Abstract
Intracellular factors are involved in and essential for hematopoiesis. HIV-1 Tat-interacting protein of 110 kDa (TIP110; p110nrb/SART3/p110) is an RNA-binding nuclear protein implicated in the regulation of HIV-1 gene and host gene transcription, pre-mRNA splicing, and cancer immunology. In the present study, we demonstrate a role for TIP110 in the regulation of hematopoiesis. TIP110 was expressed in human CD34+ cells and decreased with differentiation of CD34+ cells. TIP110 mRNA was also expressed in phenotyped mouse marrow hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). Using TIP110 transgenic (TIP110TG) and haploinsufficient (TIP110+/−) mice, we found that increased TIP110 expression enhanced HPC numbers, survival, and cell cycling, whereas decreased TIP110 expression had the opposite effects. Moreover, TIP110+/− bone marrow HPCs responded more effectively, and TIP110TG HPCs less effectively, than those of wild-type control mice to recovery from the cell-cycle–active drug 5-fluorouracil (5-FU). Unexplained sex differences were noted in HSC competitive repopulating ability, but not HPC numbers, in TIP110TG mice. Intracellularly, TIP110 regulated CMYC and GATA2 expression at the transcriptional level, and TIP110 and CMYC reciprocally regulated the expression of each other. These results demonstrate a role for TIP110 in the regulation of hematopoiesis, effects that are likely linked to TIP110 regulation of CMYC.
Introduction
Hematopoiesis is regulated at the level of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) via intracellular and extracellular molecules.1-3 Homeostasis is crucial for normal hematopoiesis.2 Cell cycling of HSCs/HPCs is regulated by growth factors/cytokines, as well as by transcription factors such as C-MYB, CMYC, GATA2, HOX family proteins, and BMI-1. C-MYB promotes HSC growth through induced CMYC.4-6 CMYC induces the expression of positive regulators involved in G1/S cell-cycle transition, and also represses negative regulators of the cell cycle, events required for the proliferation of hematopoietic progenitors.7 CMYC is involved in cell proliferation and growth, angiogenesis, apoptosis, differentiation, and regulation of chromatin structure,8-11 and plays a role in controlling self-renewal and differentiation of HSCs.12
Tat-interacting protein of 110 kDa (TIP110), which was initially cloned from a human KG-1 myeloid cell line cDNA library,13 is implicated in RNA metabolism and tumor-antigen presentation.14-17 We demonstrated previously that TIP110 regulates the transcription of HIV-1 and cellular genes.18,19 It is considered a mammalian homolog of the yeast Prp24 protein, and functions as a general pre-mRNA splicing factor.20 Using molecular, cellular, and genetic strategies, including TIP110 transgenic (TIP110TG) and conditional knockout (TIP110+/−) mice, ectopic expression, and shRNA knockdown, we found that TIP110 regulates HPC/HSC function, effects likely involving cell-cycle regulation, reciprocal control of the expression of TIP110 and CMYC, and GATA2 control of TIP110 expression.
Methods
Human cord blood CD34+ cells
Human cord blood was collected and used according to institutional guidelines. CD34+, CD15+, CD19+, and CD36+ cells were purified within 24 hours of collection using immunomagnetic selection. CD34+ cells were further purified for CD34+CD133+, CD34+CD38−, and CD34+CD38+ cells using immunomagnetic selection (Miltenyi Biotec). CD34+ cells ( > 93% pure) were cultured in 1% or 10% FBS with or without the cytokine combination of: 100 ng/mL of stem cell factor, 100 ng/mL FLT3 ligand, and 20 ng/mL of thrombopoietin (SFT; R&D Systems).
Mouse bone marrow HSCs and HPCs
Common myeloid progenitors (CMPs), megakaryocyte/erythroid progenitors (MEPs), granulocyte/macrophage progenitors (GMPs), long-term repopulating stem cells (HSCs), and short-term repopulating stem cells (HSPCs) were counted 21,22 with an LSRII flow cytometer (BD Biosciences). Purified populations were obtained with a FACSAria flow cytometric sorter (BD Biosciences), centrifuged to a pellet, and prepared for RNA analysis. Long-term repopulating HSCs were Lin−Sca1+c-kit+ (LSK)/CD34−; short-term repopulating HSCs were CD34+/LSK; CMPs were LSK/CD34−/FcγR+/hi; MEPs were LSK/CD34−/FcγR−/lo; and GMPs were LSK/CD34+/FcγR+/hi.
Plasmids
pTIP110.GFP was constructed by cloning human TIP110 cDNA in-frame into a replication-defective, self-inactivation HIV-based pCSCGW vector.23 The empty vector served as control. pshTIP110 was constructed by annealing a pair of TIP110-targeted oligonucleotides (5′-GAT CCG CGA GAG TTT GAA AGT GCG ATT CTC GAG AAT CGC ACT TTC AAA CTC TCG CTT TTT TGG TAC CG-3′ and 5′-AAT TCG GTA CCA AAA AAG CGA GAG TTT GAA AGT GCG ATT CTC GAG AAT CGC ACT TTC AAA CTC TCG CG-3′), followed by cloning into RNAi-Ready pSIREN-RetroQ-ZsGreen vector (Clontech). An 849-bp genomic DNA fragment encompassing the promoter region of TIP110 (chromosome 12, region 107478959-107479807 immediately upstream of TIP110 cDNA) and a 929-bp genomic DNA fragment encompassing the promoter region of CMYC (chromosome 8, region 128816972-128817901 upstream of CMYC cDNA) were separately cloned into a promoterless luciferase construct pGL3basic vector (Promega) using a standard PCR cloning strategy. Human GATA2 promoter (S110428) was purchased from SwitchGear Genomics. Human sh–CMYC (shRNA) and sh-GATA2 plasmids (sc44248SH and sc37228SH) were purchased from Santa Cruz Biotechnology.
