Abstract
The stem cell factor (SCF)/Kit system has served as a classic model in deciphering molecular signaling events in the hematopoietic compartment, and Kit expression is a most critical marker for hematopoietic stem cells (HSCs) and progenitors. However, it remains to be elucidated how Kit expression is regulated in HSCs. Herein we report that a cytoplasmic tyrosine phosphatase Shp2, acting downstream of Kit and other RTKs, promotes Kit gene expression, constituting a Kit-Shp2-Kit signaling axis. Inducible ablation of PTPN11/Shp2 resulted in severe cytopenia in BM, spleen, and peripheral blood in mice. Shp2 removal suppressed the functional pool of HSCs/progenitors, and Shp2-deficient HSCs failed to reconstitute lethally irradiated recipients because of defects in homing, self-renewal, and survival. We show that Shp2 regulates coordinately multiple signals involving up-regulation of Kit expression via Gata2. Therefore, this study reveals a critical role of Shp2 in maintenance of a functional HSC/progenitor pool in adult mammals, at least in part through a kinase-phosphatase-kinase cascade.
Introduction
The quiescence and entry into cycling of hematopoietic stem cells (HSCs) are tightly controlled and maintained in postnatal hematopoiesis by cytokines/growth factors and other signals in the microenvironment.1,2 Stem cell factor, SCF (also known as Kit ligand, mast cell factor, or Steel factor) is encoded by the mouse Steel locus, and its receptor Kit (also called c-Kit) is encoded by the mouse White locus.3,4 The biologic significance of Kit in hematopoiesis was first revealed in the white spotting (W) mutant mice.5 Several W mutations with different levels of Kit kinase deficiency were found to correlate with the phenotype severity.6 W37 mutation in the conserved tyrosine kinase domain leads to complete loss of the kinase activity, and W37-homozygous mutant mice are embryonic lethal because of severe macrocytic anemia. A similar phenotype was found in the mouse strain with mutations at the Steel locus. Recently Waskow et al7 investigated mild W mutants, identifying a pivotal role of Kit in development of multiple hematopoietic lineages. Kit is highly expressed in HSCs and is currently used as a phenotypic marker for HSCs.8 Studies in the viable primary W mutant mice or in mice after transplantation indicate the importance of Kit signaling in maintaining adult HSC quiescence and survival.9-11 Despite the wealth of knowledge on SCF/Kit signaling, it is poorly understood how Kit expression is regulated in HSCs and progenitors.
Shp2, a tyrosine phosphatase with 2 Src-homology 2 (SH2) domains, is highly expressed in hematopoietic cells and has been shown to physically associate with ligand-activated Kit and other cell surface receptors.12 Germline-dominant active mutations in PTPN11/Shp2 are found in approximately 50% of Noonan syndrome patients, who have a high risk of juvenile myelomonocytic leukemia.13,14 Somatic gain-of-function mutations in PTPN11 have also been detected in nonsyndromic juvenile myelomonocytic leukemia, pediatric acute myeloid leukemia, B-cell precursor acute lymphoblastic leukemia, and myelodysplastic syndromes.15-17 The expression of activated Shp2 leads to lethal myeloproliferative disease in transgenic or knockin mouse models.18-20 Thus, PTPN11 has been proposed as the first proto-oncogene that encodes a tyrosine phosphatase.21 However, the physiologic function of Shp2 in normal hematopoiesis in adults remains to be elucidated.
Here we report a functional requirement for Shp2 in adult HSC maintenance and functions. Notably, we observed a prominent ablation of the Kit-positive stem/progenitor cell subpopulations in Shp2Δ/Δ BM. Further analyses suggest that Shp2 mediates multiple signaling events resulting in increased Kit expression, at least partially through the transcription factor Gata2. Thus, Shp2, a cytoplasmic tyrosine phosphatase, is positively required for signal relay downstream of Kit and other cytokine/growth factor receptors in HSCs/progenitors, and at least one mechanism is surprisingly the up-regulation of Kit gene expression, thereby forming a positive feedback loop of Kit-Shp2-Kit in hematopoiesis.
Methods
BM transplantation
BM cells were harvested from mouse femur and tibia, and red blood cells were lysed by the NH4Cl red blood cell lysis buffer. In the competitive reconstitution assay, 5 × 105 BM nucleated cells from control or knockout animals were mixed with 5 × 105 or 2 × 106 BM nucleated cells from CD45.1 mice and transplanted into lethally irradiated recipients. For lethal irradiation of recipients, we used 2 doses of 500 rad/time with a 4-hour interval. All animal protocols were approved by the Sanford/Burnham Medical Research Institute animal care program.
Flow cytometric analysis and sorting
Lineage staining was performed by the use of mouse hematopoietic lineage flow panel from eBioscience supplemented with biotin-conjugated antibodies against mouse CD4 and CD8. Streptavidin conjugated with APC-CY7 was used as secondary staining. For further staining of HSCs, antibodies against Kit, Sca1 (both from eBioscience), and CD34 (Biolegend) were used. Lineage− cells were enriched by a lineage depletion kit (Miltenyi Biotec) according to the manufacturer's instruction.
Analysis of cell cycle and apoptosis
To assess the cell cycle, BrdU incorporation assay was performed as described previously.22 For 34−LSK or LSK cell-cycle analysis, 100 mg of BrdU was injected intraperitoneally 19 or 2 hours before euthanasia. To distinguish the G0 from G1 phase in primitive populations, sorted LSK cells were stained in 0.5 μg/mL Pyronin Y and 2 μg/mL Hoechest33342 at 45 minutes before FACS analysis. To assess cell apoptosis, annexin V-enhanced green fluorescent protein (EGFP; Biovision) was performed according to manufacturer's instruction. FACS data were analyzed by the use of the Flowjo software.
Cell lines and plasmids
The erythroid myeloid lymphoid (EML) cell line was cultured in IMDM medium with 20% horse serum and 10% BHK-KL conditioned medium. The BHK-KL cell line that expresses and secrets SCF was from Dr Schickwann Tsai. The HEK-293 cell line was maintained in DMEM medium with 10% FBS. Scramble siRNA and Shp2-specific siRNA were purchased from QIAGEN. siRNA sequences for human Shp2 are: forward 5′-UUAAUUGCCCGUGAUGUUCUU-3′; and reverse 5′-GAACAUCACGGGCAAUUAAUU-3′. siRNA sequences for mouse Shp2 are: forward 5′-GGA CUA UGA CCU CUA UTT-3′; and reverse 5′-AUA GAG GUC AUA GUA GUC CTT-3′. Jetprime (polyplus transfection) was used for siRNA and plasmid transfection into HEK293 cells, and nucleofector kit (Lonza) was used to introduce siRNA and plasmids into EML cells. GFP reporter plasmid p13kit and p70kit were generously provided by Dr Susanna Dolci. pBABE-gata2 plasmid was a gift from Dr John Crispino.
Statistical analysis
Statistical analysis was performed by the use of GraphPad Prism 5 software. The Student t test was used to calculate the P value.
Results
Shp2 ablation leads to BM failure and cytopenia in peripheral blood
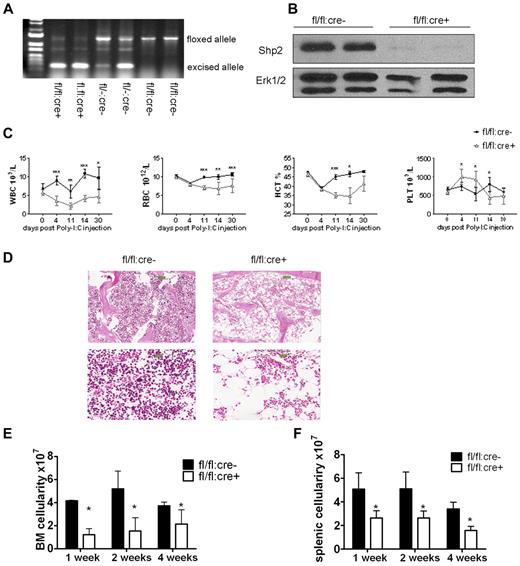
We generated an inducible Ptpn11/Shp2 knockout mouse line (Shp2flox/flox:Mx1-cre or fl/fl:cre+) in which Cre expression is transiently induced by interferon or polyinosinic:polycytidylic acid (poly-I:C). To induce Shp2 deletion, 5 doses of poly-I:C were injected intraperitoneally every other day. At 2 weeks after final injection (P.I.), genomic DNA was extracted from peripheral blood cells, and PCR analysis indicated clear excision of the floxed allele in fl/fl:cre+ animals (Figure 1A). Immunoblotting confirmed efficient Shp2 deletion in poly-I:C-treated fl/fl:cre+ (or Shp2Δ/Δ) BM cells (Figure 1B).
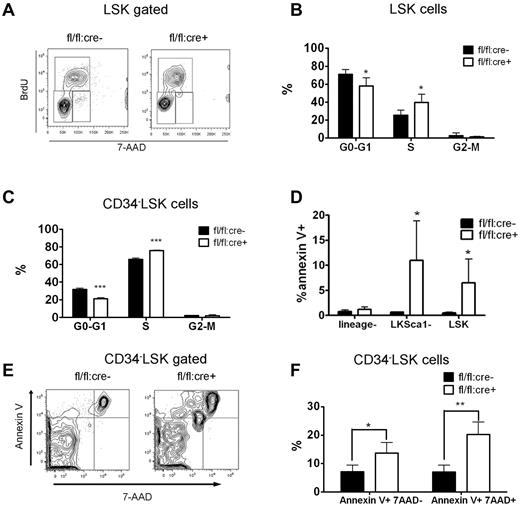
Inducible deletion of Shp2 in vivo leads to decreased cellularity in peripheral blood, BM, and spleen. (A) Deletion of the floxed region was verified by PCR analysis of peripheral blood cells isolated from mice 2 weeks after final Poly-I:C injection. (B) Deletion of Shp2 in BM was verified by immunoblotting at 2 weeks after final Poly-I:C injection. Erk was used as loading control. (C) Complete blood cell counting. Peripheral blood was collected at time points after final Poly-I:C injection (n = 5-9). HCT indicates hematocrit; PLT, platelet; RBC, red blood cells; WBC, white blood cells. (D) Representative H&E staining of trabecular femur 2 weeks after final Poly-I:C injection indicates a severe hypocellular phenotype in fl/fl:cre+ BM. Top panels show images scanned by ScanScope Digital Slide Scanners with 100× magnification. Bottom panels scanned with 400× magnification. (E) BM cellularity decreased significantly in fl/fl:cre+ mice starting from 1 week after final Poly-I:C injection (n = 3-15). (F) Splenic cellularity in the mutant animal was significantly reduced. Time points were after final Poly-I:C injection (n = 3-4). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 1.
Inducible deletion of Shp2 in vivo leads to decreased cellularity in peripheral blood, BM, and spleen. (A) Deletion of the floxed region was verified by PCR analysis of peripheral blood cells isolated from mice 2 weeks after final Poly-I:C injection. (B) Deletion of Shp2 in BM was verified by immunoblotting at 2 weeks after final Poly-I:C injection. Erk was used as loading control. (C) Complete blood cell counting. Peripheral blood was collected at time points after final Poly-I:C injection (n = 5-9). HCT indicates hematocrit; PLT, platelet; RBC, red blood cells; WBC, white blood cells. (D) Representative H&E staining of trabecular femur 2 weeks after final Poly-I:C injection indicates a severe hypocellular phenotype in fl/fl:cre+ BM. Top panels show images scanned by ScanScope Digital Slide Scanners with 100× magnification. Bottom panels scanned with 400× magnification. (E) BM cellularity decreased significantly in fl/fl:cre+ mice starting from 1 week after final Poly-I:C injection (n = 3-15). (F) Splenic cellularity in the mutant animal was significantly reduced. Time points were after final Poly-I:C injection (n = 3-4). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 1.
After poly-I:C treatment, all parameters except platelet in a complete blood cell count were significantly lower in fl/fl:cre+ mice than that in fl/fl:cre− mice (Figure 1C). No significant differences were observed among these groups of mice: +/+:cre−, fl/+:cre−, +/+:cre+, fl/+:cre−, and fl/fl:cre−, excluding an effect of Cre-mediated toxicity (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Therefore, fl/fl:cre− mice were used as controls throughout the study. H&E staining of the trabecular bone showed cytopenia in the BM of mutant animals (Figure 1D). Further quantification assays indicated a nearly two-thirds loss of total BM cellularity starting from 1 week after final injection in mutant mice, compared with controls (Figure 1E). Other hematopoietic organs, such as the spleen, also displayed significant loss of cellularity in mutant mice (Figure 1F), suggesting a severe defect in the hematopoietic compartment after acute deletion of Ptpn11/Shp2.
Shp2 is required for sustaining a functional HSC/progenitor pool
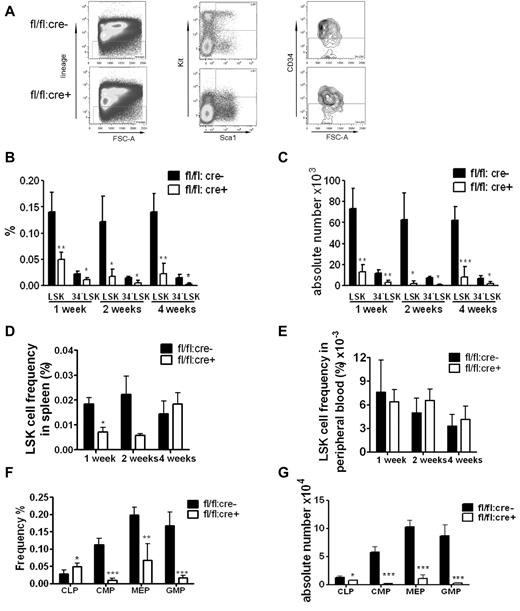
Flow cytometric analysis showed a dramatic decrease in the frequency and absolute numbers of HSC-enriched lin−Sca1+Kit+CD34− (34−LSK) cells in Shp2Δ/Δ BM (Figure 2A-C). We also carefully checked the LSK cell pool in the peripheral blood and spleen. A reduction of splenic LSK cell frequency was observed 1 week after final poly-I:C injection, and the LSK frequency in peripheral remained indistinguishable between control and mutant animals (Figure 2D-E). These results suggest that the decrease in the LSK cell pool in the BM is not because of enhanced mobilization to peripheral blood or spleen. The frequency of common lymphoid progenitors (CLPs) in the BM was elevated whereas the myeloid progenitors were significantly decreased in the knockout animals (Figure 2F). When calculated against the total BM number, the CLPs, common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs) and megakaryocyte-erythrocyte progenitors were significantly lower in mutant animals, with the most dramatic reduction observed in myeloid progenitors, indicating different roles of Shp2 in lymphoid and myeloid lineage commitment/differentiation (Figure 2G).
Loss of phenotypic HSPCs and myeloid progenitors in Shp2 knockout animals. (A) Representative FACS plots for lin−Kit+Sca1+ CD34− (34−LSK) staining 2 weeks after final Poly-I:C injection. (B) Quantification of CD34−LSK cell frequency per 2 hindlimbs at indicated time points after final Poly-I:C injection (n = 3-4). (C) Quantification of CD34−LSK cell number per 2 hindlimbs at indicated time points after final Poly-I:C injection (n = 3-4). (D) LSK cell frequency in the spleen at indicated time points after final Poly-I:C injection (n = 3-4). (E) LSK cell frequency in the peripheral blood was not changed in mutant animal. Time points were after final Poly-I:C injection (n = 3-4). (F) CMPs, GMPs, MEPs, and CLPs frequency in the BM at 2 weeks after final Poly-I:C injection. CMPs were gated as Lin−Sca-1−Kit+FcgRloCD34+. CLPs were gated as Lin−IL-7R− Sca-1loKitlo. GMPs were gated as Lin−Sca-1−Kit+FcgRhiCD34+. Megakaryocyte-erythrocyte progenitors were gated as Lin−Sca-1−Kit+FcgRloCD34−. (G) Absolute numbers of CMPs, GMPs, MEPs, and CLPs at 2 weeks after final Poly-I:C injection (n = 4). ***P < .001, **P < .01,*P < .05, data are presented as mean ± SD.
Loss of phenotypic HSPCs and myeloid progenitors in Shp2 knockout animals. (A) Representative FACS plots for lin−Kit+Sca1+ CD34− (34−LSK) staining 2 weeks after final Poly-I:C injection. (B) Quantification of CD34−LSK cell frequency per 2 hindlimbs at indicated time points after final Poly-I:C injection (n = 3-4). (C) Quantification of CD34−LSK cell number per 2 hindlimbs at indicated time points after final Poly-I:C injection (n = 3-4). (D) LSK cell frequency in the spleen at indicated time points after final Poly-I:C injection (n = 3-4). (E) LSK cell frequency in the peripheral blood was not changed in mutant animal. Time points were after final Poly-I:C injection (n = 3-4). (F) CMPs, GMPs, MEPs, and CLPs frequency in the BM at 2 weeks after final Poly-I:C injection. CMPs were gated as Lin−Sca-1−Kit+FcgRloCD34+. CLPs were gated as Lin−IL-7R− Sca-1loKitlo. GMPs were gated as Lin−Sca-1−Kit+FcgRhiCD34+. Megakaryocyte-erythrocyte progenitors were gated as Lin−Sca-1−Kit+FcgRloCD34−. (G) Absolute numbers of CMPs, GMPs, MEPs, and CLPs at 2 weeks after final Poly-I:C injection (n = 4). ***P < .001, **P < .01,*P < .05, data are presented as mean ± SD.
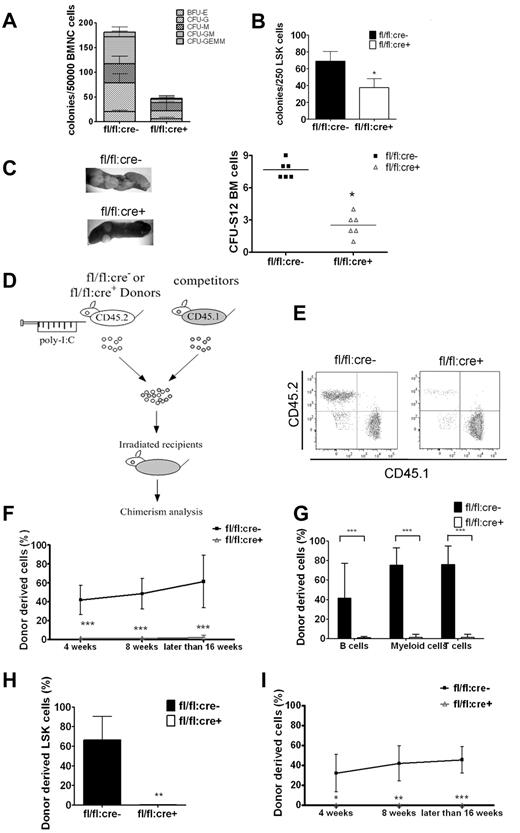
We performed colony-forming unit (CFU) assay to examine HSC/progenitor functions in BM and spleen. As shown in Figure 3A, Shp2 removal severely suppressed the total colony numbers. An unbiased suppression was detected in all colony types, including BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-GEMM. Similar results were obtained in assays by the use of sorted LSK cells (Figure 3B), suggesting impaired differentiation capacity of mutant LSK cells. Therefore, Shp2 ablation resulted in decrease in the size and differentiation potential of the LSK pool, as revealed by the CFU assay in vitro. Consistently, in vivo CFU-spleen assay performed on day 12 after BM transplantation demonstrated a significant decrease of HSCs and multipotent progenitors derived from mutant animals (Figure 3C).
Shp2 ablation results in significant decrease in BM HSCs/progenitors. (A, B) In vitro CFU assays for whole BM cells or purified LSK cells. 50 000 BM (A) or 250 sorted LSK (B) cells were seeded on methylcellulose medium Methocult 3434 (StemCell Technologies Inc) with cytokines for 14 days before colony numeration. (n = 3-6). (C) Representative spleen colonies from CFU-S day 12 assay. Quantification of CFU-S12 suggests a decrease of HSCs and multipotent progenitors (n = 5-6). (D) Competitive reconstitution strategy 1: injection first/transplantation later. Donor mice were treated with poly-I:C before BM transplantation. (E) Representative FACS plots illustrate BM chimerism in 1:1 donor versus competitor reconstitution assay. (F) Percentage of CD45.2 donor derived cells using 1:1 donor to competitor ratio (n = 6-10). (G) Percentage of CD45.2 donor-derived cells within different lineages in 1:1 ratio competitive reconstitution (n = 4-5). (H) Shp2Δ/Δ BM contributes to less than 1% of LSK chimerism in 1:1 ratio competitive reconstitution (n = 4). (I) 500 sorted LSK cells were transplanted into lethally irradiated recipients in competition with 5 × 105 CD45.1 cells in the injection first/transplantation later setting. Peripheral chimerism was analyzed at indicated time points. fl/fl:cre− LSK cells barely gave rise to any peripheral blood cells (n = 4-5). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 2.
Shp2 ablation results in significant decrease in BM HSCs/progenitors. (A, B) In vitro CFU assays for whole BM cells or purified LSK cells. 50 000 BM (A) or 250 sorted LSK (B) cells were seeded on methylcellulose medium Methocult 3434 (StemCell Technologies Inc) with cytokines for 14 days before colony numeration. (n = 3-6). (C) Representative spleen colonies from CFU-S day 12 assay. Quantification of CFU-S12 suggests a decrease of HSCs and multipotent progenitors (n = 5-6). (D) Competitive reconstitution strategy 1: injection first/transplantation later. Donor mice were treated with poly-I:C before BM transplantation. (E) Representative FACS plots illustrate BM chimerism in 1:1 donor versus competitor reconstitution assay. (F) Percentage of CD45.2 donor derived cells using 1:1 donor to competitor ratio (n = 6-10). (G) Percentage of CD45.2 donor-derived cells within different lineages in 1:1 ratio competitive reconstitution (n = 4-5). (H) Shp2Δ/Δ BM contributes to less than 1% of LSK chimerism in 1:1 ratio competitive reconstitution (n = 4). (I) 500 sorted LSK cells were transplanted into lethally irradiated recipients in competition with 5 × 105 CD45.1 cells in the injection first/transplantation later setting. Peripheral chimerism was analyzed at indicated time points. fl/fl:cre− LSK cells barely gave rise to any peripheral blood cells (n = 4-5). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 2.
The HSC function was further evaluated by the use of BM competitive reconstitution assay. fl/fl:cre− or fl/fl:cre+ donor mice were treated with poly-I:C, and BM cells (CD45.2+) harvested 2 weeks after final injection were transplanted into lethally irradiated recipients in competition with CD45.1+ competitor BM cells at a ratio of 1:1 or 4:1 (Figure 3D). Shp2Δ/Δ BM gave rise to few, if any, blood cells in the 1:1 ratio transplantation (Figure 3E-F) and contributed a significantly lower percentage in the 4:1 ratio set-up (supplemental Figure 2A) compared with the expected chimerism in the controls. In the 1:1 ratio experiment, specific lineage markers were used to evaluate donor-derived cells in different cell lineages after 16 weeks of transplantation. Shp2Δ/Δ BM produced < 5% of T, B lymphocytes, or myeloid cells (Figure 3G). Consistently, very few (< 1%) BM LSK cells were derived from Shp2Δ/Δ BM donors (Figure 3H), indicating a requirement for Shp2 in self-renewal of HSCs in vivo.
The reconstitution defect is LSK cell-autonomous
The next question we asked was whether Shp2 is required intrinsically for HSCs or whether Shp2 regulates the microenvironmental cues in the BM niche. To address this issue, we conducted reciprocal transplantation in which unfractionated wild-type BM cells or isolated LSK cells were transplanted into recipients with wild-type or Shp2-deficient BM microenvironment (supplemental Figure 2C). Successful reconstitution was observed in both fl/fl:cre− and fl/fl:cre+ recipients that received healthy BM cells (supplemental Figure 2D) or LSK cells (supplemental Figure 2E), suggesting that Shp2 deficiency in niche cells did not compromise donor HSC reconstitution capacity.
To unequivocally determine whether the profound reconstitution defect is HSC-autonomous, 500 LSK cells from poly-I:C-treated fl/fl:cre+ or fl/fl:cre− mice were transplanted into lethally irradiated recipients in competition with 5 × 105 CD45.1+ BM cells. Consistent with the whole BM cell data, Shp2Δ/Δ LSK cells derived from fl/fl:cre+ mice contributed to almost no peripheral blood cells in recipients, although approximately 50% chimerism was detected in recipients that received fl/fl:cre− CD45.2+ LSK and CD45.1+ BM cells (Figure 3I). Taken together, these results indicate that Shp2 acts in a cell-autonomous manner in the regulation of the HSC pool.
Shp2 enhances BM engraftment by promoting HSC homing
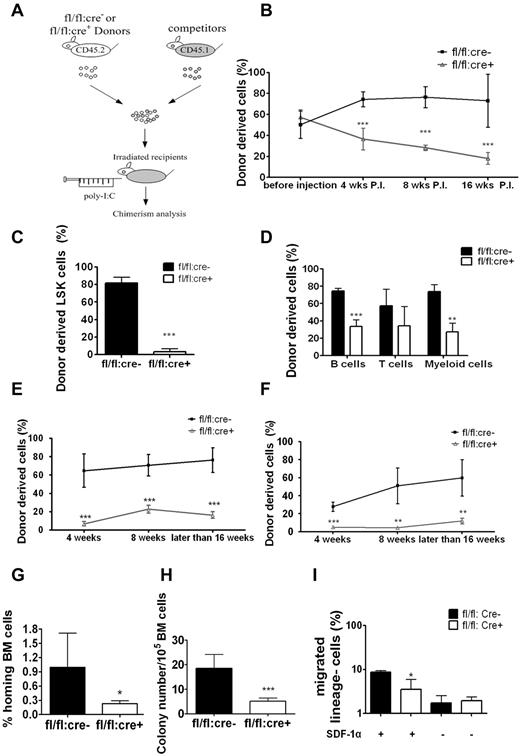
To understand the transplantation failure, we investigated whether Shp2 deficiency affected HSC homing and engraftment by inducing Shp2 deletion before (Figure 3D) or after transplantation (Figure 4A). Untreated BM cells of fl/fl:cre+ or fl/fl:cre− origin (CD45.2+) were transplanted into lethally irradiated recipients together with CD45.1+ BM cells at ratio of 1:1 or 4:1. At 4 weeks after transplantation, 5 doses of poly-I:C were given to the recipients (wild type), and the peripheral chimerism was determined at different time points. At 1:1 donor versus competitor ratio, fl/fl:cre− cells constituted the expected one-half of the peripheral blood initially and increased modestly later (Figure 4B), similar to data shown in Figure 3F, obtained in the injection first/transplantation later experiment. Approximately 20% of peripheral blood cells were derived from fl/fl:cre+ BM in the transplantation first/injection later experiment (Figure 4B), which stands in contrast to almost no fl/fl:cre+-derived blood cells in recipients in the injection first/transplantation later setting (Figure 3F).
Homing defect in Shp2Δ/Δ BM cells. (A) Competitive reconstitution strategy 2: transplantation first/injection later. The recipient mice were treated with poly-I:C after BM transplantation. (B) Percentage of CD45.2 donor-derived cells using 1:1 donor to competitor ratio with poly-I:C injection after transplantation (n = 4-7). (C) Shp2Δ/Δ BM contributed significantly less LSK cells in the 1:1 ratio competitive reconstitution experiment (n = 3). (D) Percentage of CD45.2 donor-derived cells within different lineages in 1:1 ratio competitive reconstitution (n = 3). (E) Declined reconstitution efficacy of Shp2Δ/Δ BM cells in the secondary transplantation (n = 4-6). (F) Sorted LSK cells were used as donor cells in the transplantation first/injection later competitive reconstitution assay. Peripheral chimerism was analyzed at indicated time points (n = 4). (G) In vivo homing assays. Results were shown with percentage of homed BM cells 16 hours after transplantation (n = 4, 5). (H) Homing defects in Shp2Δ/Δ progenitors. 5 × 106 wild-type or Shp2Δ/Δ BM cells were transplanted to lethally irradiated mice. Recipient BM was harvested 24 hours later and seeded in Methocult 3434 medium (StemCell Technologies Inc). Colonies were counted on day 8 (n = 7). We used lethally irradiated but nontransplanted recipients as negative control, and no colonies were detected from the BM. Significantly fewer colonies were formed from Shp2Δ/Δ marrow transplantation, suggesting that the homing defect resides in primitive progenitors. (I) Shp2 deletion severely reduces the chemotactic response of lin− cells to SDF-1α. 106 lineage-depleted BM cells were plated in the upper chamber for migration toward 5 nm SDF-1α in the lower chamber (performed in triplicate). ***P < .001, **P < .01,*P < .05; error bars are SD.
Homing defect in Shp2Δ/Δ BM cells. (A) Competitive reconstitution strategy 2: transplantation first/injection later. The recipient mice were treated with poly-I:C after BM transplantation. (B) Percentage of CD45.2 donor-derived cells using 1:1 donor to competitor ratio with poly-I:C injection after transplantation (n = 4-7). (C) Shp2Δ/Δ BM contributed significantly less LSK cells in the 1:1 ratio competitive reconstitution experiment (n = 3). (D) Percentage of CD45.2 donor-derived cells within different lineages in 1:1 ratio competitive reconstitution (n = 3). (E) Declined reconstitution efficacy of Shp2Δ/Δ BM cells in the secondary transplantation (n = 4-6). (F) Sorted LSK cells were used as donor cells in the transplantation first/injection later competitive reconstitution assay. Peripheral chimerism was analyzed at indicated time points (n = 4). (G) In vivo homing assays. Results were shown with percentage of homed BM cells 16 hours after transplantation (n = 4, 5). (H) Homing defects in Shp2Δ/Δ progenitors. 5 × 106 wild-type or Shp2Δ/Δ BM cells were transplanted to lethally irradiated mice. Recipient BM was harvested 24 hours later and seeded in Methocult 3434 medium (StemCell Technologies Inc). Colonies were counted on day 8 (n = 7). We used lethally irradiated but nontransplanted recipients as negative control, and no colonies were detected from the BM. Significantly fewer colonies were formed from Shp2Δ/Δ marrow transplantation, suggesting that the homing defect resides in primitive progenitors. (I) Shp2 deletion severely reduces the chemotactic response of lin− cells to SDF-1α. 106 lineage-depleted BM cells were plated in the upper chamber for migration toward 5 nm SDF-1α in the lower chamber (performed in triplicate). ***P < .001, **P < .01,*P < .05; error bars are SD.
At 16 weeks after transplantation, we killed the recipients and stained for CD45.2+ LSK cells. We found that LSK cells of fl/fl:cre+ origin decreased to less than 5%, reinforcing the notion that Shp2 is required for self-renewal/maintenance of HSCs/progenitors in the transplantation first/injection later scenario (Figure 4C). Similar results were obtained when we used a 4:1 ratio in transplantation (supplemental Figure 2B). B cell, T cell, or myeloid cell–specific markers were used to evaluate donor-derived cells in different lineages. Shp2Δ/Δ BM cells achieved significant lower chimerism of B and myeloid cells (Figure 4D), but the suppression was not as dramatic in the injection first/transplantation later setting (Figure 3G). We also determined the competitive reconstitution capacity of purified LSK cells in the transplantation first/injection later setting. Approximately 20% of Shp2Δ/Δ LSK cell-derived chimerism was again observed in the peripheral blood (Figure 4E) compared with the more severe phenotype in the injection first/transplantation later experiment (Figure 3I). When the isolated CD45.2+ BM cells from the primary recipients (injection later) were mixed with CD45.1+ competitor cells at 1:1 ratio for secondary transplantation, less than 5% of the CD45.2+ cells were detected in second recipients that received fl/fl:cre+ cells (Figure 4F), confirming the reconstitution failure of Shp2Δ/Δ HSCs.
The improved reconstitution efficiency observed when Ptpn11/Shp2 was deleted after transplantation suggests a possible role of Shp2 in engrafted HSC homing and the establishment of new HSC-niche interaction. In the transplantation first/injection later experiment, untreated fl/fl:cre+ BM cells were able to home and occupy the BM niche in the first place, thus compromising the effect of Shp2 deletion by subsequent poly-I:C treatment. To test this hypothesis, we evaluated directly the homing capacity of Shp2Δ/Δ BM cells in vivo. Purified control and Shp2Δ/Δ lineage− BM cells were labeled with CDFA-SE and injected into lethally irradiated recipients. At 16 hours after transplantation, recipient mice were euthanized for BM isolation and CDFA-SE+ cell quantification. As shown in Figure 4G, Shp2 removal resulted in a dramatic decrease in the number of donor lineage− BM cells in the recipients' BM. To further quantify the homed stem/progenitor cells, we collected the recipients' BM for CFU assay by using BM cells from lethally irradiated mice without transplantation as negative control. Consistent with the CDFA-SE staining data, BM cells from Shp2 mutant transplantation recipients gave rise to significantly fewer colonies than the wild-type controls (Figure 4H). These results suggested a critical role of Shp2 in regulating signals required for homing of engrafted stem/progenitor cells.
We next assessed the impact of Shp2 ablation on cell motility in vitro by performing a cell migration assay using a Boyden chamber. Purified lineage− BM cells from poly-I:C–treated fl/fl:cre+ or fl/fl:cre− mice were seeded into the upper chamber and were allowed to migrate toward chemokine SDF-1α (5nM) for 3 hours. Compared with controls, Shp2Δ/Δ BM cells showed a significantly impaired response to SDF-1α-mediated chemotaxis (Figure 4I).
Aberrant proliferation and enhanced apoptosis of Shp2-deficient HSCs/progenitors
The majority of adult HSCs remain in a dormant/quiescent status for hematopoietic homeostasis.23 We assessed the ratio of actively cycling cells by a BrdU incorporation assay. At 2 weeks after the final poly-I:C treatment, fl/fl:cre+ or fl/fl:cre− mice received intraperitoneal injections of BrdU before they were killed. BM cells were then collected and stained for surface CD34 LSK markers. BrdU and DNA staining were performed sequentially. As shown in Figure 5A through C, significantly more 34−LSK or LSK cells in S phase were observed in Shp2Δ/Δ BM than in control, whereas fewer mutant 34−LSK cells or LSK cells resided in G0/G1 quiescent phase. Pyronnin Y and Hoechst 33 342 costaining were used to distinguish the G0 phase from G1 phase, and Shp2 ablation reduced the number of LSK cells in the quiescent G0 phase (supplemental Figure 3A-B). The enhanced cell-cycle entry of Shp2Δ/Δ HSCs is further substantiated by accelerated mouse death upon 5-fluorouracil (5-FU) treatment. 5-FU is a cell cycle–specific myeloablative agent and causes depletion of cycling HSCs. Three sequential sublethal doses of 5-FU were injected to poly-I:C–treated fl/fl:cre+ or fl/fl:cre− mice every 7 days. A remarkably greater rate of mortality was observed in fl/fl:cre+ animals than controls (supplemental Figure 3C).
Disturbed quiescence and increased apoptosis for LSK cells derived from Shp2Δ/Δ BM. (A) Representative FACS plots illustrate BrdU/7-aminoactinomycin D (7-AAD) staining in LSK cells. (B) Quantification of S-phase or G0/G1-phase cell percentage in LSK subpopulation indicating more actively proliferating LSK cells in Shp2Δ/Δ animals. Results were obtained from 3 independent experiments. (C) Quantification of G0-G1, S, and G2-M phase cell percentage in 34−LSK subpopulation. (D) lin−ckit+Sca1− and LSK cell but not lin− population contained more Annexin V+ cells. (E, F) Elevated Annexin V+ 7-AAD− and Annexin V+ 7-AAD+ percentage in Shp2Δ/Δ 34−LSK cells (n = 4). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 3.
Disturbed quiescence and increased apoptosis for LSK cells derived from Shp2Δ/Δ BM. (A) Representative FACS plots illustrate BrdU/7-aminoactinomycin D (7-AAD) staining in LSK cells. (B) Quantification of S-phase or G0/G1-phase cell percentage in LSK subpopulation indicating more actively proliferating LSK cells in Shp2Δ/Δ animals. Results were obtained from 3 independent experiments. (C) Quantification of G0-G1, S, and G2-M phase cell percentage in 34−LSK subpopulation. (D) lin−ckit+Sca1− and LSK cell but not lin− population contained more Annexin V+ cells. (E, F) Elevated Annexin V+ 7-AAD− and Annexin V+ 7-AAD+ percentage in Shp2Δ/Δ 34−LSK cells (n = 4). ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 3.
We performed annexin V staining to assess HSC/progenitor cell apoptosis. No difference in cell apoptosis was detected in lineage− BM cell pool. Shp2Δ/Δ lin−ckit+Sca1− or LSK cells contained a greater percentage of Annexin V+ cells than the control (Figure 5D). Significantly increased apoptosis rate was also seen in CD34−LSK cells (Figure 5E-F). These results indicate a more stringent requirement for Shp2 in promoting cell survival in the BM HSCs/progenitors than in more developed/committed precursor cells. Collectively, the data shown here indicate that disruption of quiescence and increased apoptosis contribute to the severe loss of HSCs in the conditional Shp2 knockout animals.
Shp2 removal results in down-regulation of Kit expression directly
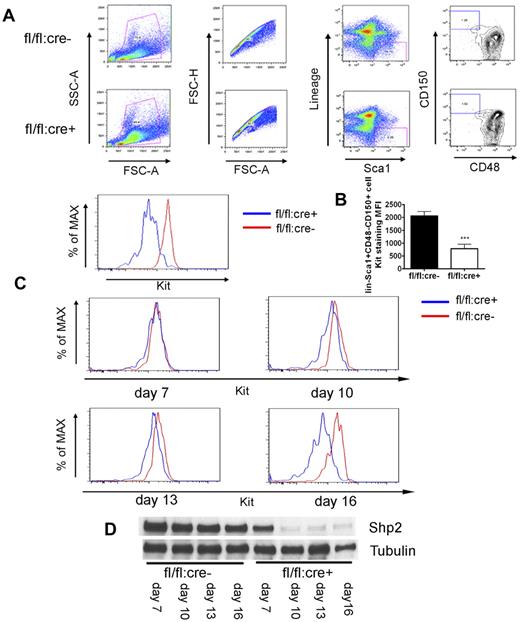
In exploring the molecular mechanism underlying the HSC deficiency induced by Shp2 deletion, we noticed severe ablation of Kit+ cells in the lineage− cell pool (Figure 2A) that could not be simply explained by the moderate increase in the progenitor cell apoptosis. Therefore, we tested the hypothesis that Shp2, although operating downstream of Kit receptor in cell signaling, may also mediate intracellular signaling pathways controlling Kit expression. To examine Kit expression in a stem cell pool, we used the signaling lymphocytic activation molecule (SLAM) markers CD150 and CD48 staining for further enrichment of HSCs (lin−Sca1+CD150+CD48−). As shown in Figure 6A, wild-type lin−Sca1+CD150+CD48− cells were Kit+, as expected. In contrast, Kit expression was significantly lower in lin−Sca1+CD150+CD48− cells isolated from Shp2Δ/Δ BM.
Kit down-regulation in SLAM marker-labeled HSCs. (A) Representative FACS plots for lin−Sca1+CD48−CD150+ staining 4 weeks post final injection indicates severe reduction of Kit expression in SLAM marker-enriched HSCs from fl/fl:cre+ BM. (B) Median fluorescence intensity (MFI) for Kit staining of lin−Sca1+CD48−CD150+ cells (n = 4). (C) FACS histogram plots illustrating Kit staining of lin−Sca1+CD48−CD150+ cells at different time points after first poly-I:C injection, indicating immediate Kit down-regulation after acute Shp2 deletion. (D) Immunoblotting of BM cells after first poly-I:C injection showed gradually increased deletion from day 7 to 16. Tubulin was used as loading control. ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 4.
Kit down-regulation in SLAM marker-labeled HSCs. (A) Representative FACS plots for lin−Sca1+CD48−CD150+ staining 4 weeks post final injection indicates severe reduction of Kit expression in SLAM marker-enriched HSCs from fl/fl:cre+ BM. (B) Median fluorescence intensity (MFI) for Kit staining of lin−Sca1+CD48−CD150+ cells (n = 4). (C) FACS histogram plots illustrating Kit staining of lin−Sca1+CD48−CD150+ cells at different time points after first poly-I:C injection, indicating immediate Kit down-regulation after acute Shp2 deletion. (D) Immunoblotting of BM cells after first poly-I:C injection showed gradually increased deletion from day 7 to 16. Tubulin was used as loading control. ***P < .001, **P < .01,*P < .05, error bars are SD; see also supplemental Figure 4.
To determine whether acute deletion of Shp2 results in down-regulation of Kit expression directly, we measured the kinetics of Kit expression decrease at different time points after poly-I:C injection. fl/fl:cre− and fl/fl:cre+ mice were given 3-5 doses of poly-I:C at 2-day intervals and BM was collected beginning from 7 days after the first injection. As shown in Figure 6D, immunoblotting of marrow samples showed a progressive increase of Shp2 deletion from day 7 (approximately 50%) to almost 100% on day 16. Interestingly, the decrease in Kit expression of lin−Sca1+CD150+CD48− cells correlated well with the Shp2 deletion extent (Figure 6C). These data support the hypothesis that Shp2 is positively required for Kit expression in HSCs. To define an immediate effect of Shp2 removal on Kit expression, we made use of a retrovirus expressing Cre recombinase to delete Shp2 in vitro. As shown in supplemental Figure 4A, fl/fl BM exhibited loss of Kit expression in lineage− gated cells 48 hours after infection with the Cre-expressing virus. This result excludes the possibility of poly-I:C toxicity or BM microenvironment effect on Kit expression.
We also tested functionally retroviral Cre-infected BM cells in a transplantation assay. Control (+/+) or fl/fl BM cells infected with Cre-IRES-human-CD2 retrovirus were stained with antihuman-CD2 (hCD2) antibody to distinguish the positively infected cells. Because hCD2 transcription is under control by the same promoter for Cre, hCD2 detection on the cell surface was used as a marker for Cre-expressing cells. When examined 48 hours after infection, approximately 50% of +/+ or fl/fl cells were hCD2+ (supplemental Figure 4B, right). The mixture of hCD2+ and hCD2− BM cells was transplanted into lethally irradiated recipients. Strikingly, although hCD2+ control BM cells maintained a chimerism greater than 50%, hCD2+ cells of fl/fl origin were barely detectable (supplemental Figure 4B left). These results are consistent with the data obtained in poly-I:C-treated fl/fl:cre+ mice (Figures 2A, 3F) and support the notion that Shp2 removal suppressed HSC reconstitution capacity, independent of the BM microenvironment.
Shp2 modulates Kit expression through Gata2
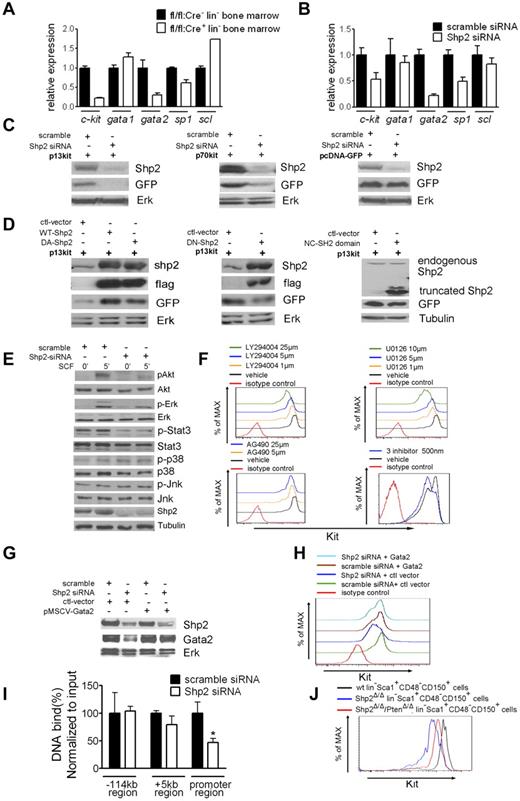
We then asked at which level Shp2 modulates Kit expression. Quantitative RT-PCR analysis demonstrated significantly reduced c-kit mRNA levels in Shp2Δ/Δ primary lineage− BM cells and also in Shp2 knockdown BM progenitor EML cells, compared with their respective controls (Figure 7A-B). These results point to a role of Shp2 in mediating intracellular signaling pathways that regulate Kit gene transcription. To test this possibility, we conducted a promoter reporter assay by using 2 constructs, p13kit and p70kit, which contain an EGFP reporter gene under control of Kit promoter only or promoter plus an enhancer sequence.24 We cotransfected HEK-293 cells with Shp2-specific siRNA or scramble siRNA with p13kit, p70kit, and control EGFP constructs, respectively, and examined the reporter expression.
Shp2 modulates Kit expression through Gata2. (A, B) qRT-PCR of Kit and Kit regulatory transcription factors Scl, Sp1, Gata2, and Gata1 mRNA demonstrated consistent suppression of Kit and Gata2 transcription in primary Shp2Δ/Δ lineage− cells and Shp2 knockdown EML cells. β-actin was used as a reference gene for normalization. (C) Shp2 knockdown repressed Kit promoter activity. Constructs with Kit regulatory elements fusion to GFP reporter were transfected into HEK293 cells. Shp2 knockdown by specific siRNA lead to GFP down-regulation in both p13kit and p70kit transfectants but not GFP vector transfectant. Erk was used as loading control. (D) Enforced expression of flag-tagged wild-type (WT) and dominant-active (DA) Shp2-enhanced, dominant-negative (DN) Shp2 suppressed Kit promoter activity, with no effect for truncated Shp2 containing the 2 SH2 domains. Erk or Tubulin was used as loading control. (E) Signaling modification in Shp2 knockdown EML cells elicited by SCF. Tubulin was used as loading control. (F) Treatment of EML cells with PI3K inhibitor LY294004, MEK inhibitor U0126, or a low dose (500 nm) of LY294004, U0126, and STAT3 inhibitor AG490 led to down-regulation of Kit expression on cell surface. (G) Shp2 knockdown in EML cells suppressed Gata2 protein expression. Tubulin was used as loading control. (H) Ectopic expression of Gata2 rescued Kit down-regulation in Shp2 knockdown EML cells. (I) Quantitative analysis of Gata2 binding to the −114 kb, +5 kb, and promoter region of Kit gene by the CHIP assay. Binding of Gata2 to Kit promoter but not −114 kb or +5 kb region was significantly reduced in Shp2 knockdown EML cells. *P < .05; error bars are SD. (J) Kit staining profile of wt lin−Sca1+CD48−CD150+ cells, Shp2Δ/Δ lin−Sca1+CD48−CD150+ cells, Shp2Δ/Δ/PtenΔ/Δ lin−Sca1+CD48−CD150+ cells. At least 3 animals were examined in each group. Representative FACS plot was shown; see also supplemental Figure 5.
Shp2 modulates Kit expression through Gata2. (A, B) qRT-PCR of Kit and Kit regulatory transcription factors Scl, Sp1, Gata2, and Gata1 mRNA demonstrated consistent suppression of Kit and Gata2 transcription in primary Shp2Δ/Δ lineage− cells and Shp2 knockdown EML cells. β-actin was used as a reference gene for normalization. (C) Shp2 knockdown repressed Kit promoter activity. Constructs with Kit regulatory elements fusion to GFP reporter were transfected into HEK293 cells. Shp2 knockdown by specific siRNA lead to GFP down-regulation in both p13kit and p70kit transfectants but not GFP vector transfectant. Erk was used as loading control. (D) Enforced expression of flag-tagged wild-type (WT) and dominant-active (DA) Shp2-enhanced, dominant-negative (DN) Shp2 suppressed Kit promoter activity, with no effect for truncated Shp2 containing the 2 SH2 domains. Erk or Tubulin was used as loading control. (E) Signaling modification in Shp2 knockdown EML cells elicited by SCF. Tubulin was used as loading control. (F) Treatment of EML cells with PI3K inhibitor LY294004, MEK inhibitor U0126, or a low dose (500 nm) of LY294004, U0126, and STAT3 inhibitor AG490 led to down-regulation of Kit expression on cell surface. (G) Shp2 knockdown in EML cells suppressed Gata2 protein expression. Tubulin was used as loading control. (H) Ectopic expression of Gata2 rescued Kit down-regulation in Shp2 knockdown EML cells. (I) Quantitative analysis of Gata2 binding to the −114 kb, +5 kb, and promoter region of Kit gene by the CHIP assay. Binding of Gata2 to Kit promoter but not −114 kb or +5 kb region was significantly reduced in Shp2 knockdown EML cells. *P < .05; error bars are SD. (J) Kit staining profile of wt lin−Sca1+CD48−CD150+ cells, Shp2Δ/Δ lin−Sca1+CD48−CD150+ cells, Shp2Δ/Δ/PtenΔ/Δ lin−Sca1+CD48−CD150+ cells. At least 3 animals were examined in each group. Representative FACS plot was shown; see also supplemental Figure 5.
As shown in Figure 7C, Shp2 knockdown strongly repressed GFP expression in cells cotransfected with p13kit and p70kit constructs but not the control vector. We also examined the Shp2 effect on Kit expression by cotransfection of p13kit with constructs expressing wild-type, dominant-active, or dominant-negative mutants. As expected, expression of wild-type Shp2 and dominant-active Shp2 enhanced the Kit reporter expression, and dominant-negative Shp2 severely suppressed the GFP intensity (Figure 7D). Consistently, ectopic expression of a truncated Shp2 that contains the 2 SH2 domains did not increase the Kit reporter GFP expression, suggesting the requirement of the PTPase activity. Together, these data indicate that Shp2 modulates the transcription of Kit in a phosphatase-dependent manner.
Previous biochemical analyses suggest Shp2 participation in intracellular signaling events immediately downstream of the Kit receptor.25 We assessed the impact of Shp2 deletion on various signaling pathways after c-kit activation by its ligand SCF. As shown in Figure 7E, SCF-stimulated p-Akt and p-Erk signals were impaired in EML cells after Shp2 knockdown. Shp2 silencing also decreased the basal level of pY-Stat3. p-JNK was unchanged, whereas p-p38 was modestly enhanced in Shp2 knockdown cells. Consistently, previous data from this and other groups have demonstrated positive roles of Shp2 in regulation of Erk and PI3K/Akt pathways.21,26 We then evaluated the effects of pathway-specific inhibitors to determine whether these signaling defects are responsible for decreased Kit expression. Treatment of EML cells with PI3K inhibitor LY294002 or MEK inhibitors U0126 led to Kit down-regulation in a dose-dependent manner. Moderate reduction of Kit expression was also detected when using a dosage (25 μm) of JAK2 inhibitor AG490. Combined treatment of these 3 inhibitors resulted in suppression of Kit expression at a very low dosage (Figure 7F, supplemental Figure 5B).
The defect in Kit gene transcription in Shp2-deficeint cells must be because of altered activity/expression in transcription factors. To address this issue, we performed qRT-PCR analysis to assess the expression of Gata1, Gata2, Scl, and Sp1, transcription factors known to regulate Kit promoter, in both primary lineage− BM and EML cells.27-30 Gata2 mRNA was decreased in Shp2Δ/Δ lineage− BM and Shp2 knockdown EML cells (Figure 7A-B). We further performed the qRT-PCR with sorted 34−LSK and 34+LSK cells, and consistent reduction of Gata2 mRNA was observed in Shp2Δ/Δ 34−LSK cells (supplemental Figure 5A). Immunoblotting results confirmed the decrease of Gata2 protein in Shp2 knockdown EML cells (Figure 7G). We further performed a ChIP assay to measure directly the amounts of Gata2 associated with the cis-regulatory elements of Kit gene.31,32 Shp2 knockdown did not affect Gata2 binding to −114 kb and +5 kb region of Kit gene but led to decreased Gata2 binding to promoter region (Figure 7I), and similarly, impaired Gata2 binding was observed when the 3 chemical inhibitors (LY294002, U0126, AG490) were used alone or in combination (supplemental Figure 5C).
Furthermore, enforced Gata2 expression rescued the phenotype of defective Kit expression in EML cells induced by Shp2 knockdown (Figure 7H). Consistently, the authors of a recent report documented regulation of Gata2 activity by Akt,33 suggesting a Shp2/PI3K/Akt/Gata2/Kit pathway. Indeed, combined deletion of Shp2 and Pten, a negative regulator of the PI3K/Akt pathway, partially restore the Kit expression in lineage− cells and SLAM marker–enriched HSCs. (Figure 7J). Therefore, although involvement of other transcription factors are not excluded, data presented here suggest a role of Shp2 in promoting Kit expression at least partially via Gata2.
Discussion
Previous investigators have shown that the cytoplasmic enzyme Shp2 physically associates with SCF-activated Kit via docking of the 2 SH2 domains on autophosphorylated tyrosyl residues in hematopoietic cells,25,34 suggesting its participation in signal relay immediately downstream of Kit. However, the biologic significance of Kit-Shp2 signaling is unclear. Here we present data suggesting a novel signaling mechanism in which Shp2 regulates HSC/progenitor activities at least in part via mediating signals controlling Kit gene expression.
A positive feedback loop consisting of Kit-Shp2-Kit
It is interesting to note that depletion of LSK cells in Shp2Δ/Δ BM was accompanied by selective loss of Kit+, but not Sca1+, subpopulation in the lin− pool. We further observed defective Kit expression in SLAM marker-labeled lin−Sca1+CD150+CD48− HSCs afterShp2 ablation. This down-regulation is an acute effect and parallels well with the kinetics of Shp2 deletion. Loss of Kit expression likely contributes to the survival defect in Shp2Δ/Δ HSCs/progenitors because the SCF/Kit pathway is required to prevent HSC apoptosis.11,35,36 The aberrant cell-cycle entry of Shp2Δ/Δ HSCs also phenocopied the disturbed HSC quiescence in viable White Spotting 41 (KitW41/W41) mice, as reported recently.10 Therefore, this study elucidates a novel molecular signaling mechanism involving a circuit of Kit-Shp2-Kit in adult HSCs, in which the receptor tyrosine kinase Kit controls its own gene expression via signaling through Shp2, an intracellular tyrosine phosphatase.
The biochemical mechanisms underlying functions of Shp2, a widely expressed enzyme, have been extensively investigated recently, leading to identification of multiple substrates in its regulation of Erk, PI3K, and Stat pathways.21,26 Molecular data shown here suggest that Shp2 mediates signaling through these pathways to transcription factors such as Gata2 in control of Kit mRNA transcription. It is likely that other transcription factors such as Egr1 and Ets1 may also be involved in Shp2 regulation of Kit expression (H.H.Z., K.J., N.A., Z.H., S.L., W.L., D.E.-Z., L.L., and G.S.-F, unpublished data, 2010).
Coordinated regulation of embryonic and adult hematopoiesis by Shp2
Although much has been learned about transcription factors required for HSC commitment, self-renewal and differentiation, relatively little is known about intracellular signaling pathways in control of HSC activities. Our previous studies suggested a critical role of Shp2 in HSC fate specification/differentiation during mouse embryogenesis, using mouse ES cells homozygous for exon 3 deletion.37-40 The heterozygous mice (exon 3+/Δ) did not display hematopoietic defects, but compromised reconstitution capacity of LSK cells was seen under stress condition after transplantation,41 which could be because of a developmental problem that arose during embryonic hematopoiesis. In this study, we developed a new mutant mouse line with Ptpn11/Shp2 deleted in the hematopoietic compartment in adults and uncovered a functional requirement for Shp2 in maintaining a functional HSC/progenitor pool in adult mammals. Together, these results suggest a concerted mechanism for control of embryonic and adult hematopoiesis by Shp2 in mammals. This is in contrast to the distinct requirement for Runx1 in the emergence and maintenance of HSCs in embryos and adults, respectively.42-44 Deletion of Shp2 not only resulted in reduced survival of HSCs but also led to impaired differentiation capacity of surviving HSCs/progenitors, as indicated by the CFU assay in vitro. Combined defects in survival and function of HSCs clearly contribute to the severe defects observed in multiple lineages of peripheral blood cells in poly-I:C–treated fl/fl:cre+ mice.
Several mechanisms are likely involved in Shp2 function in maintaining the adult HSC pool. Increased apoptosis rate is evidently responsible for reduced number of HSCs in Shp2-deficient BM. Impaired survival capacity may push quiescent HSCs forced into the cell cycle, as revealed by decrease of Shp2Δ/Δ LSK cells in G0 accompanied by increase in the S phase. Thus, the enhanced cell-cycle entry of Shp2Δ/Δ LSK cells is likely a compensatory mechanism, and this observation may not be contradictory to previous data suggesting a positive role of Shp2 in mitogenic signaling.12,26 We also present evidence here that Shp2 enhances HSC homing partially by regulating the chemotactic response to SDF-1α. Consistently, Shp2 has been shown to regulate cell motility by modulating FAK signaling.45-47 It is very likely that Shp2 coordinates multiple aspects of the homing process, including migration back to BM, extravasation to hematopoietic cord, and establishment of the HSC/niche interactions.
Opposite effects of Shp2 in embryonic and adult stem cells
The Shp2 function in HSC self-renewal and maintenance is also conserved in neural stem cells (NSCs).48 Conditional deletion of Shp2 in the brain driven by nestin-cre almost abolished the self-renewing expansion of NSCs in vitro. Thus, this signaling molecule plays a critical role in modulating pathways required for sustaining adult stem cells, such as NSCs and HSCs. Interestingly, the role of Shp2 in supporting these adult stem cells is opposite to its action in ESCs. We and others have found that homozygous Shp2 deletion or disruption of Shp2 signaling severely suppressed mouse ESC differentiation, accompanied by increased self-renewal.37,39,49,50 A similar function of Shp2 in promoting differentiation has been found in human ESCs, as Shp2 knockdown also suppressed differentiation with improved self-renewal.50 Therefore, distinct intracellular signaling mechanisms exist in regulating self-renewal and maintenance of embryonic or adult stem cells, which are yet to be elucidated.
Shp2 in hematopoiesis and leukemogenesis
The majority of leukemia-associated PTPN11 mutations disrupt the autoinhibitory interactions between its N-terminal SH2 and the phosphatase domain, resulting in elevated catalytical activity. Expression of these mutants in BM cells induced hyperproliferation and hypersensitivity of myeloid progenitors to GM-CSF and interleukin-3. Transplantation of Shp2E76K- or Shp2D61Y-expressing BM cells elicited fatal myeloid proliferative disease in recipient mice,18 and knock-in mice bearing an inducible Shp2D61Y allele displayed a similar phenotype.19 It has been recently shown that LSK cells, but not lineage-committed progenitors from Shp2D61G knock-in mice, are able to initiate myeloproliferative disease in recipient mice.20 Despite the leukemogenic property of activating PTPN11 mutations, we show in this study that Shp2 plays an essential role in maintaining the normal function of HSCs. Therefore, precise regulation of Shp2 level is pivotal for the hematopoietic homeostasis. Effective treatment of leukemia requires specific targeting of a drug to the mutant but not the wild-type Shp2 enzyme, to minimize the side effect of BM suppression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Michael David, Mark Kamps, Kenneth Kaushansky, and Robert Rickert for insightful suggestion and reagents. We thank Yoav Altman, Xi He, and Andy Chen for technical assistance and our colleagues for helpful discussion and support.
This work was supported by National Institutes of Health grant no. R01HL096125.
National Institutes of Health
Authorship
Contribution: H.H.Z. designed and performed experiments, collected data, analyzed results, and wrote the paper; K.J., N.A., Z.H., S.L., and W.L. performed experiments; D.-E.Z. and L.L. provided reagents and designed experiments; and G.-S.F. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gen-Sheng Feng, PhD, Department of Pathology, School of Medicine, University of California San Diego, 9500 Gilman Dr, MC 0864, La Jolla, CA 92093-0864; e-mail: gfeng@ucsd.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal