Abstract
We report the results of a prospective, randomized phase 3 trial evaluating the use of gemtuzumab ozogamicin (GO) in an intensive consolidation approach in 657 patients 17-60 years of age. Patients in first complete remission (CR1) after cytarabine and standard- or high-dose daunorubicin induction received 2 cycles of consolidation with high-dose cytarabine followed by peripheral blood progenitor cell collection. The 352 patients who entered consolidation were randomized to receive GO (n = 132) or not (n = 138) and then proceeded to autologous hematopoietic cell transplantation (HCT). GO was given to 67 patients. Median follow-up was 50.9 months. Results of the intention-to-treat analysis demonstrated a 4-year disease-free survival (DFS) of 33.6% versus 35.9% (P = .54) and an overall survival (OS) of 41.3% versus 41.9% (P = .52) for those randomized to receive GO versus no GO, respectively. Patients with favorable- and intermediate-risk acute myeloid leukemia (AML) treated with high-dose daunorubicin and autologous HCT had 4-year DFS rates of 60% and 40% and OS rates of 80% and 49.3%, respectively. For younger AML patients in CR1, autologous HCT should be considered in favorable- and intermediate-cytogenetic risk patients who do not have an allogeneic donor. The addition of a single dose of GO in this setting did not improve outcomes. This trial is registered at http://www.clinicaltrials.gov as NCT00049517.
Introduction
Relapse continues to be a major cause of treatment failure among patients with newly diagnosed acute myeloid leukemia (AML) despite high complete remission (CR) rates with anthracycline-based regimens. In the postremission setting, autologous hematopoietic cell transplantation (HCT) produces fewer relapses compared with intensive chemotherapy.1,2 Advances in the delivery of the conditioning regimen, supportive care, and the use of peripheral blood progenitor cells (PBPCs) in place of bone marrow as the graft source have significantly reduced the treatment-related mortality associated with this procedure.3-5 Relapse rates after autologous HCT, however, remain high, and new initiatives are needed to reduce this most important limitation of postremission therapy of AML.
One promising strategy is to administer targeted therapy specific to AML at the time of achievement of minimal residual disease to deepen the quality of remission. Targeted therapy is a strategy that could reduce relapse rates without the expense of additional toxicity to the patient. Gemtuzumab ozogamicin (GO), initially approved for the treatment of older adults with AML in first relapse, is a targeted therapy that consists of an anti-CD33 monoclonal antibody linked to calicheamicin.6,7 Before its voluntary withdrawal from the commercial market, the Eastern Cooperative Oncology Group (ECOG) used a postremission strategy incorporating GO into consolidation therapy for younger patients with de novo AML in first complete remission (CR1) to evaluate its safety and efficacy. We report the results of this randomized phase 3 trial.
Methods
Patients
This National Cancer Institute (NCI)–approved trial (NCT00049517) was conducted by the ECOG Leukemia Committee. From December 2002 through November 2008, a total of 657 patients with de novo untreated AML ranging in age from 17 to 60 years were enrolled as described previously.8 Patient bone marrow and peripheral blood samples were collected and sent to ECOG's leukemia reference laboratory (to E.M.P.) for confirmation of AML diagnosis. CD33 antigen expression by leukemic myeloblasts was analyzed by multiparameter flow cytometry. CD33 intensity was expressed by mean fluorescence channel of the specific antibody divided by the mean fluorescence channel of the isotype control, yielding the mean fluorescence intensity (MFI) ratio, as described previously.9 Initially, to be eligible for the trial, AML samples were required to demonstrate CD33 positivity (arbitrarily defined as > 20% CD33+ gated myeloblasts); the trial subsequently was amended to allow AML patients regardless of their CD33 status. Internal tandem duplication (ITD) alterations in the Fms-like tyrosine kinase 3 (FLT3) gene were detected centrally by amplifying the entire transmembrane domain and the MJ domain of the FLT3 gene.10 MLL partial tandem duplications (MLL-PTDs) were detected in a one-step PCR reaction.11 Cytogenetic data were reviewed by ECOG's Cytogenetics Committee. Data were collected and certified by the ECOG Data Coordinating Center and analyzed by the authors. The study was approved by the institutional review board at the National Cancer Institute and at each of the study centers. All patients provided written informed consent in accordance with the Declaration of Helsinki and had to be candidates for subsequent autologous HCT.
The primary objective of the consolidation portion of this trial was to compare disease-free survival (DFS) rates after 2 cycles of high-dose cytarabine consolidation with or without the additional course of GO, followed by autologous HCT.
Treatment
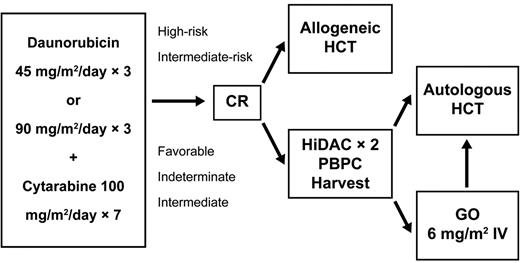
Eligible patients were randomly assigned to receive intravenous cytarabine 100 mg/m2/d infused continuously for 7 days plus intravenous daunorubicin daily for 3 days at a dose of either 45 or 90 mg/m2 (Figure 1). A second cycle of the same drugs but at a reduced dose of daunorubicin (45 mg/m2) for 3 days was prescribed if the nadir biopsy unequivocally demonstrated residual leukemia. CR was confirmed by bone marrow biopsy after recovery of all blood counts after induction therapy and before assignment to consolidation therapy.