Generation of TIP110TG and TIP110+/− mice
To generate TIP110TG mice, human TIP110 cDNA was cloned into pcDNA3 (Invitrogen). The TG cassette (Figure 2A) consisting of the cytomegalovirus (CMV) promoter, TIP110 cDNA, and the bovine growth hormone poly(A) tail was used for embryo injection. Three independent lines of TG mice were obtained and used with similar results. To generate TIP110+/− mice, genomic loci containing TIP110 exons 9-12, exons 13-18, and exon 19 were obtained by high-fidelity genomic DNA PCR (Roche) and cloned into pFlox vector24 to obtain a targeting vector (Figure 3A). EIIa-Cre mice25 were bred with founder mice to segregate and obtain TIP110 conditional-knockout mice, which were generated because complete TIP110 deletion causes embryonic lethality.26 We created a floxed conditional allele using a knockout gene cassette containing 3 appropriately positioned loxP sites in the TIP110 gene (Tri-lox strategy; Figure 3A). To eliminate the floxed marker (neo) fragment in vivo, recombinase expression of EIIaCreTG mice was used.27 Enhancer elements from the Tie2 gene are capable of directing tissue-specific expression in both endothelium and hematopoietic cells.28 Tie2 CreTG mice were used to excise the TIP110 gene from one allele to generate TIP110+/− mice. Mice were generated by the Transgenic and Knockout Core at Indiana University School of Medicine, and back-crossed at least 8 generations to a C57Bl/6 strain background. Genotyping was performed with genomic DNA PCR using primers for the TIP110 transgene: 5′-TAATACGACTCACTATAGGGCGA-3′ and 5′-CCAATCCCACCAACTGAGTA-3′; for the floxed TIP110 gene: 5′- CCTCACTGTGCTGCAAGCTCTG-3′ and 5′-GAATCATGGCTATAGGAGCCCCCC-3′, and for the Cre transgene: 5′-CAGGGTGTTATAAGCAATCCC-3′ and 5′-CCTGGAAAATGCTTCTGTCCG-3′, which resulted in respective PCR products of 650, 350, and 500 bp. All mouse experiments were approved by the Indiana University School of Medicine Institutional Review Board.
RNA isolation and semiquantitative RT-PCR analysis
Total RNA was isolated from cells using the TRIzol RNA isolation kit (Invitrogen). Before RNA precipitation, RNA was extracted with acid phenol:chloroform:isoalcohol (125:24:1), pH 4.5, to eliminate RT-PCR amplification of remaining genomic DNA in the RNA preparations. RT-PCR was performed using a One-Tube Titan RT-PCR kit with primers for human TIP110: 5′-atg gcg act gcg gcc gaa acc-3′ and 5′-ttg tcg atc tag gta ctg act-3′; for mouse TIP110: 5′-ATG GCG ACG ACG GCC GCA TCT TCG GC-3′ and 5′-GTT GTA ATC ATA GCC ATT GAT GGA CA-3′; for CMYC: 5′-AGC ATA CAT CCT GTC CGT CC-3′ and 5′-CTC AGC CAA GGT TGT GAG GT-3′; for GATA2: 5′-CAT CAA GCC CAA GCG AAG A-3′ and 5′-TTT GAC AGC TCC TCG AAG CA-3′; and for β-actin: 5′-CAG CAC TGT GTT GGC GTA CAG GTC-3′ and 5′-CAG GTC ATC ACC ATT GGC AAT CAG-3′. These primers produced PCR products of 1050, 186, 226, 212, and 280 bp, respectively. The RT-PCR program consisted of 1 cycle at 50°C for 30 minutes and 94°C for 2 minutes, followed by 10 cycles at 94°C for 30 seconds, 60°C for 45 seconds, 68°C for 1 minute, and 25 cycles at 94°C for 30 seconds, 60°C for 45 seconds, 68°C for 1 minute, plus a 5-second cycle elongation for each successive cycle, and 1 cycle at 68°C for 7 minutes.
Recombinant TIP110 protein purification and anti-TIP110 monoclonal antibody production
His-tagged TIP110 protein was expressed in 293T cells by transfection of the pTIP110.His plasmid using calcium phosphate precipitation.18 Cells harvested 48 hours after transfection were lysed in buffer containing 50mM Tris-HCl, 30mM NaCl, 10% glycerol, and 1% NP-40 plus protease inhibitor. His-tagged TIP110 protein was obtained by passing lysates through Ni-bead columns (QIAGEN), followed by elution with 0.25M imidazole. Imidazole was removed from the protein preparation by extensive dialysis against PBS. Purity of the recombinant protein was greater than 95%, as shown by SDS-PAGE and Coomassie blue staining. Balb/c mice were immunized with protein, and spleen cells were isolated and fused with SP2/0 myeloma cells to obtain anti-TIP110 monoclonal antibody.
Mouse HPC colony assay
Unseparated mouse femoral bone marrow and spleen cells from TIP110TG and TIP110+/− mice or age-matched wild-type (WT) littermates were plated in 1% methylcellulose culture medium with 30% FBS (Hyclone) at 5 × 104 cells/mL and 5 × 105 cells/mL, respectively, in the presence of recombinant human erythropoietin (1 U/mL; Amgen), stem cell factor (50 ng/mL; R&D Systems), pokeweed mitogen mouse spleen cell conditioned medium (5% vol/vol), and hemin (0.1mM; Eastman Kodak), and incubated at 37°C in 5% CO2 and 5% O2 for 7 days.29 Progenitor cells for HPCs were granulocyte-macrophage colony-forming units (CFU-GMs), erythroid burst-forming units (BFU-Es), and erythroid-granulocyte-macrophage-megakaryocyte colony-forming units (CFU-GEMMs), which were calculated per femur or spleen.29 The percentage of progenitors in the S phase of the cell cycle (cycling status) was estimated using the high–specific activity tritiated thymidine kill assay.29 Mice were assessed individually before and at selected times after IP injection of 5-FU (150 mg/kg body weight; Sigma-Aldrich).30 Days chosen for evaluation after 5-FU administration were those at the beginning of recovery and at the peaks of HPC numbers.
Competitive repopulating mouse bone marrow
Bone marrow (CD45.2+) of TIP110TG and TIP110+/− or WT littermate controls was mixed with bone marrow of nonirradiated competitor B6.Boy J (CD45.1+) mice at a 1:1 ratio (5 × 105 donor and 5 × 105 competitor cells) and transplanted into lethally irradiated (950 cGy) B6.Boy J mice. Percentages of CD45.2 donor cells and CD45.1 competitor cells were determined after transplantation by flow cytometry.29
Cell cycle, proliferation, and luciferase reporter gene assay
CD34+ cells were transfected with expression plasmids and cultured in IMDM with 1% FBS for 48 hours. Cells were harvested, stained for CD34-PE, and fixed/permeabilized for propidium iodide staining. Proliferation was determined with the MTT assay. Absorbance was measured with a SmartSpec 3000 spectrophotometer (Bio-Rad) at a test wavelength of 570 nm with a reference wavelength of 690 nm. Optical density was the difference between the absorbance and the test wavelength. Luciferase assays were performed using the dual luciferase system (Promega). The appropriate control plasmids were used to equalize total transfected DNA. TKβgal was included to normalize transfection efficiency variations.
Statistical analysis
Data are expressed as means ± SD unless noted as SEM. Comparisons among groups were made using the Student t test.
Results
TIP110 expression
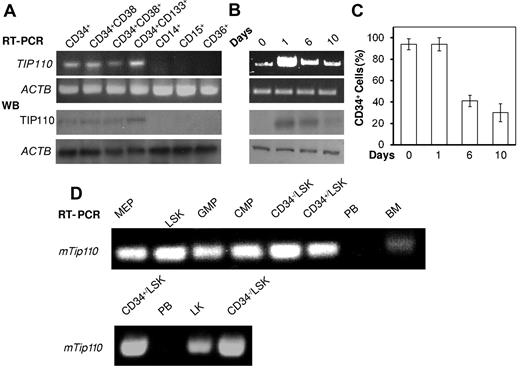
CD34+ cord blood contains HSCs and HPCs.2,3 Total RNA was isolated from cord blood CD34+, CD34+CD38−, CD34+CD38+, CD34+CD133+, CD14+, CD15+, and CD36+ cells. TIP110 mRNA (Figure 1A top panel) and protein (Figure 1A middle panel) expression was noted in all CD34+ cell populations, with slightly higher TIP110 mRNA and protein expression in CD34+CD133+ cells compared with the other 3 populations. TIP110 mRNA or protein was not detected in mature CD14+, CD15+, or CD36+ cells, suggesting a role for TIP110 in the regulation of HSCs/HPCs. Cord blood CD34+ cells were cultured in SFT medium for up to 10 days. TIP110 mRNA and protein expression rapidly peaked at day 1 and then gradually decreased (Figure 1B). TIP110 protein was expressed in CD34+ cells at time 0, as shown in Figure 1A, but not in Figure 1B lane 1. This was likely due to the CD34+ cells shown in Figure 1A having been cultured in IMDM medium with 1% FBS and SFT for 5 hours, whereas the CD34+ cells shown in Figure 1B lane 1 were analyzed immediately after separation. CD34+ cells were actively cycling after 1 day in SFT medium (data not shown). Significant induction of TIP110 expression from freshly isolated quiescent CD34+ cells to cycling CD34+ cells indicated a possible effect of TIP110 expression on induced CD34+ cell cycling.
TIP110 expression in phenotyped cord blood CD34+ cells and mouse bone marrow HSCs and HPCs. (A) Freshly isolated CD34+ cells were cultured in IMDM medium supplied with FBS and SFT for 5 hours and sorted into CD34+CD38−, CD34+CD38+, CD34+CD133+, CD14+, CD15+, and CD36+ subpopulations. TIP110 expression in each of these subpopulations was determined by semiquantitative RT-PCR and Western blotting (WB). (B-C) Freshly isolated CD34+ cells were cultured in IMDM medium supplemented with FBS and SFT, and harvested at days 0, 1, 6, and 10 to assess TIP110 expression by RT-PCR, Western blotting (B), and flow cytometry using anti-CD34 antibody staining (C). Results shown in panels A-C are from 1 experiment representative of 3 separate reproducible experiments. (D) Tip100 mRNA expression, as assessed by semiquantitative RT-PCR, in purified populations of HSCs (CD34−LSK, CD34+LSK, and LSK cells) and HPCs (MEPs, GMPs, and CMPs) compared with bone marrow (BM) and peripheral blood (PB).
TIP110 expression in phenotyped cord blood CD34+ cells and mouse bone marrow HSCs and HPCs. (A) Freshly isolated CD34+ cells were cultured in IMDM medium supplied with FBS and SFT for 5 hours and sorted into CD34+CD38−, CD34+CD38+, CD34+CD133+, CD14+, CD15+, and CD36+ subpopulations. TIP110 expression in each of these subpopulations was determined by semiquantitative RT-PCR and Western blotting (WB). (B-C) Freshly isolated CD34+ cells were cultured in IMDM medium supplemented with FBS and SFT, and harvested at days 0, 1, 6, and 10 to assess TIP110 expression by RT-PCR, Western blotting (B), and flow cytometry using anti-CD34 antibody staining (C). Results shown in panels A-C are from 1 experiment representative of 3 separate reproducible experiments. (D) Tip100 mRNA expression, as assessed by semiquantitative RT-PCR, in purified populations of HSCs (CD34−LSK, CD34+LSK, and LSK cells) and HPCs (MEPs, GMPs, and CMPs) compared with bone marrow (BM) and peripheral blood (PB).
TIP110 mRNA expression was also noted by semiquantitative RT-PCR analysis in phenotypically defined mouse bone marrow HSCs (CD34−LSK, CD34+LSK, and total LSK) and HPCs (CMPs, MEPs, and GMPs; Figure 1D), with much lower expression noted in total bone marrow, and even less in peripheral nucleated cells. Large differences in expression between mouse bone marrow HSC and HPC populations were not detected.
Increased HPC numbers and cycling in TIP110TG mice
TIP110TG mice were generated with human TIP110 expression under the control of a CMV promoter (Figure 2A). Increased TIP110 mRNA (Figure 2B) and protein (Figure 2C) expression was similar for all 3 TG mouse lines. Mice did not exhibit apparent developmental, behavioral, or pathologic changes from birth up to age 4 months, when they were killed. Compared with age-matched WT littermate controls, TIP110TG mice had normal circulating levels of blood cells, a slight, nonsignificant decrease in nucleated femoral marrow cellularity (WT: 21 ± 2 vs TG: 18 ± 3 × 106) and a slight, nonsignificant increase in nucleated spleen cellularity (WT: 82 ± 21, TG: 94 ± 26 × 106). However, TIP110TG mice had significantly higher numbers of CFU-GMs, BFU-Es, and CFU-GEMMs per femur and spleen than littermate controls (Figure 2D) and a significantly higher percentage of marrow and spleen HPCs in the S phase of the cell cycle (Figure 2E). These results demonstrate a positive role for TIP110 in regulating HPC numbers, effects likely mediated through increased HPC cycling.
Effects of the human TIP110-expressing transgene on HPCs. (A) Diagram of the TIP110 transgene cassette consisting of the CMV promoter, human TIP110 cDNA (NM_014706), and bovine growth hormone poly(A) tail. (B) Bone marrow (BM) and spleen cells were harvested from TIP110TG mice for genomic DNA isolation and PCR genotyping for the TIP110 transgene. (C) Murine embryonic fibroblasts were prepared from embryos of TIP110-transgene–expressing mice and analyzed for TIP110 protein expression by Western blotting. Cells were treated with MG132 (a proteasome inhibitor) to stabilize Tip10 protein (unpublished data). *Endogenous TIP110 expression; **transgene expression including a 6× His tag. (D-E) Cells were isolated from BM and spleens of age-matched C57B/L6 control mice and TIP110TG mice and assessed for HPCs by colony assay with progenitors expressed as absolute numbers per organ (D) or progenitors were evaluated for percentage of cells in the S phase using the tritiated thymidine kill assay for determination of cycling status (E). Open bars show WT cells; closed bars, TIP110TG cells. Data for panels B and C are from 1 of at least 3 reproducible experiments. Data in panels D and E are shown as means ± SEM for 8 individually assessed mice for each group in a total of 2 independent experiments.
Effects of the human TIP110-expressing transgene on HPCs. (A) Diagram of the TIP110 transgene cassette consisting of the CMV promoter, human TIP110 cDNA (NM_014706), and bovine growth hormone poly(A) tail. (B) Bone marrow (BM) and spleen cells were harvested from TIP110TG mice for genomic DNA isolation and PCR genotyping for the TIP110 transgene. (C) Murine embryonic fibroblasts were prepared from embryos of TIP110-transgene–expressing mice and analyzed for TIP110 protein expression by Western blotting. Cells were treated with MG132 (a proteasome inhibitor) to stabilize Tip10 protein (unpublished data). *Endogenous TIP110 expression; **transgene expression including a 6× His tag. (D-E) Cells were isolated from BM and spleens of age-matched C57B/L6 control mice and TIP110TG mice and assessed for HPCs by colony assay with progenitors expressed as absolute numbers per organ (D) or progenitors were evaluated for percentage of cells in the S phase using the tritiated thymidine kill assay for determination of cycling status (E). Open bars show WT cells; closed bars, TIP110TG cells. Data for panels B and C are from 1 of at least 3 reproducible experiments. Data in panels D and E are shown as means ± SEM for 8 individually assessed mice for each group in a total of 2 independent experiments.
Decreased HPC numbers and cycling in TIP110+/− mice
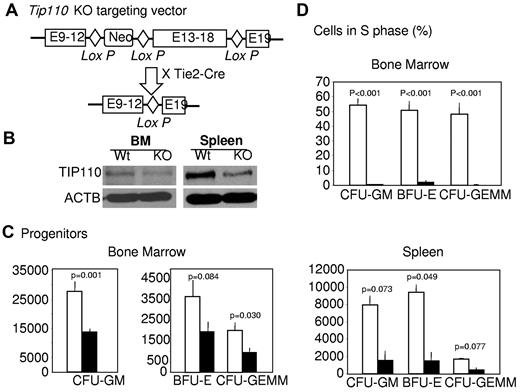
We designed and constructed a targeting vector to specifically disrupt TIP110 gene expression and function (Figure 3A). We reasoned that removal of TIP110 exons 13-18, which encode the putative nuclear localization signal and RNA recognition motif of TIP110, would lead to inactivation of TIP110 function. We bred TIP110 conditional knockout mice with hematopoietic lineage-specific promoter Tie2-driven CreTG mice (Tie2-Cre) to obtain TIP110+/− mice, because TIP110 total knockout causes embryonic lethality. Western blotting confirmed a nearly 50% reduction of TIP110 expression in the marrow and spleens of TIP110+/− mice (Figure 3B). TIP110+/− mice had a normal level of circulating blood cells from birth to 4 months, when they were killed for experiments. There were slight but not statistically significant changes in nucleated cellularity in femoral marrow (WT: 24.3 ± 3 vs knockout: 28.5 ± 4 × 106, P > .05) and spleen (WT: 127.8 ± 11, knockout: 103.1 ± 26 × 106, P > .05). In contrast to TIP110TG mice (Figure 2), TIP110+/− mice manifested significantly decreased CFU-GMs, BFU-Es, and CFU-GEMMs in the bone marrow and spleen compared with controls (Figure 3C), and TIP110+/− bone marrow HPCs were slowly or noncycling (Figure 3D). Spleen HPCs of TIP110+/− and WT mice were slowly or noncycling (data not shown). The above TIP110TG and TIP110+/− effects document a positive role for TIP110 expression on the regulation of HPC proliferation.
Effects of TIP110 haploinsufficiency on HPCs. (A) Diagram of TIP110 conditional knockout cassette consisting of 3 TIP110 genomic loci: exons 9-12, 13-18, and 19, 3 loxP sites in between, and a neo selection marker. Breeding of TIP110 conditional knockout animals with hematopoietic linage-promoter Tie2-driven CreTG mice led to TIP110 haploinsufficiency (+/−) in hematopoietic organs. (B) Bone marrow and spleen cells were harvested from age-matched C57B/L6 and TIP110+/− mice to determine TIP110 expression by Western blotting. (C-D) Cells were isolated from bone marrow and spleens of age-matched C57B/L6 and TIP110+/− mice and assayed for absolute numbers (C) and cycling status (D) of HPCs, as described in the legend to Figure 2. Open bars show WT cells; closed bars, TIP110+/− cells. Data shown in panel B are from 1 of 3 reproducible experiments. Data in panels C and D are expressed as means ± SEM of 14 individually assessed mice for each group from a total of 3 independent experiments.
Effects of TIP110 haploinsufficiency on HPCs. (A) Diagram of TIP110 conditional knockout cassette consisting of 3 TIP110 genomic loci: exons 9-12, 13-18, and 19, 3 loxP sites in between, and a neo selection marker. Breeding of TIP110 conditional knockout animals with hematopoietic linage-promoter Tie2-driven CreTG mice led to TIP110 haploinsufficiency (+/−) in hematopoietic organs. (B) Bone marrow and spleen cells were harvested from age-matched C57B/L6 and TIP110+/− mice to determine TIP110 expression by Western blotting. (C-D) Cells were isolated from bone marrow and spleens of age-matched C57B/L6 and TIP110+/− mice and assayed for absolute numbers (C) and cycling status (D) of HPCs, as described in the legend to Figure 2. Open bars show WT cells; closed bars, TIP110+/− cells. Data shown in panel B are from 1 of 3 reproducible experiments. Data in panels C and D are expressed as means ± SEM of 14 individually assessed mice for each group from a total of 3 independent experiments.
Recovery of HPCs from TIP110+/− and TIP110TG mice administered 5-FU
We assessed the recovery of nucleated cells and HPCs and the percentage of HPCs in the S phase in the bone marrow after administration of 5-FU, a cell-cycle–specific drug (Figure 4). Mice were assessed on days 7 and 11 after 5-FU administration, compared with day 0 in the no-treatment group. Based on previous recovery patterns for control mice,30 HPC numbers and cycling in TIP110+/− bone marrow were significantly lower than in control WT mice at day 0 (Figure 4A), as shown in Figure 3. The bone marrow of WT mice receiving 5-FU was significantly reduced in nucleated cellularity and HPCs at day 7, with return to normal cell numbers, an increase in CFU-GMs, a return to untreated numbers of BFU-Es, and a deficit of CFU-GEMMs at day 11. This was associated with enhanced HPC cycling at day 7 and decreased HPC cycling to a slow or noncycling state at day 11 compared with untreated WT controls. Cellularity of TIP110+/− bone marrow was decreased at day 7 and returned to control (untreated) levels at day 11 after 5-FU administration, similar to that of WT control mice. TIP110+/− HPCs were in a more protected state from the effects of 5-FU: CFU-GM numbers were not decreased by day 7, compared with 74.3% decrease at day 7 for WT controls. By day 11 after 5-FU administration, CFU-GM numbers in TIP110+/− bone marrow were 14.1-fold elevated compared with untreated TIP110+/− mice, whereas WT CFU-GMs were only 5-fold elevated compared with WT controls. BFU-Es from both WT and TIP110+/− bone marrow were similarly decreased by 80% and 84% at day 7 compared with their respective pretreatment levels. Whereas WT BFU-E numbers returned to pretreatment levels at day 11, there was a 1.9-fold increase on day 11 for TIP110+/− mice. CFU-GEMMs of WT and TIP110+/− mice were both decreased (81% and 86%, respectively) by day 7 after 5-FU administration. Whereas WT CFU-GEMMs were decreased by 53% at day 11, TIP110+/− CFU-GEMMs were at pretreatment levels. Cycling of WT and TIP110+/− bone marrow HPCs was accelerated at day 7. However, by day 11 after 5-FU administration, when TIP110+/− HPCs were still rapidly cycling (68%-72%), WT bone marrow HPCs were in a slow or noncycling state, suggesting continued activity/production of TIP110+/− HPCs at this time. Phenotypically, there were statistically significant decreases in absolute numbers of long (CD34−LSK)- and short (CD34+LSK)-term repopulating populations (LSK) of HSCs and GMPs in TIP110+/− bone marrow (Figure 4B). No differences were noted in numbers of CMPs and MEPs. Recovery and increases of phenotyped CD34−LSK, CD34+LSK, and total LSK cells, CMPs, and MEPs were evident on day 7 for both WT and TIP110+/− mice, with less rebound for GMPs detected on this day. This increase (Figure 4B) occurred before that of the functionally assessed HPCs (CFU-GMs, BFU-Es, CFU-GEMMs; Figure 4A). By day 11 after 5-FU administration, CD34−LSK cells were decreased compared with day 7, but the other HSC and HPC populations at day 11 were equal to or higher than that at day 7, suggesting differentiation of long-term repopulating (CD34−LSK) HSCs. However, whereas the phenotyped HPCs for TIP110+/− CMPs, MEPs, and GMPs were lower than WT HPCs at day 11, it is important to stress that the functionally assessed CFU-GMs, BFU-Es, and CFU-GEMMs from unseparated bone marrow of TIP110+/− mice were similar to those of WT mice, suggesting a greater ratio of functionally active to phenotypically defined HPCs for TIP110+/− compared with WT mice. The relatively more protected functional bone marrow HPCs of TIP110+/− WT mice were likely due at least in part to TIP110+/− HPCs at time 0 being in a slow or noncycling, less responsive state to killing by 5-FU.
Recovery of nucleated cells, CFU-GMs, BFU-Es, and CFU-GEMMs from C57Bl/6 WT control and TIP110+/− mice administered 5-FU. Results are for 1 experiment in which 4 mice were individually assessed per group and per day that the mice were killed. Closed bars show WT mice; open bars, TIP110+/− mice. aP < .05 for WT compared with day 0 untreated WT; bP < .05 for TIP110+/− compared with untreated TIP110+/−; cP < .05 compared with WT of that day.
Recovery of nucleated cells, CFU-GMs, BFU-Es, and CFU-GEMMs from C57Bl/6 WT control and TIP110+/− mice administered 5-FU. Results are for 1 experiment in which 4 mice were individually assessed per group and per day that the mice were killed. Closed bars show WT mice; open bars, TIP110+/− mice. aP < .05 for WT compared with day 0 untreated WT; bP < .05 for TIP110+/− compared with untreated TIP110+/−; cP < .05 compared with WT of that day.
We also evaluated TIP110TG mice in which HPC numbers and cycling status were enhanced at pre-5-FU treatment levels compared with WT control mice (Figure 2). In contrast to enhanced recovery of TIP110+/− compared with WT bone marrow HPCs (Figure 4) at day 11 after 5-FU administration treatment, the only time point we evaluated for TIP110TG mice, TIP110TG CFU-GMs, BFU-Es, and CFU-GEMMs were decreased by 38%, 93%, and 93%, respectively, compared with pretreatment TIP110TG values, and these HPCs were in a slow or noncycling state (0%-8% in the S phase, data not shown). Therefore, functional TIP110TG HPCs appear to be much less protected in response to 5-FU than TIP110+/− and WT cells, effects likely attributable to enhanced cycling state of TIP110TG cells at time 0 compared with TIP110+/− and WT HPCs. We did not assess phenotyped TIP110TG bone marrow HSCs/HPCs at this time.
Competitive HSC repopulation of TIP110TG and TIP110+/− bone marrow
To determine whether TIP110 expression influences HSC function, we performed competitive repopulating assays using bone marrow from TIP110TG or TIP110+/− mice. Whereas no significant differences were noted overall in the engraftment of TIP110TG compared with WT mice (data not shown), sex differences in engrafting capability were uncovered (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). There was a statistically significant increase in male donor cell chimerism of TIP110TG longer-term engrafting HSCs at months 2-4 (supplemental Figure 1A), but a trend to a significant decrease in longer-term female donor cell chimerism (supplemental Figure 1B). This sex difference trend, for which we do not yet have an adequate explanation, was also apparent when absolute numbers of LSK cells were assessed per femur (supplemental Figure 1C). Interestingly, there were no differences in the engrafting capability of TIP110 knockout compared with control HSCs when male and female donor cells were assessed separately (supplemental Figure 1D), even though phenotyped short- and long-engrafting HSCs were significantly decreased in TIP110+/− compared with WT mice (Figure 4B). This suggests, but does not prove, that TIP110+/− HSCs may be more functionally potent at the per-cell level. No significant sex differences were detected between functional HPCs (as assessed by colony assay for CFU-GMs, BFU-Es, and CFU-GEMMs).
Modulation of TIP110 expression in CD34+ cells influences proliferation and survival
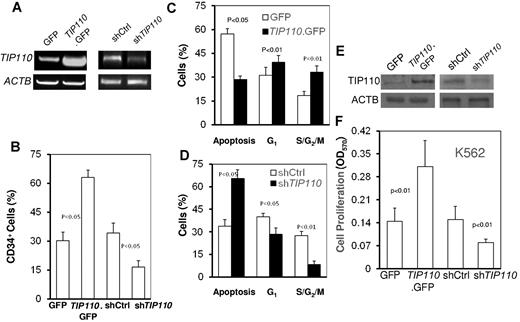
Cord blood CD34+ cells are in a quiescent state and require cytokines for survival and proliferation. Low-level TIP110 expression in cord blood CD34+ cells (Figure 1B) and a role for TIP110 induction of cell cycling of HPCs in TIP110TG mice (Figure 2E) raised the possibility that TIP110 expression may facilitate CD34+ cells to exit a quiescent state and may enhance CD34+ cell survival. Cord blood CD34+ cells were transfected with expression plasmids for TIP110 or TIP110 siRNA to induce over- or underexpression, respectively, of TIP110. Because cytokines affect CD34+ viability and CD34+ cell-surface marker expression and induce quiescent CD34+ cells to cycle, we cultured these cells in IMDM medium with 1% FBS but without any cytokines for 2 days. Semiquantitative RT-PCR showed increased TIP110 expression in cells that were transfected with the TIP110 expression plasmid and decreased TIP110 expression in cells transfected with the TIP110 siRNA expression plasmid (Figure 5A). Ectopic TIP110 expression resulted in more than 63% CD34+ cells after 2 days of culture without any cytokines, compared with approximately 30% CD34+ cells in green fluorescent protein (GFP) transfection controls (Figure 5B). In contrast, greatly reduced TIP110 expression gave rise to 16.8% CD34+ cells, significantly lower than control (37.5%) values (Figure 5B). In the absence of added growth factors, TIP110 overexpression resulted in decreased apoptosis and increased cell cycling (Figure 5C), whereas decreased expression of TIP110 resulted in enhanced apoptosis and decreased cell cycling (Figure 5D).
Effects of TIP110 expression on differentiation, cell-cycle status, and apoptosis of CD34+ cells. Freshly isolated CD34+ cells were immediately transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). These cells were cultured in IMDM with 1% FBS without the addition of any growth factors for 2 days, then harvested for TIP110 mRNA expression by semiquantitative RT-PCR (A), percentage of CD34-expressing cells by FACS (B), and cell-cycle analysis and apoptosis by flow cytometry for TIP110 (C-D). Shown are GFP-overexpressing (C) and shTIP110-underexpressing (D) CD34+ cells. Data are shown as means ± SEM for 3 experiments, each done in triplicate. K562 cells were transiently transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). Cells were cultured for 48 hours and harvested for evaluation of TIP110 expression by Western blotting (E) or assessed by the MTT assay for cell proliferation (F). Data in panels E and F are from 1 of at least 3 reproducible experiments.
Effects of TIP110 expression on differentiation, cell-cycle status, and apoptosis of CD34+ cells. Freshly isolated CD34+ cells were immediately transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). These cells were cultured in IMDM with 1% FBS without the addition of any growth factors for 2 days, then harvested for TIP110 mRNA expression by semiquantitative RT-PCR (A), percentage of CD34-expressing cells by FACS (B), and cell-cycle analysis and apoptosis by flow cytometry for TIP110 (C-D). Shown are GFP-overexpressing (C) and shTIP110-underexpressing (D) CD34+ cells. Data are shown as means ± SEM for 3 experiments, each done in triplicate. K562 cells were transiently transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). Cells were cultured for 48 hours and harvested for evaluation of TIP110 expression by Western blotting (E) or assessed by the MTT assay for cell proliferation (F). Data in panels E and F are from 1 of at least 3 reproducible experiments.
To determine whether TIP110 affects cell proliferation, we transfected TIP110 expression plasmid or TIP110 shRNA expression plasmid into the human myeloid cell line K562, and performed MTT analysis for cell proliferation (Figure 5E-F). We used K562 cells because more cells were obtainable than with cord blood CD34+ cells. Compared with controls, TIP110 overexpression significantly increased the numbers of cells generated, whereas TIP110 silencing resulted in significantly fewer cells (Figure 5F) compared with controls, suggesting that TIP110 regulates CD34+ cell proliferation and survival.
TIP110 regulation of CMYC and GATA2
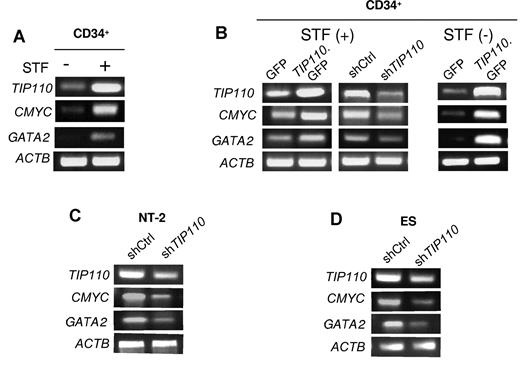
CMYC is an essential regulator of hematopoiesis31 and GATA2 is required for the maintenance and expansion of HSCs.32-34 We analyzed CMYC and GATA2 expression in cytokine-stimulated CD34+ cord blood cells to determine whether CMYC and GATA2 are involved in TIP110 regulation of hematopoiesis. CMYC and GATA2 were expressed at very low levels in freshly isolated CD34+ cells, but there was greatly enhanced expression of TIP110, CMYC, and GATA-2 in CD34+ cells that were stimulated with cytokines for 24 hours (Figure 6A), prompting us to evaluate a relationship between CMYC/GATA2 and TIP110 expression in CD34+ cells. We transfected freshly isolated cord blood CD34+ cells with TIP110 GFP or TIP110 siRNA expression plasmids, cultured the cells in IMDM medium with 1% FBS supplemented with STF for 2 days, and isolated total RNA for CMYC and GATA2 mRNA expression by RT-PCR. Increased expression of CMYC and GATA2 was apparent in TIP110-transfected CD34+ cells, and decreased expression of CMYC and GATA2 was seen in TIP110 siRNA–transfected cells (Figure 6B left). Because cytokines induce quiescent CD34+ cells into cycle and affect CMYC and GATA2 expression, we transfected CD CD34+ cells with the TIP110 GFP expression plasmid, cultured the cells in IMDM medium with 1% FBS without cytokines for 2 days, and isolated total RNA for CMYC and GATA2 mRNA expression by RT-PCR. Significantly increased expression of CMYC and GATA2 was observed in TIP110-transfected CD34+ cells (Figure 6B right), and an association of CMYC, GATA2, and TIP110 expression was confirmed in NT-2 cells (Figure 6C) and human embryonic stem cells (Figure 6D).
Effects of TIP110 on expression of CMYC and GATA2. (A) Total RNA was isolated from freshly isolated cord blood CD34+ cells, and the progeny of these cells were cultured overnight in IMDM medium supplied with 10% FBS and STF, and assessed by semiquantitative RT-PCR for TIP110, CMYC, and GATA2 gene expression. (B) Freshly isolated CD34+ cells were transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). Cells were cultured in IMDM supplemented with FBS and STF (left) or without STF factors (right) for 48 hours. Total RNA was isolated by RT-PCR for TIP110, CMYC, and GATA2 expression. (C) NT-2 cells were transfected with shRNA control (shCtrl) or shRNA for TIP110 (shTIP110), cultured in DMEM supplemented with 10% FBS for 48 hours, and then harvested for TIP110, CMYC, and GATA2 expression by RT-PCR. (D) Human embryonic stem cells were transfected with shRNA control (shCtrl) or shRNA for TIP110 (shTIP110), cultured in HEScGRO hES cell medium for 72 hours, and then harvested for assay of TIP110, CMYC, and GATA2 expression by RT-PCR. Data for panels A through D are from 1 of at least 3 reproducible experiments.
Effects of TIP110 on expression of CMYC and GATA2. (A) Total RNA was isolated from freshly isolated cord blood CD34+ cells, and the progeny of these cells were cultured overnight in IMDM medium supplied with 10% FBS and STF, and assessed by semiquantitative RT-PCR for TIP110, CMYC, and GATA2 gene expression. (B) Freshly isolated CD34+ cells were transfected with expression plasmids for GFP, TIP110.GFP, shRNA control (shCtrl), or shRNA for TIP110 (shTIP110). Cells were cultured in IMDM supplemented with FBS and STF (left) or without STF factors (right) for 48 hours. Total RNA was isolated by RT-PCR for TIP110, CMYC, and GATA2 expression. (C) NT-2 cells were transfected with shRNA control (shCtrl) or shRNA for TIP110 (shTIP110), cultured in DMEM supplemented with 10% FBS for 48 hours, and then harvested for TIP110, CMYC, and GATA2 expression by RT-PCR. (D) Human embryonic stem cells were transfected with shRNA control (shCtrl) or shRNA for TIP110 (shTIP110), cultured in HEScGRO hES cell medium for 72 hours, and then harvested for assay of TIP110, CMYC, and GATA2 expression by RT-PCR. Data for panels A through D are from 1 of at least 3 reproducible experiments.
A CMYC promoter–driven luciferase reporter gene was used to determine the effects of TIP110 on CMYC expression. 293T cells were transfected with the CMYC reporter gene and TIP110 GFP or TIP110 siRNA expression plasmids. TIP110 expression activated luciferase reporter gene expression of CMYC in a dose-dependent manner (Figure 7A), demonstrating that TIP110 overexpression triggers CMYC gene expression. Conversely, TIP110 silencing inhibited luciferase reporter gene expression of CMYC in a dose-dependent manner (Figure 7B). Similar experiments were performed with a human TIP110 promoter–driven luciferase reporter gene and CMYC expression plasmid to determine whether CMYC could reciprocally influence TIP110 gene expression. CMYC expression activated TIP110 promoter–driven luciferase reporter gene expression in a dose-dependent manner (Figure 7C). CMYC silencing inhibited luciferase reporter gene expression of TIP110 in a dose-dependent manner (Figure 7D). In addition, GATA2 expression activated TIP110 promoter–driven luciferase reporter gene expression in a dose-dependent manner (Figure 7E), and GATA2 silencing inhibited luciferase reporter gene expression of TIP110, also in a dose-dependent manner (Figure 7F). However, TIP110 did not influence GATA2 promoter activity (data not shown). These results demonstrate that TIP110 and CMYC reciprocally regulate the other's gene expression, and that GATA2 regulates TIP110 gene expression, suggesting that positive feedback regulation between TIP110 and CMYC, as well as GATA2, may account for TIP110 regulation of HPC numbers and proliferation.
Reciprocal regulation of TIP110 and CMYC and GATA2 regulation of TIP110. (A-B) 293T cells were transfected with pGL3.CMYCProm-Luc and increasing amounts of TIP110 expression plasmid (A) or shTIP110 plasmid (B). 293T cells were transfected with pGL3.TIP110Prom-Luc and increasing amounts of CMYC expression plasmid (C) or shCMYC plasmid (D), and increasing amounts of GATA2 expression plasmid (E) or shGATA2 plasmid (F). Transfected cells were cultured for 48 hours and then harvested for luciferase reporter gene assay. In all transfections, either pc.DNA3 or shCtrl, as indicated, was included to equalize the total amount of transfected DNA in each experiment, and the pTK-βgal plasmid was included to normalize the transfection efficiency variations among all transfections. Data represent the means ± SEM of 3 representative experiments, each done in triplicate.
Reciprocal regulation of TIP110 and CMYC and GATA2 regulation of TIP110. (A-B) 293T cells were transfected with pGL3.CMYCProm-Luc and increasing amounts of TIP110 expression plasmid (A) or shTIP110 plasmid (B). 293T cells were transfected with pGL3.TIP110Prom-Luc and increasing amounts of CMYC expression plasmid (C) or shCMYC plasmid (D), and increasing amounts of GATA2 expression plasmid (E) or shGATA2 plasmid (F). Transfected cells were cultured for 48 hours and then harvested for luciferase reporter gene assay. In all transfections, either pc.DNA3 or shCtrl, as indicated, was included to equalize the total amount of transfected DNA in each experiment, and the pTK-βgal plasmid was included to normalize the transfection efficiency variations among all transfections. Data represent the means ± SEM of 3 representative experiments, each done in triplicate.
Discussion
We defined a role for TIP110, which is expressed at very low levels in terminal cells or tissues and at higher levels in tumor cells,18 in regulating the proliferation of HPCs. These effects were cell-cycle related and involved CMYC and GATA-2. The opposite outcomes of TIP110TG and TIP110+/− regarding absolute numbers and cell-cycle status of HPCs demonstrated that TIP110 is an essential gene regulating hematopoiesis. Cell-cycle–regulatory effects of TIP110 expression were confirmed by recovery patterns of HSCs and HPCs of TIP110+/− compared with WT bone marrow cells. More effective recovery of TIP110+/− bone marrow HPCs in response to 5-FU administration likely reflects, at least in part, the slower cycling status of TIP110+/− HPCs. The reasons for the striking gender-specific effects of TIP110 overexpression on longer-term HSC engraftment remain to be determined. We did not detect differences with regard to gender in the numbers or proliferation of HPCs.
Quiescence is critical in preventing erosion of HSC numbers, and may limit propagation of accumulated DNA damage.5,35,36 Mechanisms involved in immature hematopoietic cell exit into the cell cycle are not completely clear. Our data indicate that TIP110, CMYC, and GATA2 are expressed at very low levels when CD34+ cord blood cells are first isolated. Expression levels are up-regulated rapidly when CD34+ cells are cultured in vitro with a combination of cytokines, including stem cell factor, FLT3 ligand, and thrombopoietin. Our results suggest that TIP110 expression is strictly correlated with HPC proliferation. When CD34+ cells exogenously express TIP110, the expression of CMYC and GATA2 is up-regulated. This CMYC and GATA2 up-regulated expression by TIP110 happens regardless of the presence or absence of SFT, suggesting that TIP110 positively regulates CMYC and GATA2 expression. Enforced TIP110 expression in quiescent CD34+ cord blood cells decreased apoptosis in the absence of growth factors and enhanced the numbers of cells entering into cycle, suggesting that TIP110 levels may be important factors regulating hematopoiesis through triggering CD34+ from a quiescent to a cycling state.
To explore how TIP110 activates the CMYC gene, we built a CMYC and TIP110 promoter and used a promoter reporter gene assay system. TIP110 activates CMYC through its promoter; CMYC also could activate TIP110 through the TIP110 promoter. Identity of CMYC as a TIP110 target gene strongly supports a means by which TIP110 functions in hematopoiesis. CMYC is a critical transcription factor that regulates hematopoiesis, and its deficiency severely impairs definitive hematopoiesis.37 CMYC−/− HSCs/progenitors are unable to expand in number and differentiate to generate a significant number of differentiated progeny.38 We now demonstrate that TIP110 and CMYC reciprocally regulate each other at the promoter level, thus forming a self-regulating feedback system. Overexpression or reduced expression of TIP110 causes changes in CMYC expression that modulate hematopoiesis. TIP110 also regulates GATA2 expression. GATA2 is a member of the GATA transcription factor family, crucial regulators of hematopoiesis.39,40 GATA2 is expressed in HSCs and certain HPCs, and uniquely promotes the proliferation, survival, and function of these cells.32-34 To determine whether and how TIP110 and GATA2 regulate each other, we built a GATA2 promoter and used a TIP110 promoter reporter gene assay. GATA2 activates TIP110 through its promoter, whereas TIP110 activation of GATA2 is not through its promoter, suggesting that the effects of TIP110 on hematopoiesis may also involve TIP110 regulation of GATA2. Overexpression or reduced expression of TIP110 causes changes in CMYC and GATA2 expression that may co-modulate hematopoiesis.
In conclusion, TIP110 is a newly identified essential gene expressed in the earliest cells of adult bone marrow hematopoietic development. TIP110 regulates hematopoiesis, HSCs, and HPCs, as well as CMYC and GATA2 expression. Whereas the relationship of TIP110 to CMYC and cell-cycle regulation of HPCs seems clear, more work is necessary to determine the role(s) of TIP110 and GATA2 in the regulation and homeostasis of HPCs and hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank ManRyul Lee for providing human embryonic stem cells and Sara Rohrabaugh for help with phenotyping of cells.
This study was supported by National Institutes of Health (NIH) Public Health Service grants NIH R01 HL056416 and NIH R01 HL067384 and by NIH grant P01 HL053586 to H.E.B.
National Institutes of Health
Authorship
Contribution: Y.L. designed research, performed research, analyzed and interpreted data, and wrote the manuscript; K.T., Y.F., C.M., G.H., and S.C performed research; C.M. performed research and analyzed data; J.J.H. interpreted data and wrote the manuscript; and H.E.B. performed research, designed research, analyzed and interpreted data, supported the research, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ying Liu, MD, or Hal E. Broxmeyer, PhD, Department of Microbiology and Immunology, Indiana University School of Medicine, R2 302, 950 W Walnut St, Indianapolis, IN 46202; e-mail: yliu2@iupui.edu or hbroxmey@iupui.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal