Smad4 and activates apoptosis
Compared with the other truncated MH1 portions tested, the increased effect of MH1-c was correlated to its capacity to bind Hoxa9, as assessed by FLAG immunoprecipitation of the different portions of MH1 (Figure 6C). The increased effect of MH1-c was furthermore correlated to its capacity to induce apoptosis, as assessed by up-regulation of the pro-Caspase 3, activation of the cleaved-Caspase 3 and a reduction in Bcl2 expression (Figure 6D). Using the TUNEL assay, we confirmed that > 80% of hematopoietic cells expressing MH1-c were apoptotic (Figure 6E).
To understand the mechanism behind the effect of MH1-c, we looked at the localization of Smad4 by immunostaining. Expression of MH1-c was observed to induce a robust nuclear translocation of the Smad4 protein, while Smad4 remained accumulated in the cytoplasm of cells transduced with the empty control vector (MH1-e; Figure 6F). The nuclear translocation of Smad4 is generally thought to be ligand-dependent. Therefore, we tested production of TGF-β by the transduced hematopoietic cells and could confirm the autocrine production of TGF-β by HOXA9- and NUP98-HOXA9–transduced cells (supplemental Figure 16). MH1-c disrupts the physical interaction between Hoxa9 and Smad4; the Smad4 subcellular distribution pattern was modified and the nuclear translocation clearly activated the TGF-β pathway, which was confirmed by an increased presence of Smad4 on the chromatin of cells transduced with MH1-c as assessed by chromatin immunoprecipitation using the anti-Smad4 antibody (Figure 6G) and by an increase in transcription of specific targets of the Smad4 pathway (p15, p21, and p27), involved in growth control (Figure 6H).
The coexpression of a truncated Smad4 blocks the leukemic transformation of primitive hematopoietic cells in vivo
Next, we asked whether MH1-c might affect the development of leukemia in vivo. When HSPCs were cotransduced with HOXA9 and the MH1-e, and transplanted into the tail vein of lethally irradiated recipient mice, the transduced HSPCs developed AML. In contrast, HSPCs cotransduced with HOXA9 and MH1-c remained healthy more than 1 year (Figure 6I), without any detectable GFP+ RFP+ cells in BM when they were killed. All mice transplanted with HSPCs expressing NUP98-HOXA9 and MH1-c stayed healthy and there was no detectable GFP+ RFP+ cells in BM, while mice transplanted with HSPCs cotransduced with NUP98-HOXA9 and the empty control vector (MH1-e) developed myeloproliferative disease and splenomegaly (Figure 6J). Taken together, these findings show that active Smad4 that can freely translocate to the nucleus of HSPCs in vivo can prevent or profoundly delay the initiation of malignant transformation.
Discussion
Several conserved signaling pathways have emerged as important regulatory circuits to govern fate options of HSCs, including Wingless-type (Wnt),32 Notch,33 Sonic hedgehog (Shh),34 and Smad pathways.31 Importantly, while these signaling pathways control HSC self-renewal, they are often dysregulated in leukemia and during leukemogenesis.35 For example, the Wnt/β-Catenin pathway has recently been shown to play an important role for HOXA9-mediated transformation of HSCs in AML.36 Overall, our findings support the concept that Smad4 is also a critical regulatory molecule involved in transformation of primitive hematopoietic cells and growth maintenance of leukemia stem cells.
In this study, we used an inducible Smad4 knockout (Smad4−/−) mouse model,29 to evaluate the role of Smad4 in immortalization/transformation of hematopoietic progenitor and stem cells. Deletion of the Smad4 gene induces a robust immortalization of Smad4−/− primitive hematopoietic cells expressing HOXA9 or NUP98-HOXA9 in vitro. Leukemia transformation induced by HOXA9 or NUP98-HOXA9 in vivo was increased when mice where transplanted with Smad4−/− HSPCs. Together, these data clearly demonstrate that in HOXA9/NUP98-HOXA9–induced leukemia Smad4 plays an important negative regulatory role to prevent the initiation of leukemic transformation of primitive hematopoietic cells and to robustly impede the growth of LICs once the leukemia is established.
Smad4 is a key molecule in the TGF-β signaling pathway where its role on complex formation with activated receptor Smads is to translocate to the nucleus37 and regulate gene expression involved in cell cycle and apoptosis.11 Therefore we first wanted to address if the complex between Smad4-Hoxa9 could block the Smad4 signaling and consequently Smad4-induced differentiation/apoptosis of primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9. This hypothesis was plausible because Smad4−/−-transduced cells show increased immortalization compared with Wt-transduced cells. However, in our investigation, we discovered that the canonical function of Smad4 was not directly involved in this phenotype.
First of all, our previous study on normal BM shows that Smad4 signaling does not induce differentiation/apoptosis of primitive hematopoietic cells. This is evident because Smad4−/− BM (untransduced cells) generated normal numbers of CFU-GM colonies when cultured in semisolid medium showing that the clonogenicity and proliferation of hematopoietic stem and progenitor cells was unaffected by the Smad4 deficiency.29
Secondly, when we used TGF-β receptor kinase inhibitors to block TGF-β/Activin receptors and therefore block pathways activating Smad4 signaling, we could not observe as much increase in immortalization of Wt primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9 as was observed for Smad4−/− cells transduced with the same oncogenes.
Finally, other fusion proteins, like TEL-AML138 and AML1-EVI1,39-41 have been described to bind to Smad3 and impair TGF-β signaling and apoptosis of transduced hematopoietic stem cells in vitro. Therefore primitive hematopoietic cells transduced with these oncogenes are insensitive to recombinant TGF-β. In contrast, the TGF-β signaling was not impaired in hematopoietic cells expressing HOXA9 or NUP98-HOXA9 oncogenes, because we clearly observed that transduced HSPCs were sensitive to TGF-β growth inhibition when recombinant TGF-β was supplemented in the medium.
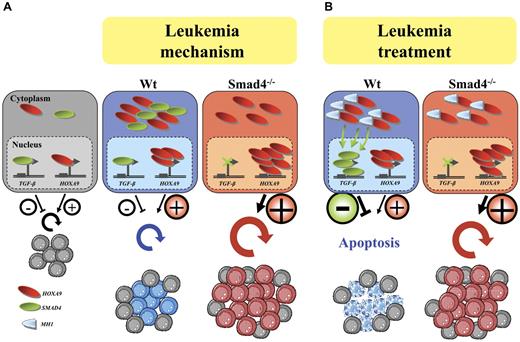
All of these findings suggest that Smad4-Hoxa9 cannot block the Smad4 signaling, and consequently, Smad4-induced differentiation/apoptosis of primitive hematopoietic cells transduced with HOXA9 or NUP98-HOXA9. To define the mechanism, we used microscopy after immunostaining and observed that in Smad4−/− cells, the Smad4 molecule is nonexistent and simultaneously the stabilization of Hoxa9 does not take place leading to increased Hoxa9 in the nucleus, greater activity on target genes and amplified immortalization/transformation of hematopoietic progenitors. Therefore the Smad4-Hoxa9 complexes accumulate in the cytoplasm, suggesting that the role of Smad4 is to stabilize Hoxa9 to the cytoplasm and consequently restrain Hoxa9 activity in the nucleus of primitive hematopoietic cells at moderate levels (Figure 7A; leukemia mechanism).
Smad4 stabilizes Hoxa9 proteins to the cytoplasm suggesting a protective role of Smad4 against nuclear activation by Hoxa9 and leukemia transformation. (A) Illustration of the model of leukemogenesis involving regulation of two pathways; the TGF-β and the Hoxa9 pathways as respectively negative and positive regulators of primitive hematopoietic cell expansion in normal conditions (normal). Under forced expression of HOXA9 in Wt cells, Smad4 stabilizes Hoxa9 to the cytoplasm and therefore protects primitive hematopoietic cells against increased nuclear activation by Hoxa9. In Smad4−/− primitive hematopoietic cells, this protective mechanism cannot be operated which leads to increased concentration of Hoxa9 in the nucleus; increased transcriptional activation by Hoxa9 in primitive hematopoietic cells is increased making them vulnerable to leukemia transformation (leukemia mechanism). (B) Reactivation of the Smad pathway ensues on expression of a 20aa peptide mimicking a small portion of the MH1 domain by releasing the Smad4 to activate its targets and to induce apoptosis (leukemia treatment).
Smad4 stabilizes Hoxa9 proteins to the cytoplasm suggesting a protective role of Smad4 against nuclear activation by Hoxa9 and leukemia transformation. (A) Illustration of the model of leukemogenesis involving regulation of two pathways; the TGF-β and the Hoxa9 pathways as respectively negative and positive regulators of primitive hematopoietic cell expansion in normal conditions (normal). Under forced expression of HOXA9 in Wt cells, Smad4 stabilizes Hoxa9 to the cytoplasm and therefore protects primitive hematopoietic cells against increased nuclear activation by Hoxa9. In Smad4−/− primitive hematopoietic cells, this protective mechanism cannot be operated which leads to increased concentration of Hoxa9 in the nucleus; increased transcriptional activation by Hoxa9 in primitive hematopoietic cells is increased making them vulnerable to leukemia transformation (leukemia mechanism). (B) Reactivation of the Smad pathway ensues on expression of a 20aa peptide mimicking a small portion of the MH1 domain by releasing the Smad4 to activate its targets and to induce apoptosis (leukemia treatment).
In our study, the cytosolic presence of Hoxa9 was observed in early passage progenitors (immortalized 2 or 3 weeks in vitro). This is corroborated by previous experiments showing also predominant presence of Hoxa9 in the cytoplasm of hematopoietic stem and primitive progenitor cells.42 AML blasts are generated from the transformation of primitive hematopoietic cells. Interestingly we observed that both murine and human leukemic cells predominantly expressed Smad4 and Hoxa9 in the cytoplasm and importantly HOXA9 was reported to reside principally in the cytosol of human AML cells.43 However, nuclear localization of HOXA9 has been observed in cell lines, like in HOXA9HF1 cells, a HOXA9-immortalized cell line,44 or in the human myelomonocytic leukemia U-937 cell line, known to express HOXA9.42 Therefore, we maintained NUP98-HOXA9–transduced cells more than 3 months in liquid culture in vitro to establish a cell line that has > 95% blast morphology and observed that Hoxa9 was more expressed in the nucleus (supplemental Figure 17). It is therefore possible that the cytosolic expression of Hoxa9 is specific to early transduced primitive hematopoietic cells and that the Hoxa9 shift toward the nucleus as the cells become more established in vitro.
The lack of leukemogenesis in TGF-β signaling-deficient mice models29,45-47 suggests that loss of TGF-β responsiveness is more important for progression rather than initiation of leukemogenesis. However, TGF-β is believed to be an important player in leukemogenesis because different mechanisms rendering loss of TGF-β responsiveness have been demonstrated in leukemic cells.21 Loss-of-function mutations that disrupt the TGF-β pathway involving the SMAD4 gene23,24 or the TGFBR2 gene25,26 have been reported in patients with AML. Using ultra-dense array comparative genomic hybridization on 86 AML genomes, 18 copy number alteration (CNA) regions were found recurrently altered, and interestingly, 1 of these CNAs represents the deletion of the SMAD4 gene,27 demonstrating that SMAD4 is an AML-associated gene. This relates Smad4-Hoxa9 interaction to potentially relevant biology, where alteration in expression of the Smad4 gene would disrupt interaction of Smad4 with Hoxa9 and then augment the activity of Hoxa9.
In myeloid leukemic cells, autocrine TGF-β and/or its Smad signals control the ability of leukemic cells to respond to various differentiation inducers, suggesting that this pathway plays a role in determining the cell fate of leukemic cells.22 In that case, changing the subcellular distribution of endogenous Smad4 protein through counteracting the stabilization introduced by high levels of Hoxa9 in primitive hematopoietic cells would provide a relevant mechanism to induce differentiation/apoptosis and block expansion of transformed primitive hematopoietic cells (Figure 7B; leukemia treatment). The coexpression of a truncated Smad4 (MH1-c), which corresponds to the MH1 part of the Smad4 protein that binds to the Hoxa9 homeodomain,28 blocks the interaction between endogenous Smad4 and Hoxa9. Disruption of Smad4 stabilization is confirmed by robust nuclear translocation of the Smad4 protein, which automatically activates target genes of the TGF-β pathway and promotes apoptosis. The activation of TGF-β signaling considerably affects immortalization of primitive hematopoietic cells expressing the oncogene in vitro. Furthermore, we demonstrate that expression of a competitor molecule (MH1-c) leads to a loss of leukemia stem cells and blocks the development of leukemia in vivo.
Different studies described interactions between several other Hox and Smad proteins,48-50 suggesting that the mechanisms behind this are probably complex. Importantly, the specific portion of Smad4 (MH1-c) encodes a peptide of 20 amino acids (127-147 of Smad4) which correspond to the region within the MH1 amino-terminus of Smad4 (101-148 of Smad4) that has been described in vitro to interact with Hoxa9.28 However, while we clearly describe that the truncated Smad4 affects the subcellular distribution of the endogenous Smad4 protein leading to activation of TGF-β signaling, Wang et al reported that this truncated Smad4 blocks the immortalization capability of Hoxa9 by repressing the DNA binding activity of Hoxa9 on its target genes.28 We believe that both mechanisms are cooperative as we also observed down-regulation of Hoxa9 targets involved in BM transformation in Wt cells transduced with MH1-c (supplemental Figure 18). Importantly, our study shows for the first time that expression of the truncated Smad4 blocks not only the in vitro immortalization capacity the oncogenes have on hematopoietic primitive cells, but also blocks the in vivo transformation capacity. These findings open new avenues to treat HOXA9-induced leukemias and demonstrate in general how activation of a negative regulatory pathway that prevents leukemogenesis may be a feasible approach to improve leukemia treatment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Guy Sauvageau (Institute for Research in Immunology and Cancer, Quebec, QC) and Dr Gary Gilliland (Harvard University, Boston, MA) for providing us with retroviral vectors. We thank Zhi Ma for assistance in cell sorting, Ariane Tormin for providing with human BM samples, and Ingbritt Åstrand-Grundström for expert confirmation of differential counts of hematopoietic cells. We thank Matilda Nifelt Hägerström, Virginie Carmignac, Kenichi Miharada, and Kristian Reckzeh for technical help. We thank Johan Richter, Bertil Johansson, Carin Lassen, and Florence Nguyen Khac for their help to obtain human AML samples.
This work was supported by the Hemato-Linné grant (Swedish Research Council Linnaeus), the Swedish Cancer Foundation (Cancerfonden), The Swedish Cancer Society (S.K.), the Swedish Children's Cancer Society (S.K.), the Swedish Medical Research Council (S.K.), and the EU project grants CONSERT, STEMEXPAND, and PERSIST. R.Q. was supported by fellowships from the Association pour la Recherche contre le Cancer (ARC, France) and the Swedish Children's Cancer Society (Barncancerfonden). This work was supported by traveling grants from John and Augusta Perssons foundation (R.Q.). P.V. is a senior clinical investigator of FWO-Vlaanderen. The Lund Stem Cell Center was supported by a Center of Excellence grant in life sciences from the Swedish Foundation for Strategic Research.
Authorship
Contribution: R.Q. designed the research, performed experiments, analyzed data, and wrote the paper; G.K. designed the research, analyzed data, and contributed to writing the paper; F.H. performed experiments on the FACS and microscopy analysis for immunostaining; M.R. and B.L. made retroviral supernatant for transduction; T.F., P.V., and M.L.S. provided AML samples and advice; J.C. provided advice and discussion; and S.K. directed the research, analyzed data, and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.L.S. is Quest Diagnostics Nichols Institute, Chantilly, VA.
Correspondence: Prof Stefan Karlsson, Molecular Medicine and Gene Therapy, Lund Stem Cell Center, BMC A12, Sölvegatan 17, Lund 22184, Sweden; e-mail: Stefan.Karlsson@med.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal