To the editor:
Clinical studies have indicated that human leukocyte antigen (HLA)–DPB1 functions as a classical transplantation antigen in allogeneic stem cell transplantation (SCT). Mismatching for HLA-DPB1 was associated with an increased risk of graft-versus-host disease (GVHD) but also a decreased risk of disease relapse.1,2 However, some studies showed that specific HLA-DPB1 mismatches were associated with poor clinical outcome.3 It was suggested that this unfavorable effect was caused by differences in immunogenicity between HLA-DPB1 alleles. An algorithm defining permissive and nonpermissive HLA-DPB1 mismatches was developed based on cross-reactive T-cell reactivity patterns. It was suggested that permissive mismatches would not result in T-cell responses, whereas strong T-cell responses were expected to be generated against nonpermissive mismatches.3,4 Clinical analysis of different patient cohorts indeed showed a significant higher risk of mortality in the nonpermissive mismatched group compared with the permissive mismatched group. However, the risk of GVHD and relapse did not significantly differ between these groups, implying that the effect on overall survival may not have directly resulted from differences in alloreactivity.3,5,6
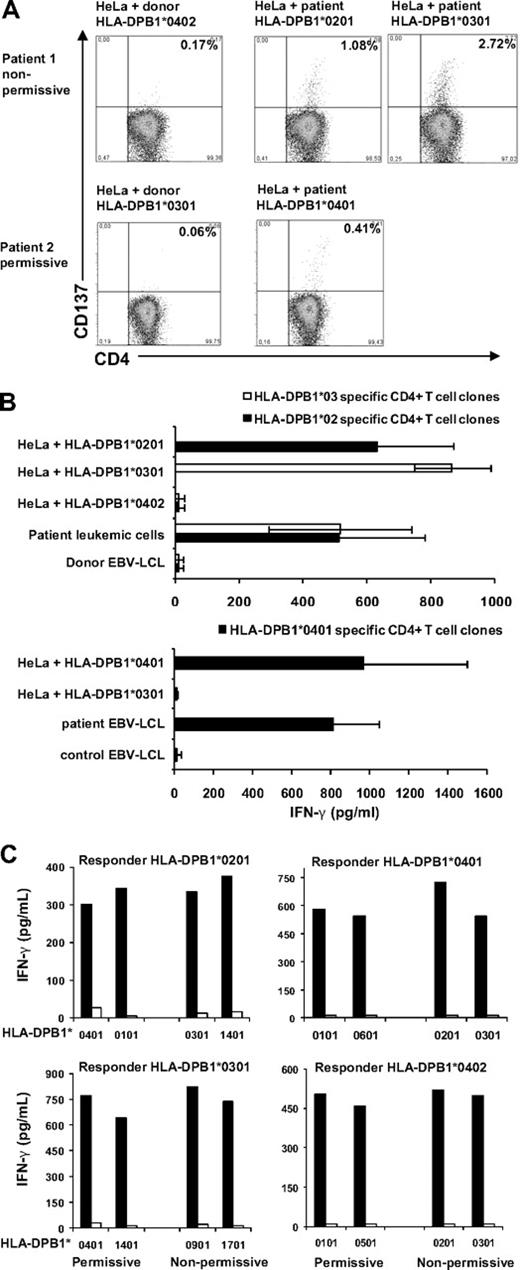
To analyze whether permissive HLA-DPB1 specific immune responses occur in vivo, we analyzed immune responses in 2 patients responding to donor lymphocyte infusion (DLI) after HLA-A, -B, -C, -DRB1, -DQB1 matched, HLA-DPB1 mismatched SCT. The patients received a permissive or nonpermissive HLA-DPB1 mismatched SCT, respectively. CD4+ T cells were isolated from peripheral blood obtained during the clinical immune response to DLI, and stimulated with HLA-class II negative HeLa cells transduced with the specific HLA-DP molecules derived from patient or donor. In both patients, HLA-DP specific CD4+ T cells were demonstrated by CD137 up-regulation in response to stimulation with HeLa cells transduced with patient and not donor HLA-DP molecules (Figure 1A). CD137 expressing CD4+ T cells were clonally isolated and specific recognition of patient HLA-DP molecules was confirmed for 58% to 78% of the T-cell clones (Figure 1B). T-cell receptor-Vβ analysis showed a polyclonal origin of these HLA-DPB1 specific CD4+ T-cell responses.
Permissive and nonpermissive HLA-DPB1 mismatches induced HLA-DP specific immune responses in vivo and in vitro. Two patients transplanted with an HLA-A, -B, -C, -DRB1, -DQB1 matched, HLA-DPB1 mismatched SCT were analyzed for the presence of HLA-DP specific CD4+ T cells after DLI. Patient 1 (HLA-DPB1*0201,0301) was transplanted for a chronic B-cell leukemia with a nonpermissive HLA-DPB1 mismatched donor (HLA-DPB1*0402,0501). Patient 2 (HLA-DPB1*0401) was transplanted for multiple myeloma with a permissive HLA-DPB1 mismatched donor (HLA-DPB1*0301,0402) in the GVH direction. (A) Purified CD4+ T cells obtained during the clinical immune response to DLI were stimulated with HLA class II negative HeLa cells transduced with either donor or patient HLA-DP molecules. After 48 hours, CD137 expression on CD4+ T cells was determined using flow cytometry. Percentages of CD137 positive CD4+ T cells in response to stimulation with different HLA-DP molecules are shown. (B) CD137 expressing CD4+ T cells shown in panel A were clonally isolated and tested for recognition of different target cells. IFN-γ production (pg/mL) was determined in 50 μL of supernatant. Mean results ± SD of a selection of 11 HLA-DPB1*0201 specific CD4+ T-cell clones, 15 HLA-DPB1*0301 specific CD4+ T-cell clones, and 15 HLA-DPB1*0401 specific CD4+ T-cell clones are shown. (C) Purified CD4+ T cells derived from 4 different healthy persons were stimulated with HeLa cells transduced with permissive mismatched HLA-DPB1 molecules (n = 2) or nonpermissive mismatched HLA-DPB1 molecules (n = 2). At day 14, 25 000 CD4+ T cells from each cell line were restimulated with 50 000 HLA-DP transduced HeLa cells used for stimulation (■) or HeLa cells transduced with control donor HLA-DP molecules (□). IFN-γ release (pg/mL) measured in 50 μL of supernatant upon restimulation is shown.
Permissive and nonpermissive HLA-DPB1 mismatches induced HLA-DP specific immune responses in vivo and in vitro. Two patients transplanted with an HLA-A, -B, -C, -DRB1, -DQB1 matched, HLA-DPB1 mismatched SCT were analyzed for the presence of HLA-DP specific CD4+ T cells after DLI. Patient 1 (HLA-DPB1*0201,0301) was transplanted for a chronic B-cell leukemia with a nonpermissive HLA-DPB1 mismatched donor (HLA-DPB1*0402,0501). Patient 2 (HLA-DPB1*0401) was transplanted for multiple myeloma with a permissive HLA-DPB1 mismatched donor (HLA-DPB1*0301,0402) in the GVH direction. (A) Purified CD4+ T cells obtained during the clinical immune response to DLI were stimulated with HLA class II negative HeLa cells transduced with either donor or patient HLA-DP molecules. After 48 hours, CD137 expression on CD4+ T cells was determined using flow cytometry. Percentages of CD137 positive CD4+ T cells in response to stimulation with different HLA-DP molecules are shown. (B) CD137 expressing CD4+ T cells shown in panel A were clonally isolated and tested for recognition of different target cells. IFN-γ production (pg/mL) was determined in 50 μL of supernatant. Mean results ± SD of a selection of 11 HLA-DPB1*0201 specific CD4+ T-cell clones, 15 HLA-DPB1*0301 specific CD4+ T-cell clones, and 15 HLA-DPB1*0401 specific CD4+ T-cell clones are shown. (C) Purified CD4+ T cells derived from 4 different healthy persons were stimulated with HeLa cells transduced with permissive mismatched HLA-DPB1 molecules (n = 2) or nonpermissive mismatched HLA-DPB1 molecules (n = 2). At day 14, 25 000 CD4+ T cells from each cell line were restimulated with 50 000 HLA-DP transduced HeLa cells used for stimulation (■) or HeLa cells transduced with control donor HLA-DP molecules (□). IFN-γ release (pg/mL) measured in 50 μL of supernatant upon restimulation is shown.
To demonstrate the generation of HLA-DP specific immune responses directed against permissive and nonpermissive HLA-DPB1 mismatches in different persons, we developed a model to generate allo–HLA-DP responses in vitro. Purified CD4+ T cells of 4 different responders were stimulated with HeLa cells transduced with permissive (n = 2) or nonpermissive (n = 2) HLA-DPB1 molecules. Fourteen days after stimulation, CD4+ T cells were restimulated with HeLa cells transduced with HLA-DP molecules used for stimulation and HeLa cells transduced with responder HLA-DP molecules. Stimulation with permissive and nonpermissive mismatched HLA-DPB1 molecules both resulted in the generation of HLA-DP specific CD4+ T cells as measured by specific interferon γ (IFN–γ) production in response to the HLA-DP molecules used for stimulation (Figure 1C).
In conclusion, we demonstrated that in vivo both permissive and nonpermissive HLA-DPB1 mismatches resulted in strong polyclonal immune responses. Furthermore, we demonstrated in vitro for 4 additional persons that permissive and nonpermissive HLA-DPB1 responses were equally effectively generated. These data show immunogenicity of permissive mismatched HLA-DPB1 alleles. We suggest that the difference in overall survival between patients transplanted with permissive and nonpermissive HLA-DPB1 mismatched donors observed in clinical studies3,4,6 may not result directly from differences in alloreactivity.
Authorship
Acknowledgments: This work has been supported by grants from the Dutch Cancer Society (05-3267) and the European Union (6th Framework Program Allostem).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline E. Rutten, Leiden University Medical Center, Albinusdreef 2, postbus 9600, Leiden 2300 RC, The Netherlands; e-mail: c.e.rutten@lumc.nl.