Abstract
To reduce doxorubicin, bleomycin, vinblastine and dacarbazine toxicity, the Cancer and Leukemia Group B conducted a phase 2 trial of doxorubicin, vinblastine, and gemcitabine for newly diagnosed, nonbulky stages I and II Hodgkin lymphoma. Ninety-nine assessable patients received 6 cycles of doxorubicin 25 mg/m2, vinblastine 6 mg/m2, and gemcitabine 800 mg/m2 (1000 mg/m2 in first 6) on days 1 and 15 every 28 days. Computed tomography (CT) and positron emission tomography (PET) were performed before and after 2 and 6 cycles. Complete remission (CR)/CR unconfirmed was achieved in 72 of 99 patients (72.7%) and partial remission in 24 of 99 patients (24.2%). The CR rate was 81% when using PET criteria. Two patients have died of Hodgkin lymphoma progression. Median follow-up for nonprogressing patients is 3.3 years. The progression-free survival (PFS) at 3 years was 77% (95% confidence interval, 68%-84%). The relapse rate was less than 10% for patients with favorable prognostic factors. The 2-year PFS for cycle 2 PET-negative and -positive patients was 88% and 54%, respectively (P = .0009), compared with 89% and 27% for cycle 6 PET-negative and -positive patients (P = .0001). Although the CR rate and PFS were lower than anticipated, patients with favorable prognostic features had a low rate of relapse. Cycle 2 PET and cycle 6 PET were predictive of PFS. This clinical trial is registered at www.clinicaltrials.gov as #NCT00086801.
Introduction
Most patients with early-stage Hodgkin lymphoma (HL) are cured with treatment strategies, including radiation therapy (RT), chemotherapy such as doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), or combined modality treatment,1 with relatively similar results.2,3 Thus, the current challenge is to maintain excellent treatment results while reducing the serious and potentially life-threatening early and late toxicities. RT has been the major cause of late morbidity and mortality related to treatment.4-6 Pulmonary toxicity from bleomycin has been the major cause of acute toxicity and rare mortality with ABVD.2,7 Gemcitabine is one of the most active single agents for relapsed and refractory HL with response rates of 39% to 43%.8,9 An impressive overall response rate of 70% with 19% complete remissions (CRs) was achieved in a Cancer and Leukemia Group B (CALGB) phase 2 trial of gemcitabine combined with vinorelbine and pegylated liposomal doxorubicin.10 Based on these results, CALGB conducted a phase 2 trial of a variant of ABVD, doxorubicin, vinblastine, and gemcitabine (AVG), for newly diagnosed patients with stage I and II nonbulky classic HL in which gemcitabine replaced bleomycin, and dacarbazine was omitted because of the limited phase 2 data documenting its activity.11 The goal was to assess the CR rate and progression-free survival (PFS) of AVG without RT with an attempt to reduce acute and long-term toxicity. A secondary objective was to evaluate positron emission tomography (PET) in predicting outcome after 2 cycles of AVG and at the completion of treatment because several reports have suggested that early interim PET is highly predictive of PFS.12-15
Methods
Twenty-nine CALGB institutions enrolled patients on this phase 2 clinical trial. The institutional review board of each institution approved the protocol, and all patients gave written informed consent in accordance with the Declaration of Helsinki.
Patient population
Previously untreated patients with histologically documented, nonbulky, classic HL with clinical stages I or II, and measurable disease on physical examination or imaging studies were enrolled. The diagnosis of classic HL by site pathologists was required for enrollment. The diagnosis was subsequently confirmed by central pathology review requiring agreement by 2 hematopathologists after independent review of hematoxylin and eosin stains, pathology report, and selected immunohistochemical stains when required.
On-study procedures
At time of enrollment, patients underwent history and physical examination, laboratory studies including complete blood count, differential count, erythrocyte sedimentation rate (ESR), serum creatinine, glucose, aspartate aminotransferase, alkaline phosphatase, bilirubin, lactic acid dehydrogenase, HIV serology if there were risk factors, left ventricular ejection fraction determination by echocardiogram or multigated acquisition scan, pulmonary function tests including diffusing capacity of the lung for carbon monoxide (DLCO) and forced vital capacity, and bone marrow biopsy. Pretreatment imaging studies consisted of chest x-ray, computerized tomography (CT) of the chest, abdomen, and pelvis, and PET. Complete blood count was performed on day 1 and day 15 of each cycle as well as serum creatinine, aspartate aminotransferase, alkaline phosphatase, and bilirubin on day 1. CT of chest, abdomen, and pelvis and PET was performed after 2 cycles of AVG for interim response assessment and after completion of cycle 6. Pulmonary function tests were also repeated after the fourth cycle of AVG.
Protocol treatment
Each cycle of AVG consisted of doxorubicin 25 mg/m2, vinblastine 6 mg/m2, and gemcitabine 1000 mg/m2 administered intravenously on days 1 and 15. Based on hematologic toxicity observed in the first 6 patients as stipulated in the protocol, subsequent patients received gemcitabine at a dose of 800 mg/m2 intravenously per protocol-specified dose de-escalation. Cycles were repeated every 28 days for a total of 6 cycles. Toxicity was assessed according to the Common Terminology Criteria for Adverse Events, Version 3.0. If the absolute neutrophil count (ANC) was < 1000/μL on a treatment day, treatment was delayed for one week to allow ANC recovery; or if there was an episode of febrile neutropenia (temperature ≥ 38.5°C with ANC < 500/μL), filgrastim, peg-filgrastim, or sargramostim was administered prophylactically with subsequent treatments according to institutional guidelines. Dose reductions to 75% of gemcitabine, vinblastine, and doxorubicin were made for platelet counts ≤ 75 000/μL. Doxorubicin and vinblastine doses were reduced to 50% for bilirubin > 1.5 and ≤ 3.0 mg/dL, and doxorubin and vinblastine doses were reduced to 25% and gemcitabine dose to 50% for bilirubin > 3.0 and ≤ 5.0 mg/dL. Vinblastine dose was decreased to 75% for grade 2 and 50% for grade 3 peripheral neuropathy or constipation. Doses of doxorubicin and vinblastine were held for ≥ grade 3 mucositis, dysphagia, or diarrhea and restarted at 75% of full dose when toxicity cleared. Gemcitabine-related pneumonitis ≥ grade 2 prompted discontinuation of gemcitabine and removal from protocol therapy.
Response assessment
Responses were determined after 2 cycles and after completion of AVG (6 cycles) by history and physical examination, routine blood test, and contrast-enhanced CT scans of the chest, abdomen, and pelvis according to the 1999 version of the International Lymphoma Workshop (IWG) criteria.19 A secondary determination of response was performed according to the PET criteria of the International Harmonization Project of the IWG for response criteria for malignant lymphomas.20
PET data analysis.
PET data that were obtained before treatment, 1 to 2 weeks after 2 and after 6 cycles of AVG were centrally read by 2 independent reviewers and an adjudicator, all of whom were blinded to each other and to the clinical information. Visual assessment was performed using International Harmonization Project criteria.20 Analysis of response on PET was separate and not an official criterion for treatment response.
Statistical considerations.
Patient registration and data collection were managed by the CALGB Statistical Center. This study was designed to accrue a maximum of 98 patients for a one-sided α of 10% with respect to 85% CR rate and 80% power with respect to 92% CR rate. All submitted data were reviewed by the staff of the Statistical Center and the study chair. Analyses were performed by CALGB statisticians. Members of the Audit Committee visit all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements, including eligibility, treatment, adverse events, response, and outcome. Such on-site review of medical records was performed for 27 (27%) of the 104 participants registered to this study. The Kaplan-Meier method21 was used to estimate PFS measured from the time of trial entry until progression or death. Toxicity was reported according to National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0. Two-sided log-rank test22 was used to compare PFS between PET-negative and PET-positive patient groups. The dose intensity (DI) of each agent was calculated by dividing the total received dose by the number of weeks of treatment. The relative total dose intensity of each agent was calculated by expressing the total dose of agent per week (relative total dose intensity) as a percentage of the target dose per week. The relative dose intensity (RDI) was calculated by averaging the relative total dose intensities of the 3 agents over all treatment courses.23
Endpoints
The primary endpoint of this trial was complete response defined as CR or CR unconfirmed after 6 cycles of chemotherapy. PFS measured from study entry until relapse was a secondary endpoint. Residual disease was assessed among the PET-positive patients by biopsy, if clinically feasible, or by following until relapse. Toxicity endpoints included grade 3 or greater myelosuppression.
Determination of CR rate
A target of 98 patients treated was to be enrolled in this trial and treated with AVG. The null hypothesis (H0) that the CR rate (P) is ≤ .85 versus the alternative (HA) that P ≥ .92 was tested while monitoring for lack of efficacy. Once 98 patients were accrued, the treatment was to be considered efficacious if at least 88 patients achieve a CR. Based on simulations, the approximate significance level and power under this design are .10 and .80, respectively, to test the null hypothesis that the CR rate is 85% versus the alternative that it is 92%.
Predictive value of FDG-PET
The positive predictive values (number of PET-positive progressions/number of PET-positive) and negative predictive values (number of PET-negative remissions/number of PET-negative) for patients with PET (after 2 and 6 cycles of therapy) for 2-year PFS were estimated. One-sided P values were determined in comparisons of sensitivity (number of PET-positive progressions/number of progressions) and specificity (number of PET-negative remissions/number in remission) of cycle 2 PET and cycle 6 PET.24
Results
Patient characteristics
A total of 104 patients were enrolled between May 15, 2004 and September 29, 2006; 5 were never treated (2 withdrew consent, 1 non-Hodgkin lymphoma, 1 stage III, 1 poor liver function). Thus, 99 patients were assessable for outcomes and toxicity. There were 49 males and 50 females. The median age was 37 years (range, 18-80 years). Stages and histologies are listed in Table 1. Adverse risk factors for stage I and II disease included age ≥ to 40 years in 44 of 99 (44%), ≥ 3 sites of involvement in 63 of 93 (68%), and ≥ 4 sites of involvement in 42 of 93 (45%).
Patient characteristics
| . | No. (%) of patients . |
|---|---|
| Stage | |
| IA | 10 (10.1) |
| IIA | 70 (70.7) |
| IIB | 19 (19.2) |
| Histology (central pathologic review) | |
| NS | 68 (73.1) |
| MC | 14 (15.0) |
| cHL-u | 10 (10.8) |
| L-R cHL | 1 (1.1) |
| Missing | 6 |
| . | No. (%) of patients . |
|---|---|
| Stage | |
| IA | 10 (10.1) |
| IIA | 70 (70.7) |
| IIB | 19 (19.2) |
| Histology (central pathologic review) | |
| NS | 68 (73.1) |
| MC | 14 (15.0) |
| cHL-u | 10 (10.8) |
| L-R cHL | 1 (1.1) |
| Missing | 6 |
NS indicates nodular sclerosis; MC, mixed cellularity; cHL-u, classic Hodgkin lymphoma-unspecified; and L-R cHL, lymphocyte-rich classic Hodgkin lymphoma.
Prognostic features are summarized in Table 2. For patients with complete data, 69 of 80 (86.2%) were in an unfavorable category according to the criteria of the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) and Eastern Cooperative Oncology Group that include age ≥ 40 years, ESR ≥ 50 mm/hr for stage IA and IIA and ≥ 30 mm/hr for stages IB and IIB, mixed cellularity histology, and ≥ 4 sites of involvement.3 According to the criteria of the European Organization for Research and Treatment of Cancer (EORTC), which include age ≥ 40 years, male gender, stage, number of sites of involvement, B symptoms, ESR, and histology, 62 of 86 (72.1%) were in an unfavorable category.17 For the criteria of the German Hodgkin Lymphoma Study Group (GHSG), which includes ≥ 3 sites of involvement, large mediastinal mass, extranodal extension of nodal disease, and elevated ESR, 66 of 86 (76.7%) were in an unfavorable category.18
Risk factors and outcome
| Risk group . | n (%) . | No. (%) of progressions . |
|---|---|---|
| EORTC | ||
| Favorable | 24 (27.9) | 2 (8.3) |
| Unfavorable | 62 (72.1) | 17 (27.4) |
| Missing | 13 | 4 |
| NCIC-CTG | ||
| Favorable | 11 (13.8) | 0 (0.0) |
| Unfavorable | 69 (86.2) | 17 (24.6) |
| Missing | 19 | 6 |
| GHSG | ||
| Favorable | 20 (23.3) | 4 (20.0) |
| Unfavorable | 66 (76.7) | 15 (22.7) |
| Missing | 13 | 4 |
| Risk group . | n (%) . | No. (%) of progressions . |
|---|---|---|
| EORTC | ||
| Favorable | 24 (27.9) | 2 (8.3) |
| Unfavorable | 62 (72.1) | 17 (27.4) |
| Missing | 13 | 4 |
| NCIC-CTG | ||
| Favorable | 11 (13.8) | 0 (0.0) |
| Unfavorable | 69 (86.2) | 17 (24.6) |
| Missing | 19 | 6 |
| GHSG | ||
| Favorable | 20 (23.3) | 4 (20.0) |
| Unfavorable | 66 (76.7) | 15 (22.7) |
| Missing | 13 | 4 |
Response, relapse, and survival
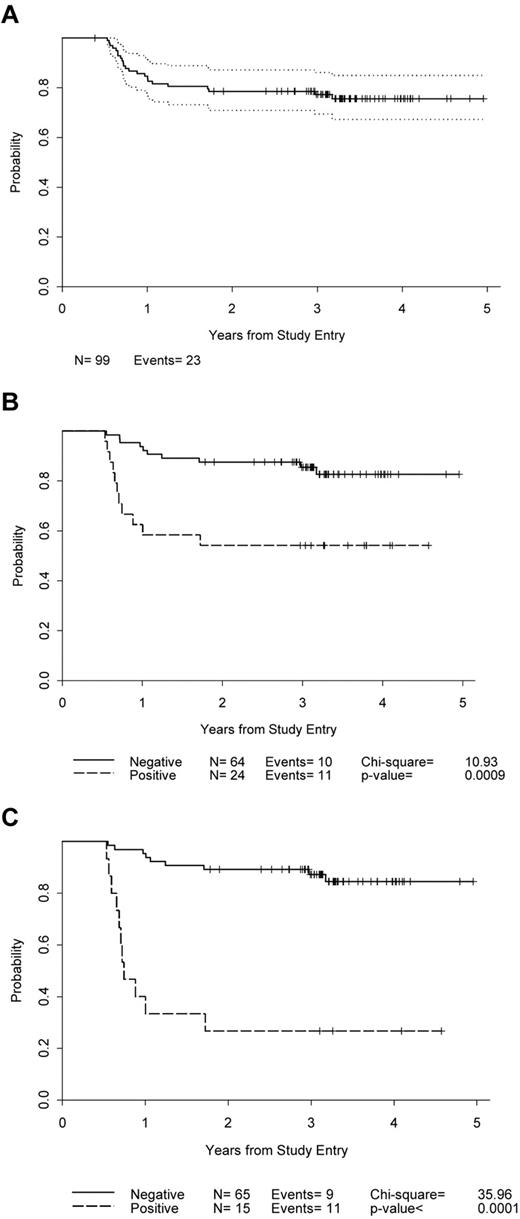
Of the 99 patients, 72.7% achieved CR or CR unconfirmed (Table 3). By current IWG criteria20 using PET, 81% (65 of 80) achieved CR (Table 4). Nine patients received less than 6 cycles of AVG: 1 progressed on treatment, 2 withdrew for unclear reasons, and 6 experienced adverse events. The median follow-up time for patients who have not progressed is 3.3 years (95% confidence interval, 0.4-5.0) and the 3-year PFS is 77% (95% confidence interval, 68%-84%) (Figure 1A). There were 23 relapses, 18 of which were documented by biopsy. Fifteen relapses were in primary sites, 7 in both primary and new sites, and 1 in a new site only. There were 24.6% of patients who relapsed in the unfavorable group using NCIC-CTG/Eastern Cooperative Oncology Group criteria compared with 0 in the favorable group, whereas 27.4% relapsed in the unfavorable group using EORTC criteria, compared with 8.3% in the favorable group (Table 2). By actuarial analysis, the differences in PFS between patients in favorable and unfavorable groups with both criteria approached, but did not achieve, statistical significance (P = .08 for both comparisons). No differences in relapses or PFS were seen between patients divided into favorable and unfavorable groups in the GHSG model, which uses only number of sites of involvement (1or 2 vs ≥ 3) and not age. None of our patients had bulky disease or extranodal extensions, 2 other risk factors used in the GHSG model. Two patients have died of progressive disease after relapsing.
Response and relapses
| . | Response, n (%) . | Relapsed, n (%) . |
|---|---|---|
| CR + CRu | 72 (72.7) | 13 (18.1) |
| PR | 24 (24.2) | 7 (29.2) |
| Stable | 3 (3) | 3 (100) |
| Total | 99 | 23 (23.2) |
| . | Response, n (%) . | Relapsed, n (%) . |
|---|---|---|
| CR + CRu | 72 (72.7) | 13 (18.1) |
| PR | 24 (24.2) | 7 (29.2) |
| Stable | 3 (3) | 3 (100) |
| Total | 99 | 23 (23.2) |
PR indicates partial response.
PET results
| . | Patient status . | Total . | |
|---|---|---|---|
| In remission . | Progressed . | ||
| Cycle 2 PET | |||
| Negative | 54 | 10 | 64 |
| Positive | 13 | 11 | 24 |
| Total | 67 | 21 | 88 |
| Cycle 6 PET | |||
| Negative | 56 | 9 | 65 |
| Positive | 4 | 11 | 15 |
| Total | 60 | 20 | 80 |
| . | Patient status . | Total . | |
|---|---|---|---|
| In remission . | Progressed . | ||
| Cycle 2 PET | |||
| Negative | 54 | 10 | 64 |
| Positive | 13 | 11 | 24 |
| Total | 67 | 21 | 88 |
| Cycle 6 PET | |||
| Negative | 56 | 9 | 65 |
| Positive | 4 | 11 | 15 |
| Total | 60 | 20 | 80 |
PFS. (A) All enrolled patients. (B) Patients according to PET after 2 cycles of chemotherapy. (C) Patients according to PET after 6 cycles of chemotherapy.
PFS. (A) All enrolled patients. (B) Patients according to PET after 2 cycles of chemotherapy. (C) Patients according to PET after 6 cycles of chemotherapy.
RDI
Overall, AVG was well tolerated with few dose reductions or significant delays in treatment. A total of 91.9% of all patients treated received the full 6 cycles of treatment. Only 9 patients had dose reductions. A total of 70.7% had one or more treatment delays, but most of these were 1 to 2 weeks overall, usually because of low ANC. Of these delays, 59 (59.6%) occurred after the first treatment cycle 1. One delay was the result of ileus and the rest to neutropenia. Subsequent delays were infrequent because of prophylactic use of growth factors to prevent neutropenia. Eighty-eight of the 91 patients receiving all 6 cycles did so within 28 weeks (ie, completed therapy on schedule or had minor delays). All patients who progressed received all 6 cycles of therapy. The median RDI was 97.2% with a range of 52.3% to 100.0%. Eight patients (8.1%) had RDIs < 85%, 16 (16.2%) had RDIs < 90%, and 36 (36.4%) had RDIs < 95%. Using a proportional hazards model, RDI was not significantly associated with time to progression.
Results of PET during and after treatment
Cycle 2 PET.
PET results after 2 cycles of AVG were available for 88 patients (74 PET/CT and 14 PET alone; Table 4). The estimated 2-year probability of PFS for the PET-negative patients was 0.88 (0.77, 0.94), compared with 0.54 (0.33, 0.71) in the PET-positive group (Figure 1B). The positive predictive value was 11 of 24 = 45.8% (25.6%, 67.2%). The negative predictive value was 54 of 64 = 84.4% (73.1%, 92.2%). The sensitivity was 11 of 21 = 52.4% (29.8%, 74.3%), and the specificity was 54 of 67 = 80.6% (60.1%, 89.2%).
Cycle 6 PET after completion of treatment.
Results of PET scanning at the completion of 6 cycles of AVG are available for 80 patients (Table 4). The estimated 2-year PFS for PET-negative patients was 0.89 (0.79, 0.95) and 0.27 (0.08, 0.50) for those who were PET-positive (Figure 1C). The positive predictive value of cycle 6 PET was 11 of 15 = 73.3% (44.9%, 92.2%), and the negative predictive value was 56 of 65 = 86.1% (75.3%, 93.5%). The sensitivity was 11 of 20 = 55.0% (31.5%, 76.9%), and the specificity was 56 of 60 = 93.3% (83.8%, 98.2%).
Comparison of sensitivity and specificity of cycle 2 PET and cycle 6 PET.
Both cycle 2 and cycle 6 PET data were available for 80 patients. Cycle 6 and cycle 2 PET had similar sensitivity (P = .75), whereas cycle 6 PET was significantly more specific than cycle 2 PET (P = .01).
Adverse events
Ninety patients completed 6 cycles of AVG. Adverse events leading to withdrawal of 6 patients from treatment were: 1 patient grade 3 pulmonary toxicity, 2 patients grade 2 pulmonary toxicity (1 of whom also had grade 4 neutropenia and grade 3 thrombocytopenia), 1 patient grade 3 neutropenia, 1 patient grade 3 cardiac ischemia, and 1 patient grade 2 decrease in DLCO (20% decrease from baseline) with grade 3 thrombocytopenia and grade 4 neutropenia. In the first 6 patients, grade 3 neutropenia in 2 of 6 and grade 4 in 3 of 6 patients led to a reduction in the dose of gemcitabine from 1000 mg/m2 to 800 mg/m2 in the subsequent 93 patients as stipulated in the protocol. With gemcitabine at 800 mg/m2, neutropenia was the most frequent adverse event: 46% had grade 3 and 28% grade 4. Febrile neutropenia occurred in 3 patients (3%). One patient had grade 3 thrombocytopenia. Pulmonary toxicity was grade 1 in 28 patients (28%), grade 2 in 16 (16%), and grade 3 in 2 patients (1 pneumonitis who went on to complete treatment, 1 decreased DLCO who discontinued treatment; 2%). There was no grade 4 pulmonary toxicity. There were 2 other nonhematologic grade 4 adverse events (1 hyperuricemia, 1 hypocalcemia). The only other grade 3 nonhematologic adverse events seen in more than 3% of patients were elevations of alanine aminotransferase (6 patients; 6%) and pain felt to be possibly related to treatment in 8% (8 patients: 1 bone, 1 myalgia, 1 abdominal, 1 extremity, 1 esophageal, 2 chest [1 angina], 1 headache).
Discussion
The AVG regimen produced complete responses in 72.7% and durable remissions for 77% of patients with early-stage, nonbulky, classic HL. The CR/CR unconfirmed rate using the response criteria available at the time the study was designed19 failed to meet the protocol-defined efficacy endpoint of 85% to 92%. Using current PET-based IWG criteria,20 the CR rate was 81%. These results are inferior to most recent results in early-stage HL patients with combined modality treatment in favorable and unfavorable risk groups18,25 and ABVD alone.26-28 This finding may be at least partially explained by differences in the patient risk factors in the AVG trial compared with other reports in the literature.
Although a CR rate of 94% has been reported for 6 cycles of ABVD alone for patients with stages I, II, and IIIA HL,2,27 it is not clear whether the risk factors in the population of patients treated in the AVG trial were comparable with those reported for ABVD alone. The median age was higher (37 years) in the AVG trial than in 2 trials of ABVD alone trials for early-stage disease (33 and 30 years, respectively),2,27 and 44% of patients were at least 40 years old. In the AVG trial, 68% of patients had at least 3 and 45% at least 4 initial sites of involvement compared with 17% and 7%, respectively, in the ABVD trial that included only stages I and II.27 In the AVG trial, 72% of patients were in an unfavorable risk category according to EORTC criteria compared with 48% in the trial of ABVD alone for stage I and II HL.27
These adverse risk factors may have also affected the PFS seen with AVG. The 3-year PFS of 77% is slightly below the PFS of approximately 80% to 90% reported with ABVD alone in unselected nonbulky stage I and II patients.2,27-29 Only 2 patients have died. In the one ABVD trial where EORTC prognostic criteria were reported, 48% of stages I and II HL patients were in the unfavorable risk group27 compared with 72% in the AVG trial. In the AVG study, 8% of patients in the EORTC favorable risk category relapsed compared with 27% in the unfavorable risk category. In the AVG trial, the difference in PFS between patients in the EORTC favorable group compared with the unfavorable group approached but did not achieve statistical significance (P = .08), possibly because of small numbers (Table 2).
The results of PET imaging after 2 cycles of AVG and at the completion of treatment were also predictive of PFS. At 2 years, PFS was approximately 50% for patients who were PET-positive after 2 cycles compared with almost 90% for those who were PET-negative. The only published results with PET after 2 or 3 cycles of chemotherapy in stage I and II patients had only 7 of 57 patients who were PET-positive, 2 of whom relapsed compared with 1 relapse in 50 patients who were PET-negative. The chemotherapy was ABVD in the majority of these patients, and 42 of 57 also received RT after chemotherapy.12 Our results with AVG should be compared with other trials for stages I and II HL, which used chemotherapy only. Unfortunately, PET data for such trials are lacking. Although the percentage of stage I and II patients who are PET-positive after 2 or 3 cycles of ABVD (7 of 57, 12%)12 seems to be less than that with 2 cycles of AVG (24 of 88, 27%, Table 4), the PFS for patients who are PET-positive after 2 cycles of ABVD who never receive RT is unknown. It is noteworthy that stage I and II PET-negative patients after 2 cycles of AVG have a 2-year PFS close to 90%, which is comparable with published results with ABVD alone.2,3,26-28
The differences in 2-year PFS were greater between PET-positive and PET-negative patients after 6 cycles of AVG than after 2 cycles. The 2-year PFS for postcycle 6 PET-positive patients was 27% compared with 89% for cycle 6 PET-negative patients. The positive predictive value for PET at completion of treatment was increased to 73% compared with 46% for PET after 2 cycles of AVG. Cycle 6 PET had a sensitivity similar to cycle 2 PET, but with a significantly higher specificity. These findings contrast with the results reported comparing PET after 2 and 4 cycles and at the completion of anthracycline-containing chemotherapy for all HL stages where the PFS according to PET was similar at all 3 PET time points.13 Interpretation criteria for PET in that study appear to have been different from those used in the AVG trial. Recently, a retrospective analysis of interim PET during chemotherapy versus end-of-treatment PET in early-stage nonbulky HL patients demonstrated that end-of-treatment, but not interim, PET was highly predictive of PFS and overall survival.30 The patients had interim PET performed at various times during treatment, received 6 or 4 cycles of ABVD, and involved field (IF) RT was administered to 15 of 17 patients who were interim PET-positive. The addition of IF RT to patients with interim-positive PET may have improved their outcome and contributed to the lack of predictability of interim PET.
Excessive pulmonary toxicity has been reported for combination chemotherapy containing both gemcitabine and bleomycin,31,32 but it is difficult to compare the incidence of pulmonary toxicity of 6 cycles AVG with 6 cycles of ABVD. There were 2 patients (2%) with grade 3 pulmonary toxicity with AVG, and one of these was able to complete treatment. Two other patients with grade 2 pulmonary toxicity were withdrawn from treatment by decision of their treating physicians. With gemcitabine combined with vinorelbine and pegylated liposomal doxorubicin for relapsed HL, the model for AVG, in which most patients had received prior ABVD, approximately 40% prior autologous stem cell transplantation and an unknown number chest irradiation, the incidence of grade 3 pulmonary toxicity was 10% and grade 4, 2.5%. There was one fatality from alveolar hemorrhage in a patient who had received prior ABVD, mediastinal RT, and autologous stem cell transplantation.10 In the phase 2 study of 6 cycles of ABVD alone for newly diagnosed stages I and II HL, 1 patient (1%) had grade 1 and 1 patient (1%) grade 3 pulmonary toxicity.27 In the ABVD trial at Memorial Sloan-Kettering Cancer Center, pulmonary toxicity was not graded according to the Common Terminology Criteria for Adverse Events, but 22% of patients on ABVD had bleomycin discontinued during treatment because of a decrease in DLCO, 7% had pulmonary symptoms, and there was 1 death related to bleomycin.26 In the retrospective experience of the Mayo Clinic, 27% of patients developed bleomycin pulmonary toxicity with ABVD. The mortality resulting from bleomycin for all patients who received any initial regimen containing bleomycin was 4.2% and was 24% in patients who developed bleomycin pulmonary toxicity.7 It is difficult to draw conclusions from the relatively small number of patients in the AVG trial, but it is noteworthy that there was no instance of grade 4 pulmonary toxicity and no pulmonary fatalities.
Grade 3 and 4 neutropenia were the most frequent grade 3 and 4 adverse events and were comparable with those reported with ABVD.2 Data from observational trials for ABVD treatment have been recently reported. It was suggested that an RDI of less than or equal to 85% is suboptimal. Approximately 20% of patients receiving ABVD therapy have RDIs less than 85% compared with 8% with AVG. RDI has not been established as an important factor in determining remission in HL.33 There were few drug dose reductions, and the overall RDI was high and was not associated with time to progression in our study.
The aim of most recently completed and current clinical trials in early-stage HL has been to maintain high cure rates while reducing short- and long-term morbidity and mortality. One approach with combined modality therapy has been to reduce the number of cycles of chemotherapy and the size and doses of RT fields.18 In the HD10 trial of the GHSG, freedom from treatment failure rate at 5 years was 90% to 93% for 2 or 4 cycles of ABVD with both 20 or 30 Gy IF RT for favorable stage I and II HL patients (1 or 2 sites without bulk, extranodal extension, or elevated ESR). None of the trials with chemotherapy only was restricted to a comparable population of the most favorable early-stage HL patients, and results of this approach in such a population would be of interest. Furthermore, it is noteworthy that the rate of secondary neoplasia at a median follow-up of 7.5 years is 4.6%, similar in all 4 arms of the HD10 regimen, and it may increase with further follow-up. Secondary neoplasia rates at 20 years after more extensive RT have been approximately 20%.34 In addition, of 57 deaths (4.8%) in the HD10 trial, 10 were the result of HL, 11 secondary neoplasias, 9 cardiovascular events, 7 acute toxicity of treatment, and 5 salvage therapy.
Excellent results in early-stage unfavorable HL with combined modality treatment in the HD11 study of the GHSG, which compared 4 cycles of ABVD with 4 cycles of standard-dose bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procarbazine, and prednisone (BEACOPP) and then a second comparison of 30 Gy versus 20 Gy IF RT have recently been recently reported. The best 5-year freedom-from-treatment-failures were similar in 3 of the arms (ABVD times 4 + 30 Gy IF RT, 4 standard BEACOPP + 30 Gy IF RT, and 4 standard BEACOPP + 20 Gy IF RT). For the entire trial at 5 years, freedom-from-treatment-failure was 85.0%, overall survival 94.5%, and PFS 86%.25 These results are clearly a benchmark for comparison with other trials in similar populations. There have been 52 second cancers (3.7%) at a median follow-up time of 6.8 years, At a median follow-up time for survival of 7.6 years, 105 patients have died (7.5%). Causes of deaths were HL in 36% and other causes in 64% (18% second malignancies, 13% cardiovascular events). Both secondary neoplasia and cardiovascular morbidity and mortality are the most frequent late complications after RT for HL,5 and morbidity and mortality from both causes may increase with further follow-up time in the HD11 trial. Further follow-up will also be necessary to determine the long-term morbidity and mortality of combined modality programs in other trials with reduced chemotherapy and in more recent trials with further reduced “involved-nodal RT.”35,36
Chemotherapy alone is an alternative to combined modality therapy for nonbulky early-stage HL, which reduces the risk of late morbidity and mortality associated with RT.3,26-28 The AVG regimen attempted to reduce pulmonary toxicity by omitting bleomycin. There are data suggesting that dacarbazine, but not bleomycin, is a critical component of the ABVD regimen. It has been found that the outcome of patients for whom bleomycin had to be omitted from ABVD at some time during treatment because of toxicity have a similar outcome to those who received a full course of ABVD.2,7,37 In the current GHSG 4-arm HD13 trial comparing 2 cycles of chemotherapy with IF RT for favorable early-stage HL, the AV and ABV arms were closed because of “safety reasons,” whereas the ABVD and AVD arms remain open.38 The final results of this trial will be of interest, and other trials to look further at the AVD regimen could be considered.
Although the AVG results may be inferior those achievable with ABVD, it would require a randomized comparison with ABVD to establish its true efficacy. ABVD alone has results that are comparable with those with combined modality therapy in stages I and II HL, although there may be a slightly higher relapse rate. No difference in survival has been demonstrated.3 Current trials are investigating the use of chemotherapy only in most early-stage patients while reintroducing IF RT only for the minority who are PET-positive during treatment. The interim PET results during AVG treatment provide a rationale for this approach in HL patients with nonbulky stage I and II disease. The successor to the AVG study in the same patient population, CALGB 50604 (www.cancer.gov/clinicaltrials; NTC01132807), which employs interim PET to determine treatment, is currently open in the United States Intergroup.
In conclusion, results with AVG were below expectations, although few relapses were seen in patients with favorable prognostic features. An appreciation of pretreatment prognostic features is important in interpreting results of clinical trials in patients with stages I and II HL. Use of AVG outside the setting of clinical trials is not recommended, although accurate comparison of the efficacy of AVG versus ABVD with or without RT would require randomized trials with balanced prognostic factors. This study provides the largest prospectively obtained dataset, which demonstrates the value of interim and post-treatment PET in predicting PFS in early-stage nonbulky HL patients.
Presented in part at the Annual Meeting of the American Society of Hematology, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Appendix
Christiana Care Health Services, Inc, CCOP, Wilmington, DE (Stephen Grubbs, supported by CA45418); Dana-Farber Cancer Institute, Boston, MA (Harold J. Burstein, supported by CA32291); Georgetown University Medical Center, Washington, DC (Minetta C. Liu, supported by CA77597); Cancer Centers of the Carolinas, Greenville, SC (Jeffrey K. Giguere, supported by CA29165); Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY (Jeffrey Kirshner, supported by CA45389); Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO (Rakesh Gaur); Massachusetts General Hospital, Boston, MA (Jeffrey W. Clark, supported by CA32291); Memorial Sloan-Kettering Cancer Center, New York, NY (Clifford A. Hudis, supported by CA77651); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Rafat Ansari, supported by CA86726); Roswell Park Cancer Institute, Buffalo, NY (Ellis Levine, supported by CA59518); Southeast Cancer Control Consortium Inc, CCOP, Goldsboro, NC (James N. Atkins, supported by CA45808); State University of New York Upstate Medical University, Syracuse, NY (Stephen L. Graziano, supported by CA21060); Ohio State University Medical Center, Columbus, OH (Clara D. Bloomfield, supported by CA77658); University of California at San Francisco, San Francisco, CA (Charles J. Ryan, supported by CA60138); University of Chicago, Chicago, IL (Hedy L. Kindler, supported by CA41287); University of Iowa, Iowa City, IA (Daniel A. Vaena, supported by CA47642); University of Maryland Greenebaum Cancer Center, Baltimore, MD (Martin Edelman, supported by CA31983); University of Nebraska Medical Center, Omaha, NE (Anne Kessinger, supported by CA77298); University of North Carolina at Chapel Hill, Chapel Hill, NC (Thomas C. Shea, supported by CA47559); University of Vermont, Burlington, VT (Steven M. Grunberg, supported by CA77406); Wake Forest University School of Medicine, Winston-Salem, NC (David D. Hurd, supported by CA03927); Walter Reed Army Medical Center, Washington, DC (Brendan M. Weiss, supported by CA26806); Washington University School of Medicine, St Louis, MO (Nancy Bartlett, supported by CA77440); and Weill Medical College of Cornell University, New York, NY (John Leonard, supported by CA07968).
Acknowledgments
This work was supported by the National Cancer Institute: CA77651 (D.J.S., H.S.), CA33601 (J.L.J.), CA32291 (A.S.L., G.P.C.), CA77440 (N.L.B.), CA04457 (L.K.), CA77658 (N.C.H., S.-H.J.), CA32291 (R.W.T.), CA47642 (M.E.J.), and CA77597 (B.D.C.). This work was supported in part by the Lymphoma Foundation, Adam Spector Fund for Hodgkin Research, the Ernest & Jeanette Dicker Charitable Foundation, and Mr Daniel Moon and Family (for D.J.S.). This work was also supported by CALGB (National Cancer Institute) with partial support by Eli Lilly and Company. The research for CALGB 50203 was supported in part by grants from the National Cancer Institute (CA31946) to the CALGB (Dr Monica M. Bertagnolli, Chair) and to the CALGB Statistical Center (Dr Stephen George, CA33601).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: D.J.S. designed research, provided patients, analyzed data, and wrote the manuscript; J.L.J. performed statistical analyses; A.S.L. provided patients; N.L.B. helped design research, provided administrative support, and provided patients; L.K. reviewed and analyzed imaging data and helped write the manuscript; E.D.H. performed pathologic review of diagnostic material; H.S. and N.C.H. reviewed imaging data; S.-H.J. provided statistical design and analysis; G.P.C. helped design research and provided patients; L.H.S. analyzed imaging data and provided administrative support; R.W.T. provided patients; M.E.J. designed research; and B.D.C. helped write the manuscript, provided administrative support, and provided patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the participating institutions appears in the “Appendix.”
Correspondence: David J. Straus, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 406, New York, NY 10065; e-mail: strausd@mskcc.org.