Schema of ECOG trial E1900. Patients in remission after induction therapy were allocated based on risk factors (as defined in “Treatment”). The autologous consolidation randomization to gemtuzumab ozogamicin was closed in October 2007. Eligibility requirements for autologous HCT are given in “Treatment.” HiDAC indicates high-dose cytarabine.
Schema of ECOG trial E1900. Patients in remission after induction therapy were allocated based on risk factors (as defined in “Treatment”). The autologous consolidation randomization to gemtuzumab ozogamicin was closed in October 2007. Eligibility requirements for autologous HCT are given in “Treatment.” HiDAC indicates high-dose cytarabine.
Based on karyotype12 and white blood cell count at presentation, patients in CR were assigned to receive consolidation therapy involving allogeneic or autologous HCT. Patients with an unfavorable cytogenetic profile or an initial white blood cell count > 100 000/μL were to proceed to allogeneic HCT if they had a suitable HLA-matched sibling donor available. Patients with intermediate-risk cytogenetics and an HLA-matched sibling match were offered the opportunity to undergo allogeneic HCT, but could choose to be treated with autologous HCT. The remaining patients (including those with a favorable cytogenetic risk) were assigned to intensive consolidation chemotherapy followed by autologous HCT. Molecular markers were not used to determine the postremission therapy allocation.
To proceed with consolidation treatment, patients in morphologic CR had to have fully recovered from complications of previous chemotherapy, including blood cell count recovery; a normal cardiac ejection fraction by multiple-gated acquisition scan; an ECOG performance status of 0, 1, or 2; and adequate pulmonary, hepatic, and renal function.
Before initiating consolidation therapy, patients were randomized to a standard or an investigational arm. All patients received 2 cycles of high-dose cytarabine therapy (3 g/m2 given intravenously over a 3-hour period every 12 hours every other day for a total of 6 doses),13 followed by sargramostim 250 μg/m2 until recovery of blood counts. Filgrastim-mobilized PBPCs were collected and cryopreserved after the second cycle. Patients required PBPC collections to contain more than 1.0 × 106 CD34+ cells/kg to proceed to autologous HCT. After PBPC collection, patients randomized to the investigational arm received a single dose of GO at 6 mg/m2 followed by sargramostim 250 μ/m2 until recovery of counts.
The patients undergoing autologous HCT received intravenous busulfan 0.8 mg/kg every 6 hours for 16 doses (without pharmacokinetic sampling) followed by intravenous cyclophosphamide 60 mg/kg daily for 2 days. Cryopreserved autologous PBPCs were thawed and infused on day 0 followed by sargramostim 250 μg/m2 support until the absolute neutrophil count was > 1500/μL. Patients were given vigorous supportive care with antibacterials, antifungals, antivirals, and blood component transfusions per institutional guidelines.
Statistical analysis
DFS was defined as the time from randomization at the start of consolidation until relapse or death of any cause. Overall survival (OS) was defined as the time from consolidation randomization until death from any cause. The study was designed according to a cure-rate model, and 338 patients were planned to be randomized to 1 of the 2 consolidation arms. The long-term cure rate was assumed to be 35% in the standard arm, with a 9-month median DFS in the noncured group. The long-term cure rate was anticipated to be 45% in the investigational arm, with a 14-month median DFS in the noncured group. With 338 patients and 188 events, the study had 84% power to detect this difference with the use of log-rank test at the 0.025 significance level and assuming 2 years of follow-up. The protocol provided interim efficacy analysis of DFS with the use of O'Brien-Fleming boundaries after the occurrence of 47, 94, and 141 events. The conditional power of rejecting the null hypothesis at full information was also calculated for futility monitoring. The second interim analysis demonstrated no difference in DFS for the patients whether they were treated with GO or not. Because further accrual would not demonstrate a significant advantage, the ECOG Data and Safety Monitoring Committee recommended closure of the randomization to the investigational arm in October 2007, allowing the trial to continue to accrue the induction randomization with standard consolidation after attainment of CR.
Demographic factors and disease characteristics were compared using t tests and χ2 tests. The primary comparison of DFS was performed on all 270 patients randomized before October 2007 on the intention-to-treat (ITT) principle and secondarily on those randomized patients who actually received autologous HCT. DFS and OS were compared between the 2 consolidation arms with the use of the log-rank test and a Cox proportional hazards model, stratified by induction treatment. A cumulative incidence analysis, with death without prior relapse as competing events, was performed to evaluate the treatment effect on time to relapse. All reported P values are 2-sided.
Results
CR
Of the 657 patients enrolled, 425 (64.7%) achieved CR and 352 (53.6%) entered the consolidation phase: 45 (12.8%) patients with an HLA-matched sibling donor and who had unfavorable-risk disease, as defined in “Treatment,” or intermediate-risk cytogenetics were allocated to an allogeneic HCT; 270 patients were registered to autologous HCT before October 2007: 138 were randomized to receive the investigational consolidation using GO, whereas 132 of the patients were assigned to the standard consolidation without the monoclonal antibody therapy; 37 patients were allocated to the standard consolidation with autologous HCT after October 2007. The median (range, 1.6-81.9) follow-up time among survivors was 50.9 months. There was no difference in demographics and disease characteristics between the 2 arms (Table 1).
Demographics and disease characteristics of patients randomized in consolidation
| . | Treatment . | P . | |||
|---|---|---|---|---|---|
| Standard . | Investigational . | ||||
| N . | % . | N . | % . | ||
| Age, y | |||||
| < 50 | 74 | 56.1 | 72 | 52.2 | |
| ≥ 50 | 58 | 43.9 | 66 | 47.8 | .52 |
| Median (range) | 47 (18-60) | 48 (18-60) | .74 | ||
| Sex | |||||
| Male | 64 | 48.5 | 64 | 46.4 | |
| Female | 68 | 51.5 | 74 | 53.6 | .73 |
| Peripheral WBC count, mm−3 × 1000 | |||||
| < 10 000 | 56 | 42.4 | 66 | 47.8 | |
| ≥ 10 000 | 177 | 53.6 | 171 | 52.3 | .37 |
| Median (range) | 11 (1-190) | 12 (1-179) | .69 | ||
| Hemoglobin, g/dL | |||||
| < 10 | 91 | 68.9 | 91 | 65.9 | |
| ≥ 10 | 98 | 29.7 | 91 | 27.8 | .60 |
| Median (range) | 9 (5-30) | 9 (5-15) | .86 | ||
| Peripheral platelet count mm−3 × 1000 | |||||
| < 50 000 | 68 | 51.5 | 69 | 50.0 | |
| ≥ 50 000 | 177 | 53.6 | 170 | 52.0 | .80 |
| Median (range) | 48 (1-479) | 50 (9-452) | .79 | ||
| Cytogenetics | |||||
| Favorable | 27 | 20.5 | 32 | 23.2 | |
| Indeterminate | 89 | 27.0 | 85 | 26.0 | |
| Intermediate | 44 | 33.3 | 37 | 26.8 | |
| Unfavorable | 52 | 39.4 | 59 | 42.8 | .77 |
| FLT3-ITD | |||||
| Unknown/missing | 6 | 4.5 | 11 | 8.0 | |
| Negative | 96 | 72.7 | 96 | 69.6 | |
| Positive | 30 | 22.7 | 31 | 22.5 | .91 |
| Median peripheral blasts (range) | 326 (0%-98) | 32 (0-99) | .22 | ||
| Median marrow blasts (range) | 69 (9-100) | 59 (3-100) | .13 | ||
| Median CD33 intensity, MFI ratio (range) | 48.62 (1-481) | 62.6 (3-802) | |||
| Median CD33+ blasts (range) | 99.0 (0-99) | 99.0 (70-99) | |||
| Median peripheral neutrophils (range) | 10 (0-951) | 11 (0-676) | .54 | ||
| . | Treatment . | P . | |||
|---|---|---|---|---|---|
| Standard . | Investigational . | ||||
| N . | % . | N . | % . | ||
| Age, y | |||||
| < 50 | 74 | 56.1 | 72 | 52.2 | |
| ≥ 50 | 58 | 43.9 | 66 | 47.8 | .52 |
| Median (range) | 47 (18-60) | 48 (18-60) | .74 | ||
| Sex | |||||
| Male | 64 | 48.5 | 64 | 46.4 | |
| Female | 68 | 51.5 | 74 | 53.6 | .73 |
| Peripheral WBC count, mm−3 × 1000 | |||||
| < 10 000 | 56 | 42.4 | 66 | 47.8 | |
| ≥ 10 000 | 177 | 53.6 | 171 | 52.3 | .37 |
| Median (range) | 11 (1-190) | 12 (1-179) | .69 | ||
| Hemoglobin, g/dL | |||||
| < 10 | 91 | 68.9 | 91 | 65.9 | |
| ≥ 10 | 98 | 29.7 | 91 | 27.8 | .60 |
| Median (range) | 9 (5-30) | 9 (5-15) | .86 | ||
| Peripheral platelet count mm−3 × 1000 | |||||
| < 50 000 | 68 | 51.5 | 69 | 50.0 | |
| ≥ 50 000 | 177 | 53.6 | 170 | 52.0 | .80 |
| Median (range) | 48 (1-479) | 50 (9-452) | .79 | ||
| Cytogenetics | |||||
| Favorable | 27 | 20.5 | 32 | 23.2 | |
| Indeterminate | 89 | 27.0 | 85 | 26.0 | |
| Intermediate | 44 | 33.3 | 37 | 26.8 | |
| Unfavorable | 52 | 39.4 | 59 | 42.8 | .77 |
| FLT3-ITD | |||||
| Unknown/missing | 6 | 4.5 | 11 | 8.0 | |
| Negative | 96 | 72.7 | 96 | 69.6 | |
| Positive | 30 | 22.7 | 31 | 22.5 | .91 |
| Median peripheral blasts (range) | 326 (0%-98) | 32 (0-99) | .22 | ||
| Median marrow blasts (range) | 69 (9-100) | 59 (3-100) | .13 | ||
| Median CD33 intensity, MFI ratio (range) | 48.62 (1-481) | 62.6 (3-802) | |||
| Median CD33+ blasts (range) | 99.0 (0-99) | 99.0 (70-99) | |||
| Median peripheral neutrophils (range) | 10 (0-951) | 11 (0-676) | .54 | ||
WBC indicates white blood cell; PB, peripheral blood; and BM, bone marrow.
Autologous HCT
Of the 270 patients assigned to autologous HCT before October 2007, 132 (49.3%) received the planned therapy: Table 2 shows the reasons for 138 patients (66 standard, 72 investigational consolidation) not proceeding to autologous HCT; these included disease progression (21.0%), inadequate PBPC collection (12.3%), patient withdrawal/refusal (15.9%), toxicity of consolidation therapy (9.4%), change to alternative therapy (14.5%), intercurrent illness (4.3%), death (2.9%), insurance denial (3.6%), unexpected decrease in blood counts (2.2%), never began protocol-defined consolidation therapy (6.5%), and other (7.2%).
Reasons for not proceeding to transplantation
| Reason . | Standard n, % . | Investigational n, % . | Combined n, % . |
|---|---|---|---|
| Disease progression | 16 (24.2) | 13 (18.1) | 19 (21.0) |
| Inadequate PBPC collection | 10 (15.2) | 7 (9.7) | 17 (12.3) |
| Patient withdrawal/refusal | 11 (16.7) | 11 (15.3) | 22 (15.9) |
| Toxicity | 2 (3.0) | 11 (15.3) | 13 (9.4) |
| Alternative therapy | 10 (15.2) | 10 (13.9) | 20 (14.5) |
| Intercurrent illness | 2 (3.0) | 4 (5.6) | 6 (4.3) |
| Death | 1 (1.5) | 3 (4.2) | 4 (2.9) |
| Insurance denial | 1 (1.5) | 4 (5.6) | 5 (3.6) |
| Unexpected decrease in blood counts | 2 (4.5) | 1 (1.4) | 3 (2.2) |
| Never started protocol-defined consolidation treatment | 4 (6.1) | 5 (6.9) | 9 (6.5) |
| Other | 7 (10.6) | 5 (6.9) | 12 (7.2) |
| Overall | 66 (100) | 72 (100) | 138 (100) |
| Reason . | Standard n, % . | Investigational n, % . | Combined n, % . |
|---|---|---|---|
| Disease progression | 16 (24.2) | 13 (18.1) | 19 (21.0) |
| Inadequate PBPC collection | 10 (15.2) | 7 (9.7) | 17 (12.3) |
| Patient withdrawal/refusal | 11 (16.7) | 11 (15.3) | 22 (15.9) |
| Toxicity | 2 (3.0) | 11 (15.3) | 13 (9.4) |
| Alternative therapy | 10 (15.2) | 10 (13.9) | 20 (14.5) |
| Intercurrent illness | 2 (3.0) | 4 (5.6) | 6 (4.3) |
| Death | 1 (1.5) | 3 (4.2) | 4 (2.9) |
| Insurance denial | 1 (1.5) | 4 (5.6) | 5 (3.6) |
| Unexpected decrease in blood counts | 2 (4.5) | 1 (1.4) | 3 (2.2) |
| Never started protocol-defined consolidation treatment | 4 (6.1) | 5 (6.9) | 9 (6.5) |
| Other | 7 (10.6) | 5 (6.9) | 12 (7.2) |
| Overall | 66 (100) | 72 (100) | 138 (100) |
Sixty-six patients received autologous HCT in the standard consolidation arm. Sixty-seven patients in the investigational arm received GO, but only 62 proceeded to autologous HCT. Five patients did not proceed to autologous HCT after GO because of disease progression, veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), insurance denial of HCT, or seeking alternative therapy, including allogeneic HCT. The median time from GO therapy to admission for autologous HCT was 1.5 (range, 0.9-5.5) months. Four patients randomized to the investigational arm did not receive GO before proceeding to autologous HCT but were included in the ITT analysis.
Six GO-treated patients developed reversible VOD/SOS after autologous HCT, whereas none of the patients treated with standard consolidation therapy developed this syndrome. Recovery of neutrophils (95.5% vs 87.9%; P = .06) and platelets (60.6% vs 36.4%; P = .008) occurred more rapidly in the standard arm. Overall, the autologous HCT treatment-related mortality (TRM) was 2.3%.
Outcome
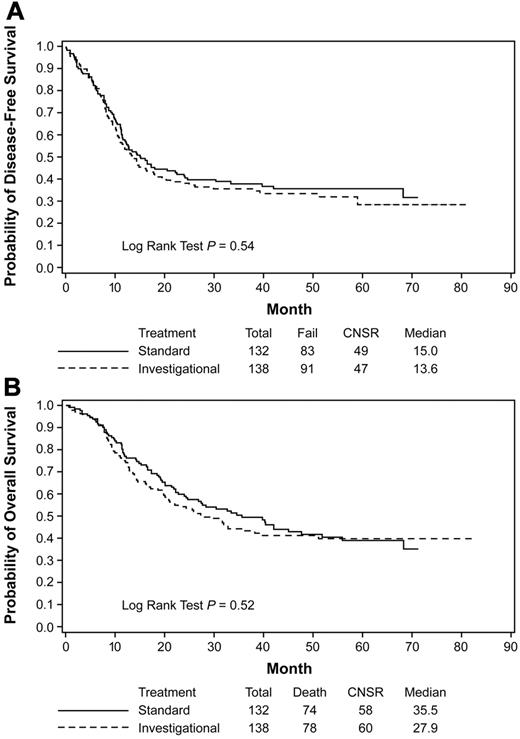
In the ITT analysis, the median DFS was 15.0 months for the standard consolidation and 13.6 months for the investigational consolidation (hazard ratio [HR] = 1.10; 95% confidence interval [CI] = 0.82-1.48; P = .54; Figure 2A); OS was 35.5 vs 27.9 months (HR = 1.11; 95% CI = 0.81-1.53; P = .52; Figure 2B), respectively. The 4-year DFS was 35.9% and 33.6% and 4-year OS was 41.9 and 41.3 for the standard and investigational arms, respectively (Table 3). No differences were observed between the 2 treatment arms in DFS (HR = 1.06; 95% CI = 0.78-1.45; P = .70) or OS (HR = 1.13; 95% CI = 0.81-1.57; P = .48) after adjusting for gender, age, initial hemoglobin concentration, leukocyte and platelet counts, cytogenetic risk, and FLT3 and MLL mutation status.
Kaplan-Meier estimates of DFS and OS. Data from the ITT analysis are shown for DFS (A) and OS (B) of all patients treated in the autologous randomization arms of the trial. Data are from the time of randomization at the start of consolidation. The investigational arm contained the additional treatment with gemtuzumab ozogamicin. CNSR indicates censured.
Kaplan-Meier estimates of DFS and OS. Data from the ITT analysis are shown for DFS (A) and OS (B) of all patients treated in the autologous randomization arms of the trial. Data are from the time of randomization at the start of consolidation. The investigational arm contained the additional treatment with gemtuzumab ozogamicin. CNSR indicates censured.
DFS and OS rates by randomization and subgroups
| . | 4-year DFS, % . | 4-year OS, % . |
|---|---|---|
| Intention to treat (Figure 2) | ||
| Investigational | 33.6 | 41.3 |
| Standard | 35.9 | 41.9 |
| Received autologous HCT (Figure 3) | ||
| Investigational | 40.8 | 50.8 |
| Standard | 41.3 | 43.9 |
| Daunorubicin dose/cytogenetic-risk/received autologous HCT (Figure 5) | ||
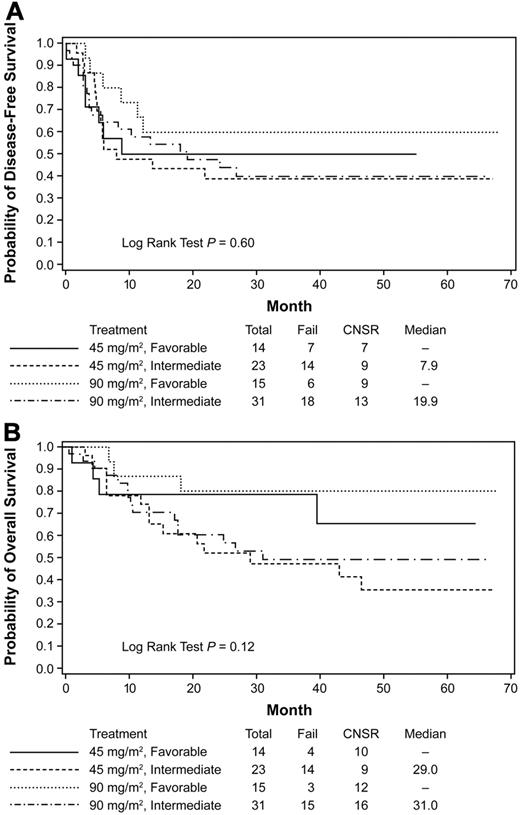
| 45mg/m2, favorable | 50 | 65.5 |
| 45mg/m2, intermediate | 39.1 | 35.6 |
| 90mg/m2, favorable | 60 | 80 |
| 90 mg/m2, intermediate | 40 | 49.3 |
| FLT3-ITD (not shown) | ||
| Positive | 23.4 | 25.7 |
| Negative | 37.0 | 45.8 |
| MLL-PTD (not shown) | ||
| Positive | 15.4 | 19.2 |
| Negative | 34.5 | 41.8 |
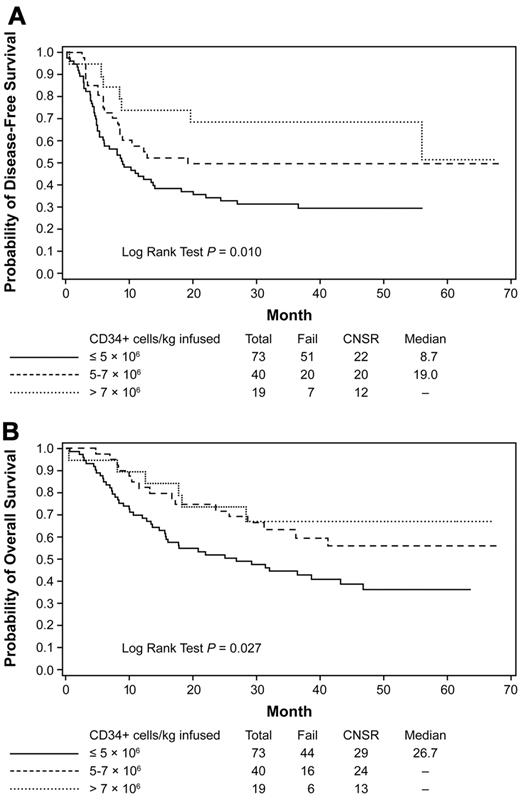
| Total CD34+ cells infused (Figure 6) | ||
| ≤ 5 × 106/kg | 29.5 | 36.4 |
| 5 × 106 to 7 × 106/kg | 49.6 | 55.9 |
| > 7 × 106/kg | 68.4 | 67.0 |
| . | 4-year DFS, % . | 4-year OS, % . |
|---|---|---|
| Intention to treat (Figure 2) | ||
| Investigational | 33.6 | 41.3 |
| Standard | 35.9 | 41.9 |
| Received autologous HCT (Figure 3) | ||
| Investigational | 40.8 | 50.8 |
| Standard | 41.3 | 43.9 |
| Daunorubicin dose/cytogenetic-risk/received autologous HCT (Figure 5) | ||
| 45mg/m2, favorable | 50 | 65.5 |
| 45mg/m2, intermediate | 39.1 | 35.6 |
| 90mg/m2, favorable | 60 | 80 |
| 90 mg/m2, intermediate | 40 | 49.3 |
| FLT3-ITD (not shown) | ||
| Positive | 23.4 | 25.7 |
| Negative | 37.0 | 45.8 |
| MLL-PTD (not shown) | ||
| Positive | 15.4 | 19.2 |
| Negative | 34.5 | 41.8 |
| Total CD34+ cells infused (Figure 6) | ||
| ≤ 5 × 106/kg | 29.5 | 36.4 |
| 5 × 106 to 7 × 106/kg | 49.6 | 55.9 |
| > 7 × 106/kg | 68.4 | 67.0 |
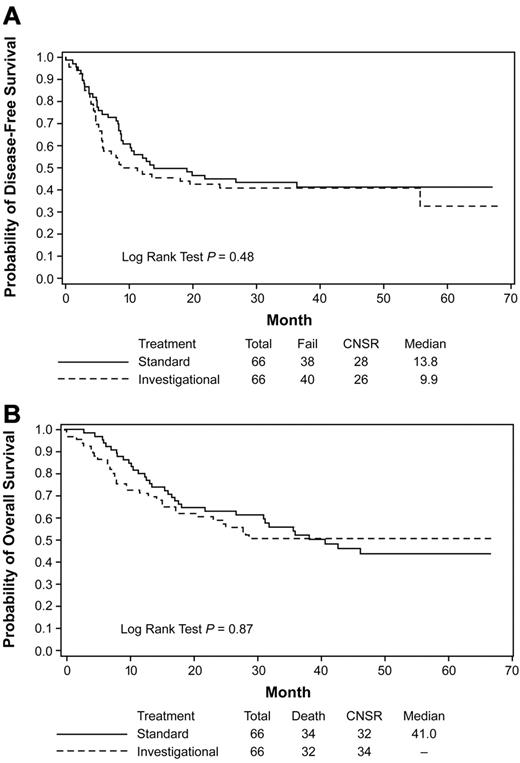
A secondary analysis of those who actually received the autologous HCT demonstrated a median DFS of 13.8 months (4-year DFS 41.3%) for the standard arm versus 9.9 months for the investigational arm (4-year DFS 40.8%; HR = 1.17; 95% CI = 0.75-1.84; P = .48; Figure 3A). The median OS after autologous HCT for the standard consolidation (41.0 months, 4-year OS 43.9%) was not significantly different from that in the investigational arm (not yet reached; 4-year OS 50.8%) (HR = 1.04; 95% CI = 0.64-1.69; P = .87; Figure 3B).
Kaplan-Meier estimates of DFS and OS. Autologous HCT data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT. Data are from the time of transplantation. The investigational arm contained the additional treatment with GO. CNSR indicates censured.
Kaplan-Meier estimates of DFS and OS. Autologous HCT data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT. Data are from the time of transplantation. The investigational arm contained the additional treatment with GO. CNSR indicates censured.
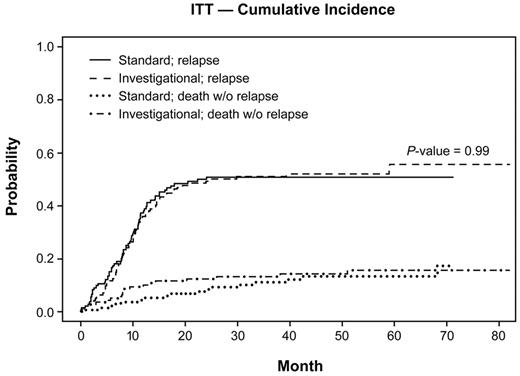
Taking into account death without prior relapse as competing events, the 2-year cumulative incidence rate of relapse was 0.50 (95% CI = 0.41-0.59) for the standard group and 0.49 (95% CI = 0.40-0.57) for the investigational group (P = .99; Figure 4).
Cumulative incidence of relapse and death without relapse. Data from the ITT analysis are shown for the cumulative incidence of relapse and for deaths without relapse of all patients allocated to the autologous randomization arms of the trial. Data are from the time of randomization at the start of consolidation. The investigational arm contained the additional treatment with GO.
Cumulative incidence of relapse and death without relapse. Data from the ITT analysis are shown for the cumulative incidence of relapse and for deaths without relapse of all patients allocated to the autologous randomization arms of the trial. Data are from the time of randomization at the start of consolidation. The investigational arm contained the additional treatment with GO.
Outcome by cytogenetic risk group, molecular markers, and CD33 expression
The median DFS in patients with favorable cytogenetics was 22.1 months (4-year DFS 46.6%) and the median OS has not yet been reached (4-year OS 56.9%). The median DFS and OS in patients with intermediate cytogenetics were 17.2 and 34.4 months (4-year DFS 36.5 and OS 43.1%), respectively. For indeterminate cytogenetics, DFS and OS were 12.4 months and 27.2 month (4-year DFS 26.0 and OS 32.8%), respectively. Finally, for unfavorable cytogenetics, DFS and OS were 6.9 and 11.1 months (4-year DFS 21.0 and OS 21.1%), respectively. Patients who had favorable-risk cytogenetics and were treated with standard-dose daunorubicin and autologous HCT had a 4-year DFS of 50% and OS of 65.5%. Patients who had favorable-risk cytogenetics and were treated with high-dose daunorubicin and autologous HCT had excellent outcomes with a trend for an improved median DFS and OS (4-year DFS 60.0 and OS 80.0%, respectively; Figure 5A-B and Table 3).
Kaplan-Meier estimates of DFS and OS. Based on induction therapy, cytogenetic risk and received autologous HCT. Data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT based on the induction therapy standard (45 mg/m2) or high-dose (90 mg/m2) and on cytogenetic risk group. The analysis is regardless of the addition of GO during consolidation. Data are from the time of randomization at the start of transplantation. CNSR indicates censured.
Kaplan-Meier estimates of DFS and OS. Based on induction therapy, cytogenetic risk and received autologous HCT. Data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT based on the induction therapy standard (45 mg/m2) or high-dose (90 mg/m2) and on cytogenetic risk group. The analysis is regardless of the addition of GO during consolidation. Data are from the time of randomization at the start of transplantation. CNSR indicates censured.
Sixty-one patients with FLT3-ITD had a median DFS of 8.1 months, 4-year DFS 23.4%, and OS of 13.4 months, 4-year OS 25.7%. Thirteen patients with the MLL-PTD had a median DFS and OS of 10.2 and 21 months (4-year DFS 15.4 and OS 19.2%), respectively. The addition of GO to consolidation did not improve the survival for these subsets of patients (curves not shown).
In the 67 GO-treated patients, the median percentage of CD33+ myeloblasts was 99% (range, 70%-99%) and the median of CD33 MFI ratio was 62.6 (range, 4.3-352.9). Patients with lower CD33 antigen density (CD33 MFI ratio ≤ 62.6) fared better than patients with higher CD33 antigen density (CD33 MFI ratio > 62.6) in DFS (HR = 0.57; 95% CI = 0.29-1.11; P = .10) and OS (HR = 0.56; 95% CI = 0.27-1.16; P = .12), but the differences were not statistically significant.
Outcome by CD34 cell dose infused
Among the 132 patients who received an autologous HCT, the median dose of CD34+ cells per kilogram infused was 4.8 × 106 (range, 1.8-41.8). Patients who received a CD34 cell dose of > 7 × 106/kg had better DFS (HR = 0.36; 95% CI = 0.17-0.81; P = .01) and OS (HR = 0.42; 95% CI = 0.18-0.99; P = .05) than patients who received a dose ≤ 5 × 106/kg. Patients who received a dose of CD34+ cells per kilogram between 5-7 × 106 also had a better DFS (HR = 0.58; 95% CI = 0.35-0.98; P = .04) and OS (HR = 0.55; 95% CI = 0.31-0.98; P = .04) than patients who received a dose ≤ 5 × 106 (Figure 6A-B and Table 3). Similar results were observed after adjusting for other risk factors.
Kaplan-Meier estimates of DFS and OS. Estimates are based on CD34 cell dose infused during autologous HCT. Data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT. Cell dose is per kilogram of body weight. Data are from the time of randomization at the start of transplantation. The analysis is regardless of the addition of GO during consolidation. CNSR indicates censured.
Kaplan-Meier estimates of DFS and OS. Estimates are based on CD34 cell dose infused during autologous HCT. Data from the subset analysis are shown for DFS (A) and OS (B) for patients who received protocol-prescribed autologous HCT. Cell dose is per kilogram of body weight. Data are from the time of randomization at the start of transplantation. The analysis is regardless of the addition of GO during consolidation. CNSR indicates censured.
Discussion
This large, prospective randomized trial showed that the administration of a single dose of GO as additional consolidation therapy before autologous HCT did not improve outcome. Furthermore, patients with favorable-risk disease who received high-dose daunorubicin followed by autologous HCT fared very well irrespective of the use of GO consolidation, suggesting that autologous HCT alone might be sufficient in this patient group, provided that remission induction is enhanced with anthracycline intensification.
Many postremission approaches have been explored to improve outcomes for younger AML patients. It does not appear, however, that intensifying consolidation using other agents improves outcomes compared with high-dose cytarabine.14 Several cooperative groups have compared autologous HCT against intensive chemotherapy with variable outcomes regarding DFS and OS.1,2,15-19 The present study sought to improve DFS and OS outcomes by reducing the leukemic cell burden before autologous HCT consolidation with GO.
The Food and Drug Administration–approved dose of GO is 9 mg/m2; however, at doses of 6 mg/m2 there is adequate CD33 epitope saturation for over 24 hours.7,20 In an effort to provide sufficient myeloblasts targeting while reducing the possibility of nonhematologic toxicity before autologous HCT, the 6 mg/m2 dose was chosen for this study. We decided not to give GO before PBPC collection because the effects of the drug on hematopoietic progenitor cell collection were unknown. Because the addition of GO was positioned after PBPC collection, this product itself was not purged before HCT. Perhaps giving the drug immediately before PBPC mobilization to reduce leukemic contamination could have improved the results of the approach. Administering GO therapy after 2 cycles of high-dose cytarabine may have diminished its effectiveness. We also did not find an association between the level of CD33 expression and the efficacy of GO. However, in retrospect, the variability of CD33 expression on leukemic stem cells21 may have limited the utility of GO as an in vivo purging agent. One could argue that a higher single dose of GO may have been more efficacious; the aforementioned concerns for toxicity resulted in the use of an attenuated dose. Finally, other studies that have attempted to incorporate GO in consolidation have not been able to demonstrate a survival benefit for younger AML patients.22 Enthusiasm for multiple cycles of GO in consolidation has been diminished by a recent study reported by Lowenberg et al that failed to show improvement in DFS or OS,23 albeit in an older group of patients with AML and myelodysplastic syndrome.
GO was believed to be an effective drug in AML with limited, but well known, side effects, particularly liver toxicity. The National Cancer Research Institute (formerly the Medical Research Council) AML 15 trial demonstrated that GO at 3 mg/m2 was a safe addition to standard induction chemotherapy and consolidation.24 The results of that trial demonstrated the addition of a lower dose of GO to induction and consolidation therapy produced a significant survival benefit for patients with favorable-risk cytogenetics and a trend for benefit in intermediate-risk patients.25 Conversely, a recently closed study reported by the Southwest Oncology Group did not demonstrate any value of adding GO 6 mg/m2 to standard induction and maintenance for younger AML patients.26 The higher induction death rate in patients who received GO in combination with chemotherapy was believed to be a contributing factor to the lack of efficacy. This negative result has led to the drug being voluntarily withdrawn from the commercial market. Several cooperative groups continue to study GO as a targeted therapy strategy in various stages of the treatment algorithm. Therefore, the best role, if any, for GO in the initial treatment of younger patients with AML remains to be determined.
In the present study, GO given in consolidation was well tolerated, although 6 additional patients developed reversible VOD/SOS after autologous HCT. VOD/SOS has been seen after the administration of GO in AML patients in relapse and in CR1 without HCT.27 Contrary to a report in which patients who received GO followed by allogeneic HCT had a high incidence of VOD/SOS,28 the incidence was only 9.0% in the autologous transplantation setting of the present study. There were no fatalities due to liver dysfunction. A negative effect of the additional therapy was slower hematopoietic recovery after transplantation. Despite the minimal additional morbidity, the lack of efficacy of this strategy makes the toxicity issue moot.
We noted encouraging outcomes in patients with favorable- and intermediate-risk cytogenetics, with the median OS not yet reached in the former group. Patients in the favorable cytogenetic risk group treated with 90 mg/m2 daunorubicin followed by intensive postremission therapy had a trend toward better outcomes than all other subgroups in this trial, irrespective of GO addition. Our data demonstrate the positive outcomes of using autologous HCT in the favorable-risk group, as has been demonstrated previously,12,29 and warrant the use of this strategy in future trials for this subset of patients. For the intermediate-risk patient, this intensive consolidation was effective and safe and should be considered for those patients who lack a suitable donor or refuse allogeneic HCT. In contrast, the intensive consolidation approach of this trial did not benefit patients who were FLT3-ITD+, emphasizing the need to treat these patients with alternative approaches such as FLT3-targeted therapy.
A limitation of this study, which has plagued many transplantation studies,1,2,15,16 was the large number of patients who failed to receive the intended treatment. As seen in other trials, many patients were negatively impacted by the nontransplantation consolidation, relapse before transplantation, or outright refusal of the procedure. This attrition possibly influenced the ITT outcomes in our trial and affected the subset analyses. However, even in those patients who received the autologous HCT per protocol, a beneficial effect from GO therapy was not present.
Earlier trials using bone marrow as the hematopoietic graft source demonstrated a high (10%-14%) TRM with autologous HCT.1,16 The ECOG-led U.S. Intergroup trial that used 4-hydroperoxycylophosphamide as a bone marrow purging agent had delayed engraftment and a high TRM.16 In the most recent ECOG trial using unpurged autologous PBPCs for patients in CR1, autologous HCT had a 0% TRM and lengthy DFS and OS.3 A recent retrospective analysis demonstrated an 8% TRM with the use of autologous PBPC infusions.4 In our study, the TRM was low at 2.3%, suggesting that autologous HCT with intravenous busulfan and cyclophosphamide is a safe consolidation approach, even after prior therapy with GO. A recent analysis from the European Group for Blood and Marrow Transplantation (EBMT) demonstrated that the use of bone marrow as the graft source was associated with a reduced relapse incidence compared with PBPCs as a hematopoietic cell source.30 In our trial, PBPCs were used as the graft source. In contrast to another published report by the EBMT,31 our patients who were infused with a higher dose of CD34+ cells had a better DFS and OS. The in vivo purging effect of the 2 cycles of intensive cytarabine before autologous HCT and a uniform approach to consolidation may have led to the differences in outcomes in our trial compared with the EBMT analysis. Patients who had a healthier marrow after the consolidation chemotherapy may have collected more PBPC product, which resulted in better outcomes. Future studies should focus on the remaining questions of hematopoietic cell dose and source. In addition, novel techniques to identify minimal residual disease and agents targeting new molecular markers may be important strategies to improving the outcome for patients with AML in CR1.32-34
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Funding for laboratory correlative studies was provided by Wyeth Pharmaceuticals and Immunex through a grant to the ECOG. This study was coordinated by the ECOG (Robert L. Comis, MD, Chair) and supported in part by grants from the Public Health Service (CA23318, CA66636, CA21115, CA13650, CA15488, CA17145, and CA14548), the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: H.F.F., M.R.L., S.M.L., J.M.R., H.M.L., and M.S.T. designed and performed research and wrote and edited the paper; Z.S. analyzed the data and edited the paper; E.M.P. performed diagnostic studies, analyzed data, and edited the paper; J.R. performed the molecular studies; and G.D. and R.P.K. performed the cytogenetic studies.
Conflict-of-interest disclosure: E.M.P. received support from Wyeth and Immunex to perform the correlative studies used in this trial. The remaining authors declare no other competing financial interests.
Correspondence: Hugo F. Fernandez, Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: hugo.fernandez@moffitt.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal