To the editor:
Human leukocyte antigen (HLA)–DPB1 functions as a classic transplantation antigen.1 In the context of hematopoietic stem cell transplantation (HSCT), when donor T cells recognize host HLA-DP, they can induce a graft-versus-host disease (GVHD) and/or a graft-versus-leukemia (GVL) effect, whereas in the opposite direction, host T cells recognizing the donor can induce rejection (HVG). Accordingly, any possibility to anticipate the nature and strength of an anti-DP T-cell response is crucial in this context.
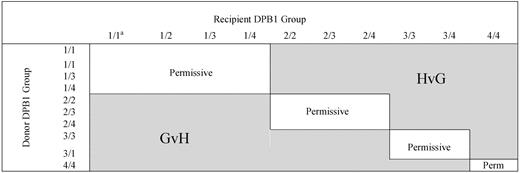
Based on the HLA-DP recognition pattern of several HLA-DPB1*0901-specific T-cell clones, Crocchiolo et al classified HLA-DPB1 alleles according to their predicted “immunogenicity,”2 and using an algorithm deduced from this classification (Figure 1), they showed that the presence of “nonpermissive” HLA-DPB1 mismatches correlated with a significantly increased hazard of acute grade 2-IV GVHD. Unexpectedly, the authors reported that the increased risk of aGVHD was detectable independently of the predicted direction (GVH/GVL or HVG) of the T-cell response. To reconcile the statistical observation with the immunologic hypothesis (the increased risk of GVH when the algorithm predicted the recognition of donor HLA-DP by host T cells), these authors considered the possibility of an indirect pathway for GVH because of cytokine release by host T cells recognizing “immunogenic” HLA-DPB1 on donor antigen-presenting cells (APC).
Algorithm for permissiveness of HLA-DPB1 mismatches in donor-recipients pairs.2 Permissive and nonpermissive HLA-DPB1 disparity according to the algorithm described by Crocchiolo et al. aNumbers indicate the group of the 2 HLA-DPB1 alleles of the donor or the recipient. Group 1: DPB1*09:01,10:01,17:01; group 2: DPB1*03:01, 14:01, 45:01; group 3: DPB1*02:01, 0202, 0203; group 4: others.3 Immunogenicity decreases from group 1 to group 4.
Algorithm for permissiveness of HLA-DPB1 mismatches in donor-recipients pairs.2 Permissive and nonpermissive HLA-DPB1 disparity according to the algorithm described by Crocchiolo et al. aNumbers indicate the group of the 2 HLA-DPB1 alleles of the donor or the recipient. Group 1: DPB1*09:01,10:01,17:01; group 2: DPB1*03:01, 14:01, 45:01; group 3: DPB1*02:01, 0202, 0203; group 4: others.3 Immunogenicity decreases from group 1 to group 4.
In 4 successive studies,3-6 we have in the past assessed the specificities of T-cell clones infiltrating skin biopsies during aGVHD (Table 1). In each situation, T-cell clones specific for host HLA-DP were isolated from the skin at the onset of aGVHD. These studies demonstrated that no mismatch could theoretically be considered as “permissive,” as confirmed in vitro by Rutten et al.7 The finding of up to a 10-fold difference observed between frequencies of T cells directed at a “permissive” versus “nonpermissive” DP mismatch, as argued by Sizzano et al,8 can hardly make a difference in the context of transplantation. T-cell frequency depends on the kinetics of the immune response and is a key physiologic factor in the race against an infection. In the present context of transplantation, the immune target (HLA-DP on host APC) remains present for weeks after the graft, and a 10-fold difference in frequency represents only 3-4 divisions for a T cell, which would take 1-2 days at most.
Host-specific cytotoxic T cells against both permissive and nonpermissive HLA-DPB1 mismatches infiltrate the skin at the onset of aGVHD
| UPN . | Donor DPB1 (group) . | Host DPB1 (group) . | Mismatches in the GVH direction . | Algorithm prediction of alloreaction . | Skin-derived T-cell clones . | Specificity . |
|---|---|---|---|---|---|---|
| UPN25,6 | 601/1001 (4/1) | 401/1001 (4/1) | B2705, DR4, DQ8 DP0401 | Permissive | HER-1, HER-28 | DP0401 |
| HER-3, HER-30 HER-27, HER-29 | B2705 DQ8 | |||||
| UPN36 | 0401/1901 (4/4) | 1301/1901 (4/4) | A1, B17, DR0402 DQ8, DP1301 | Permissive | P11 | DP1301 |
| P1, P3, P6, P7, P10 P14 | DQ8 DR0402 | |||||
| UPN15,6 | 0301/19 (2/4) | DP0101/19 (4/4) | A201, DP0101 | HVG 0301 ← | A4, D2 | DP0101 |
| TM15 | A201 | |||||
| UPN53 | 0401/0401 (4/4) | 1001/0401 (1/4) | DP1001 | GVH → 1001 | BV2S1, BV6S7, BV14S1, BV17S1, BV8S1 | DP1001 |
| UPN44 | 0301/0401 (2/4) | 0401/0501 (4/4) | DP0501 | HVG 0301← | BV6S7, BV8S1, BV13S1, BV17S1, BV22S1, BV5S2 | DP0501 |
| UPN . | Donor DPB1 (group) . | Host DPB1 (group) . | Mismatches in the GVH direction . | Algorithm prediction of alloreaction . | Skin-derived T-cell clones . | Specificity . |
|---|---|---|---|---|---|---|
| UPN25,6 | 601/1001 (4/1) | 401/1001 (4/1) | B2705, DR4, DQ8 DP0401 | Permissive | HER-1, HER-28 | DP0401 |
| HER-3, HER-30 HER-27, HER-29 | B2705 DQ8 | |||||
| UPN36 | 0401/1901 (4/4) | 1301/1901 (4/4) | A1, B17, DR0402 DQ8, DP1301 | Permissive | P11 | DP1301 |
| P1, P3, P6, P7, P10 P14 | DQ8 DR0402 | |||||
| UPN15,6 | 0301/19 (2/4) | DP0101/19 (4/4) | A201, DP0101 | HVG 0301 ← | A4, D2 | DP0101 |
| TM15 | A201 | |||||
| UPN53 | 0401/0401 (4/4) | 1001/0401 (1/4) | DP1001 | GVH → 1001 | BV2S1, BV6S7, BV14S1, BV17S1, BV8S1 | DP1001 |
| UPN44 | 0301/0401 (2/4) | 0401/0501 (4/4) | DP0501 | HVG 0301← | BV6S7, BV8S1, BV13S1, BV17S1, BV22S1, BV5S2 | DP0501 |
UPN1-5 are from References 3-6. Only case UPN5 fits the algorithm shown in Figure 1; most significantly, for UPN1 and UPN4 the T-cell reaction took place in the opposite direction of that expected by the algorithm. Data in bold indicate HLA_DPB1 mismatches in the GVHD direction.
UPN1: A 9-year-old boy with chronic myelogenous leukemia received a graft of his mother's bone marrow. The conditioning regimen consisted of cyclophosphamide (120 mg/kg) and total body irradiation (TBI): 12 Gy through 6 irradiation courses. GVHD prophylaxis consisted of cyclosporine A (CsA) and methotrexate (at days 1, 3, 6, and 11) and 5 mg/d of BB10, an anti–IL-2R antibody, for 10 days. GVHD was first suspected on day 13 and biopsy for culture performed at day 34. The patient died at day 99.
UPN2: A 7-year-old boy received a graft of his mother's bone marrow for acute myelogenous leukemia in the second complete response. The conditioning regimen consisted of TBI and high doses of cytarabine and melphalan. T-cell depletion was performed as GVHD prophylaxis using monoclonal antibody anti-CD2, anti-CD7, and rabbit complement. In addition, the patient received anti-LFA1 and anti-CD2 mAbs from day −3 to day 12. GVHD was suspected on day 19 and biopsy for culture at day 22. The patient died at day 120.
UPN3: A 10-year-old boy with idiopathic myelodysplasia and severe pancytopenia. After the patient failed to engraft with his mother's bone marrow, he received marrow from his father after the following conditioning regimen: busulfan 8 mg/kg over 2 days and cyclophosphamide 200 mg/kg over 4 days. The marrow was T cell–depleted with anti-CD2, anti-CD7, and rabbit complement. Additional in vivo immunotherapy with anti-LFA1 and anti-CD2 was performed as for UPN2. GVHD was diagnosed and skin biopsies performed at day 31 following the second transplantation. The patient died at day 89.
UPN4: A 48-year-old female with chronic myeloid leukemia received a graft from a donor from the French bone marrow transplant registry. Because of GVHD risk factors (patient age, advanced disease, and unrelated donor) and after informed consent, the patient received selected bone marrow (BM) CD34+ cells with the aim of reducing GVHD risk through T-cell reduction. No other GVHD prophylaxis was used except CD34+ selection. Only 3% CD3+ T cells contaminated the CD34 preparation corresponding to a total number of T cells reinjected of 9.4 × 104/kg. GVHD was suspected and skin biopsy performed on day 10. The patient died at day 39.
UPN5: A 42-year-old male with Richter syndrome in first partial response was grafted with unmanipulated noncryopreserved marrow from a female donor. The conditioning regimen consisted of fractionated 12 Gy TBI with lung shielding at 8 Gy followed by cyclophosphamide 60 mg/kg for 2 consecutives days. GVHD prophylaxis consisted of cyclosporine A at a dose of 3 mg/kg/d, together with methotrexate 15 mg/m2 on day 1 and 10 mg/m2 on days 3 and 6. GVHD was diagnosed and biopsies performed at day 16. The patient died of aspergillosis at day 75. (See References 3-6 for details.)
Nevertheless, a detailed analysis of anti-DP specificities remains particularly interesting and potentially useful (for example, to drive a GVL effect with DP-specific T-cell clones directed against an HLA-DP mismatch in the GVH direction, as we have previously proposed3,4,9 ). In line with HSCT, considering that “alloreactive TCR neither avoid contacting the bound peptide nor focus on the polymorphic residues that are exposed on the outer surface of the allo-MHC a-helices,”10 it would be of great interest to learn more about the set of endogenous peptides presented by HLA-DP alleles in hematopoietic and nonhematopoietic tissues. This may help to improve the targeting of anti-DP allogeneic reaction against the residual disease while avoiding the healthy tissues as much as possible.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Henri Vié, Inserm, 8 Quai Moncousu, 44007 Nantes Cedex 1, France; e-mail: hvie@nantes.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal