Abstract
Cytogenetic alterations, such as amplifications, deletions, or translocations, contribute to myeloid malignancies. MicroRNAs (miRNAs) have emerged as critical regulators of hematopoiesis, and their aberrant expression has been associated with leukemia. Genomic regions containing sequence alterations and fragile sites in cancers are enriched with miRNAs; however, the relevant miRNAs within these regions have not been evaluated on a global basis. Here, we investigated miRNAs relevant to acute myeloid leukemia (AML) by (1) mapping miRNAs within leukemia-associated genomic alterations in human AML cell lines by high-resolution genome arrays and (2) evaluating absolute expression of these miRNAs by massively parallel small RNA sequencing. Seventy-seven percent (542 of 706) of miRNAs mapped to leukemia-associated copy-number alterations in the cell lines; however, only 18% (99 of 542) of these miRNAs are expressed above background levels. As evidence that this subset of miRNAs is relevant to leukemia, we show that loss of 2 miRNAs identified in our analysis, miR-145 and miR-146a, results in leukemia in a mouse model. Small RNA sequencing identified 28 putative novel miRNAs, 18 of which map to leukemia-associated copy-number alterations. This detailed genomic and small RNA analysis points to a subset of miRNAs that may play a role in myeloid malignancies.
Introduction
Small noncoding RNAs are conserved and encoded in the genomes of invertebrates, vertebrates, and plants.1 The largest subset of naturally occurring small RNAs are microRNAs (miRNAs). Mature miRNAs are 19-24 nucleotide transcripts processed from precursor hairpin intermediate RNAs by endonuclease-mediated reactions.1 miRNAs have been implicated in critical hematopoietic processes, and their deregulation is associated with leukemogenesis. Functional validation of deregulated miRNAs in hematopoeisis has been shown for several miRNAs2 ; however, direct evidence that leukemogenesis is specifically mediated by miRNAs is lacking. In one example, overexpression of miR-155 results in a fatal and aggressive myeloproliferative disorder in mice.3 In other examples, knockout or overexpression of an miRNA has been shown to deregulate hematopoietic processes related to ≥ 1 steps of leukemogenesis.2
Hematologic malignancies often exhibit genomic alterations. Balanced and unbalanced chromosome alterations are found in patients with acute myeloid leukemia (AML). Many miRNA expression studies have been performed to identify differentially expressed miRNAs between normal and leukemic samples.4-6 These studies are extremely valuable, but they have not examined the direct relationship between genome-wide alterations and the miRNAs within these alterations. Furthermore, these studies, which rely on the annotated sequence information about the miRNAs, are not capable of identifying novel or variant miRNAs.
Analysis of human and mouse genomes reveals that miRNA genes are frequently located at fragile sites and regions of copy number alteration (CNA) associated with cancer.7 The current approximation states that 50% of miRNAs are within cancer-associated genomic regions or in fragile sites.7 These findings suggest that a mechanism of miRNA deregulation in oncogenesis is due to genomic instability. Although miRNAs have been mapped to common leukemia-associated alterations with the use of database searches, the potentially relevant miRNAs within these alterations have not been thoroughly investigated. In this study we addressed 2 main objectives: (1) identification of miRNAs that map to common leukemia-associated genomic alterations and (2) identification of relevant miRNAs within the leukemia-associated genomic alterations. We investigated miRNAs relevant to leukemogenesis by first mapping miRNAs within common leukemia-associated genomic alterations in 6 human AML cell lines by high-resolution array comparative genomic hybridization (CGH). We then determined absolute expression of these miRNAs by massively parallel small RNA sequencing. Although 77% (542 of 706) of miRNAs mapped to leukemia-associated CNAs in the 6 cell lines, only 18% (99 of 542) of these miRNAs are expressed at levels above background. In support of our conclusion, knockdown of miR-145 and miR-146a, 2 miRNAs mapping to a commonly deleted region in AML, results in a long-latency myeloid leukemia in mice. This study provides a detailed genomic and miRNA expression analysis of human leukemic cell lines to identify an enriched subset of leukemia-associated miRNAs and facilitates selection of miRNAs to investigate in human leukemogenesis or normal hematopoiesis.
Methods
Cell lines and CD34+ cells
Acute myeloid leukemic cell lines, KG-1, KG-1a, UT-7, HL-60, and THP-1, were purchased from ATCC. The myelodysplastic cell line, MDS-L, was generated in the laboratory of one of the coauthors (K.T.). CD34+ cells were positively selected from cryopreserved marrow or peripheral blood cells by immunomagnetic separation (Stem Cell Technologies).
Whole genome tiling-path aCGH analysis
Details of whole genome array construction and probe prime labeling and hybridization have been described previously.8,9 The submegabase-resolution tiling set array contains 32 433 overlapping BAC-derived DNA segments that provide tiling coverage over the entire human genome with a theoretical resolution of 50-100 kb.8 All clones were spotted in duplicate. Sample (cell line) and reference (normal diploid) genomic DNA (50-200 ng of each) were separately labeled with Cyanine 3 and Cyanine 5 deoxycytidine triphosphate fluorescence markers, respectively. The images were captured with a charge-coupled device camera and were analyzed with an ArrayWorx scanner and SoftWorx Tracker Spot Analysis software (Applied Precision). SeeGH custom software was used to visualize all data as log ratio plots.10 Clones with standard deviation between duplicate spots of > 0.1 were filtered from the raw data. To avoid false-positives due to hybridization noise, a minimum of 2 overlapping consecutive clones showing change was required for a region to be considered altered. Breakpoints of genomic alterations were identified with a hidden Markov model algorithm and were verified by visual inspection.
Small RNA library preparation
Leukemia cell lines were individually harvested, and RNA was extracted with TRIzol (Invitrogen). The extracted RNA was subjected to miRNA library construction with the use of the Illumina sequencing platform according to a published protocol.11 The sequencing library of small RNAs generated from human CD34+ bone marrow cells was obtained from a recently published analysis.12
miRNA isolation and expression analysis
The small RNA fraction was isolated with the mirVana Paris Isolation kit (Ambion). miRNA expression was quantified with miRNA-specific stem-loop primers. The small RNA fraction was used for reverse transcription with the use of reverse transcription primers specific for each independent miRNA. The cDNA synthesis reaction was subsequently used for quantitative polymerase chain reaction (PCR) with the use of miRNA-specific primers
Annotation and prediction of novel miRNAs
The annotation procedure was performed as described but used annotations form miRBase version 14 and the human genome (National Center for Biotechnology Information [NCBI] build 36.3). Novel miRNAs were predicted as previously described.11 Predicted target genes were identified with the use of TargetScan 5.1. For novel or edited miRNAs, the target genes were predicted by TargetScan Custom 5.1. Binding energies for the miRNA to its mRNA targets were calculated with IntaRNA.13
miRNA decoy retroviral vectors, packaging cell lines, and bone marrow transplantation
miRNA decoy sequences (tandem repeats each complementary to miR-145 and miR-146a, respectively) were fused to the 3′ untranslated region (UTR) of the yellow fluorescent protein (YFP) cDNA and then cloned into the dual promoter phosphoglycerate retroviral vector as previously reported.14 Virus packaging and infection of ecotropic packaging cell lines (GP+E86) were performed as previously described.14 Marrow transplantation studies were carried out with protocols approved by the University of British Columbia Animal Care Committee.
Peripheral blood and bone marrow analysis
Donor-derived engraftment and reconstitution were monitored by flow cytometric analysis of YFP expression in the peripheral blood. For immunophenotypic analysis, bone marrow cells or peripheral blood were washed and resuspended in phosphate-buffered saline (PBS) containing 4% goat serum, followed by primary monoclonal antibody (labeled with phycoerythrin or allophycocyanin) staining overnight. Details on antibodies are described elsewhere.14 Samples were run on a FACSCalibur flow cytometer (Beckman Coulter), and data were analyzed with the use of FlowJo software (Version 8.7; TreeStar Inc). Complete blood counts were performed on the peripheral blood with the use of Scil Vet ABC Hematology Analyzer (Scil Animal Care Company). Blood counts were obtained at time of death. Organs and tissues were fixed in PBS with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Microarray analysis of miRNAs
The expression data analyzed in this study were accessed at ArrayExpress (www.ebi.ac.uk/microarray-as/ae). The accession numbers for controls (n = 11) and AML patient samples (n = 54, normal karyotype; n = 4, del(5q)) are E-TABM-970 and E-TABM-405, respectively. Analyses were performed with BRB-ArrayTools developed by Dr Richard Simon and BRB-ArrayTools Development Team.15
Retroviral transduction of miR-145 and miR-146a into HL-60 myeloid leukemia cells
Amphophoenix cells were transfected with murine stem cell virus–internal ribosome entry site–green fluorescent protein (GFP) (MIG), MIG–-miR-145, or MIG–miR-146a constructs. Constructs have been previously described.14 Virus was harvested and used to infect HL-60 cells. Transduced cell lines were sorted for GFP expression and cultured in Dulbecco minimal essential medium/10% fetal bovine serum.
Apoptosis analysis
Approximately 1 × 105 HL-60 cells were washed in PBS, and resuspended in annexin V–binding buffer (10mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140mM NaCl, 2.5mM CaCl2; pH 7.4) and annexin V–conjugated antibody (1:20). After a 15-minute incubation, an additional 500 μL of annexin V–binding buffer was added and analyzed by flow cytometry.
Luciferase assay
hsa-miR-1, hsa-miR-1 (4A:G) mutant, hMEIS1-3′-UTR, and hTP53-3′-UTR were synthesized and cloned into pcDNA3.1 or pMirReport, respectively. A 95-base pair (bp) fragment (134-233) of the MEIS1 3′-UTR encompassing the miR-1 target site or 95-bp fragment (634-735) of the TP53 3′-UTR encompassing the miR-1 (4A:G) predicted target site were inserted downstream of the open reading frame of the luciferase reporter gene. For the 3′-UTR luciferase assays, 50 ng of pMirReport-3′-UTR, 250 ng of pcDNA or pcDNA-miR, and 7.5 ng of thymidine kinase-driven Renilla luciferase were cotransfected into human embryonic kidney 294 (HEK293) cells (24-well format) with the use of TransiT transfection reagent (Mirus).
5-Azacytidine treatment of myeloid leukemia cell lines
HL-60, KG-1a, and THP-1 cells were cultured in Dulbecco minimal essential medium/10% fetal bovine serum in 35-mm plates for 24 hours. After plating, cells were treated with 10μM 5-azacytidine (5-aza; Sigma) or dimethyl sulfoxide for 24 hours. Small RNAs were collected with miRVANA Paris (Ambion) isolation kit, and miR-145 and miR-146a were measured by quantitative PCR.
Statistics
Results are depicted as the mean ± standard error of the mean. Statistical analyses were performed with the Student t test. Comparison of survival between different groups was done by the Kaplan-Meier test, and P value was calculated by the log-rank test. Box-and-whisker plots were used in Figure 5C to depict the range and percentiles of values obtained. The box indicates the 75th (top), 50th (middle line; median), and 25th (bottom) percentiles of the values obtained. GraphPad Prism 4 (GraphPad) was used for statistical analysis.
Results
Mapping of CNAs in human leukemic cell lines by whole genome high-resolution array CGH
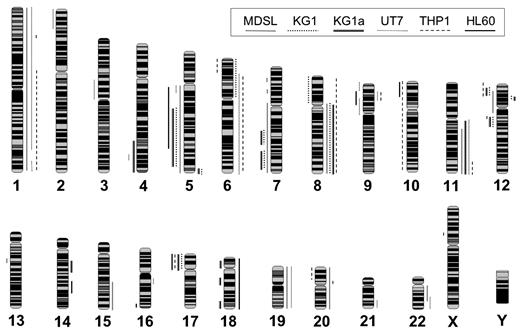
We obtained 6 human cell lines representing de novo and secondary acute myeloid malignancies: THP-1 (acute monocytic leukemia), UT-7 (acute megakaryoblastic leukemia), KG-1 (acute myeloid leukemia with myeloblast-like features), KG-1a (a subline of KG-1 that has acquired new karyotypic markers), MDSL (refractory anemia with excess blasts), and HL-60 (promyelocytic leukemia). These cell lines were chosen as models of common AML subtypes and for their large number of cytogenetic abnormalities.16 To precisely define genomic breakpoints in these leukemic model cell lines, we used high-resolution aCGH.8 Comprehensive genomic profiles of copy number gains and losses obtained from 6 leukemic cell lines are summarized in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and Figure 1. The median number of genomic alterations per cell line is 22 (range, 13-29). As expected, all cell lines exhibit genomic instability, including CNAs of all or part of chromosomal arms (Figure 1; supplemental Table 1). UT-7 exhibited the most genomic alterations (29 CNAs, and 1156 Mb of total alterations), whereas HL-60 had the least disrupted genome (429 Mb of total alterations) and KG-1 had the fewest distinct alterations (13 high level CNAs). The most recurrent alterations include gains of chromosomes 1, 6q, 8, 9p, 11q, and 19 and deletions of chromosomes 5q, 7q, 12p, and 17p. Recurring submegabase genomic alterations were also detected and include a deletion at 12q13.11-q13.12 and a gain at 12p12.1-p11.23 (Figure 1; supplemental Table 1). Most of these CNAs have been reported in patients with AML and are thought to contribute to leukemic initiation or progression.17 Application of high-resolution aCGH provides a detailed and comprehensive analysis of genomic CNAs in 6 model leukemic cell lines.
Summary of cytogenetic alterations in 6 human leukemia cell lines by aCGH. Whole-genome frequency distribution of chromosomal alterations in 6 human leukemia cell lines as detected by array CGH and visualized by SeeGH. Lines to the right of the chromosome indicate gain of chromosomal material. Lines to the left of the chromosome indicate loss of chromosomal material. Line patterns correspond to the indicated cell line.
Summary of cytogenetic alterations in 6 human leukemia cell lines by aCGH. Whole-genome frequency distribution of chromosomal alterations in 6 human leukemia cell lines as detected by array CGH and visualized by SeeGH. Lines to the right of the chromosome indicate gain of chromosomal material. Lines to the left of the chromosome indicate loss of chromosomal material. Line patterns correspond to the indicated cell line.
Discovery of expressed microRNAs mapping to leukemia-associated genomic alterations by massively parallel sequencing of small RNAs
After determining the breakpoints and regions of CNAs in the leukemic cell lines, we next sought to identify miRNAs within the identified CNA. To map miRNAs to the DNA CNA in our leukemic cell lines, we used the most recent human genome build (University of California Santa Cruz [UCSC] Human Mar. 2006) and miRNA database (miRBase 14.0) (supplemental Table 2). According to the current miRNA database, there are 706 human miRNAs.18 Of these, 542 (77%) are found within leukemia-associated CNAs in the 6 cell lines we studied (see further discussion below).
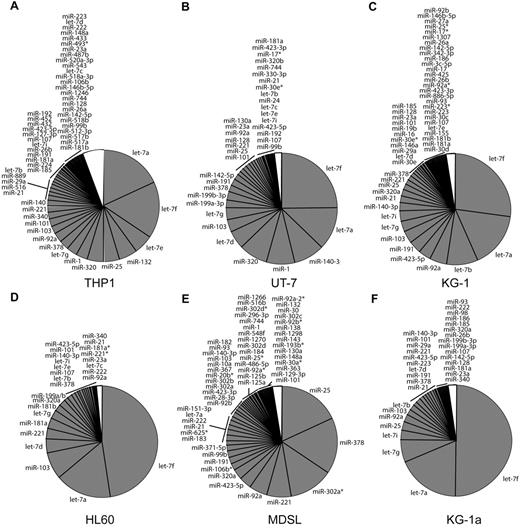
MicroRNA expression in 6 human leukemia cell lines. (A-F) Proportion (in percent) of the most abundantly expressed miRNAs in 6 human leukemia cell lines: (A) THP-1, (B) UT-7, (C) KG-1, (D) HL-60, (E) MDSL, and (F) KG-1a. White portion of the pie chart represents miRNAs reads that are expressed below background.
MicroRNA expression in 6 human leukemia cell lines. (A-F) Proportion (in percent) of the most abundantly expressed miRNAs in 6 human leukemia cell lines: (A) THP-1, (B) UT-7, (C) KG-1, (D) HL-60, (E) MDSL, and (F) KG-1a. White portion of the pie chart represents miRNAs reads that are expressed below background.
To determine which of the 542 miRNAs are expressed and potentially relevant to AML, we applied massively parallel sequencing of small RNAs to each of the 6 cell lines. We selected this approach because all variants of mature miRNA transcripts can be identified.19 In contrast, hybridization-based miRNA arrays rely on annotated miRNA sequences and therefore are not adequate to detect all miRNA variants. Small RNA fractions were isolated from each cell line and prepared for sequencing on the Illumina platform. Sequencing of small RNA libraries resulted in an average of 7.2 million reads (range, 4.5-9.5 million reads) from each of the cell lines. Sequences were mapped according to their overlap with available genomic annotations of miRNAs, resulting in an average of 0.9 million filtered reads from each cell line. The miRNA reads within the small RNA fraction of each cell line represent 40%-90% of all filtered reads (supplemental Tables 3-4). Because the cell lines exhibited numerous miRNAs with single reads, we decided to primarily focus on the miRNAs above an arbitrary background expression level, with a miRNA frequency above 0.001 (1 read per 1000 total reads; Figure 2). By this cutoff, ∼ 98% of all miRNA reads and 20% of unique miRNAs are included in our analysis from each cell line (Figure 2). miRNAs with fewer reads (less than a frequency of 0.001) were excluded from further analysis.
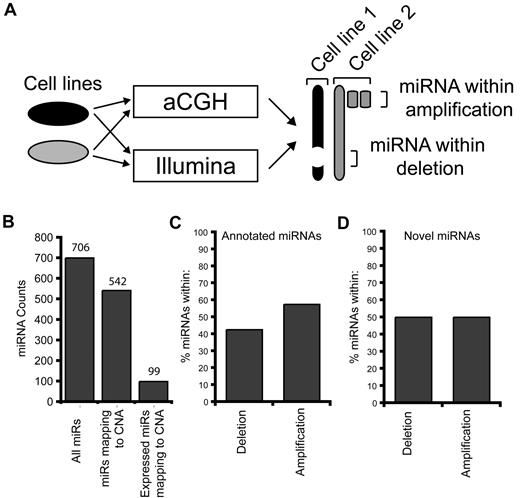
To include miRNAs relevant only to myeloid leukemic cells, we used the cell lines as controls to cross-compare absolute miRNA levels within genomic alterations, rather than nonleukemic controls (Figure 3A). By this approach, abundantly expressed miRNAs within genomic alterations that are common and relevant only to myeloid leukemia cells are identified (Figure 3A). miRNAs mapping to the leukemia-associated genomic alterations (n = 542) (supplemental Table 2) were next investigated for their absolute expression levels. Of these 542 miRNAs, 18% (n = 99) were expressed at levels above background (> 1 per 1000 expressed tags) in ≥ 1 of the cell lines (Figure 3B; Table 1). Nearly an equal distribution of miRNAs was mapped within deleted (51%) and amplified (49%) CNAs (Figure 3C-D). These findings suggest that, although many miRNAs map within CNAs, only a subset are expressed at notable levels in myeloid leukemic cell lines. We next wanted to confirm the relevance of this subset of miRNAs in primary AML samples. We used published gene expression data on miRNAs isolated from CD34+ cells from 58 patients with AML and 11 controls.4 Of 99 expressed miRNAs, 43 were evaluated by microarray analysis, of which 72% (n = 31) were significantly differentially expressed in patients with AML compared with controls (Table 1).
miRNA mapping to CNAs. (A) Schematic representation of the approach to identify miRNAs within CNAs. Each of the 6 cell lines were simultaneously analyzed for CNAs by aCGH and small RNA expression by massively parallel sequencing on the Illumina platform. The CNAs for the 6 cell lines were annotated (Table 1) and subsequently merged. Similarly, miRNA expression data from all 6 cell lines were used to identify expressed miRNAs that map to the CNAs (Table 2). By this approach, we were able to identify miRNAs that map to a homozygous deletion in one cell line by using miRNA expression values from the pooled data. (B) Distribution summary of miRNAs mapping to leukemia-associated genomic alterations found in 6 human leukemia cell lines. According to the current miRNA database (miRBase v13.0), there are 702 human miRNAs. Of these, 542 miRNAs map to CNAs identified in the 6 leukemia cell lines. After small RNA sequencing, 99 miRNAs (of 542) are expressed above background and map to CNAs. (C-D) Proportion (in percent) of the 99 expressed miRNAs within each cell line mapping to genomic regions of amplifications or deletions.
miRNA mapping to CNAs. (A) Schematic representation of the approach to identify miRNAs within CNAs. Each of the 6 cell lines were simultaneously analyzed for CNAs by aCGH and small RNA expression by massively parallel sequencing on the Illumina platform. The CNAs for the 6 cell lines were annotated (Table 1) and subsequently merged. Similarly, miRNA expression data from all 6 cell lines were used to identify expressed miRNAs that map to the CNAs (Table 2). By this approach, we were able to identify miRNAs that map to a homozygous deletion in one cell line by using miRNA expression values from the pooled data. (B) Distribution summary of miRNAs mapping to leukemia-associated genomic alterations found in 6 human leukemia cell lines. According to the current miRNA database (miRBase v13.0), there are 702 human miRNAs. Of these, 542 miRNAs map to CNAs identified in the 6 leukemia cell lines. After small RNA sequencing, 99 miRNAs (of 542) are expressed above background and map to CNAs. (C-D) Proportion (in percent) of the 99 expressed miRNAs within each cell line mapping to genomic regions of amplifications or deletions.
Expressed miRNAs mapping to copy number alterations
| Chromosome . | miR . | Bp start . | Bp end . | P* . |
|---|---|---|---|---|
| Chr 1 (amplification) | miR-30e | 40 s683 565 | 40 683 656 | .08 |
| miR-30c-1 | 40 686 494 | 40 686 582 | NA | |
| miR-101-1 | 65 296 705 | 65 296 779 | .0008 | |
| miR-186 | 71 305 902 | 71 305 987 | .32 | |
| miR-9-1 | 154 656 757 | 154 656 845 | .26 | |
| miR-181b-1 | 197 094 625 | 197 094 734 | .0001 | |
| miR-181a-1 | 197 094 796 | 197 094 905 | .0084 | |
| miR-29c | 206 041 820 | 206 041 907 | NA | |
| Chr 3 (deletion) | miR-128-2 | 35 760 972 | 35 761 055 | .0001 |
| miR-26a-1 | 37 985 899 | 37 985 975 | .0001 | |
| miR-138-1 | 44 130 708 | 44 130 806 | .11 | |
| miR-425 | 49 032 585 | 49 032 671 | NA | |
| miR-191 | 49 033 055 | 49 033 146 | .95 | |
| let-7g | 52 277 334 | 52 277 417 | .0001 | |
| miR-15b | 161 605 070 | 161 605 167 | NA | |
| miR-16-2 | 161 605 227 | 161 605 307 | .042 | |
| miR-28 | 189 889 263 | 189 889 348 | NA | |
| Chr 5 (deletion) | miR-9-2 | 87 998 427 | 87 998 513 | NA |
| miR-886 | 135 444 076 | 135 444 196 | NA | |
| miR-143 | 148 788 674 | 148 788 779 | NA | |
| miR-378 | 149 092 581 | 149 092 646 | NA | |
| miR-146a | 159 844 937 | 159 845 035 | .0008 | |
| miR-103-1 | 167 920 487 | 167 920 548 | NA | |
| miR-340 | 179 374 909 | 179 375 003 | .0001 | |
| Chr 6 (amplification) | miR-30a | 72 169 975 | 72 170 045 | NA |
| Chr 7 (deletion) | miR-148a | 25 956 064 | 25 956 131 | .0001 |
| miR-25 | 99 529 119 | 99 529 202 | NA | |
| miR-93 | 99 529 327 | 99 529 406 | NA | |
| miR-106b | 99 529 552 | 99 529 633 | NA | |
| miR-129-1 | 127 635 161 | 127 635 232 | NA | |
| miR-182 | 129 197 459 | 129 197 568 | .0002 | |
| miR-183 | 129 201 981 | 129 202 090 | .0035 | |
| miR-29a | 130 212 046 | 130 212 109 | NA | |
| miR-26b-1 | 130 212 758 | 130 212 838 | NA | |
| Chr 8 (amplification) | miR-320a | 22 158 420 | 22 158 501 | NA |
| miR-486 | 41 637 116 | 41 637 183 | NA | |
| miR-30d | 135 886 301 | 135 886 370 | NA | |
| miR-151 | 141 811 845 | 141 811 934 | .057 | |
| Chr 9 | miR-101-2 | 4 840 297 | 4 840 375 | .54 |
| miR-199b | 130 046 821 | 130 046 930 | NA | |
| Chr 10 (deletion) | miR-146b | 104 186 259 | 104 186 331 | .0001 |
| miR-1307 | 105 144 000 | 105 144 148 | NA | |
| Chr 11 (amplification) | miR-130a | 57 165 247 | 57 165 335 | .0001 |
| miR-192 | 64 415 185 | 64 415 294 | 0.68 | |
| miR-34c | 110 889 374 | 110 889 450 | .0001 | |
| let-7a-2 | 121 522 440 | 121 522 511 | .0001 | |
| Chr 12 (deletion) | miR-200c | 6 943 123 | 6 943 190 | .027 |
| miR-26a-2 | 56 504 659 | 56 504 742 | .0025 | |
| let-7i | 61 283 733 | 61 283 816 | .045 | |
| Chr 14 (amplification) | miR-342 | 99 645 745 | 99 645 843 | .0024 |
| miR-493 | 100 405 150 | 100 405 238 | NA | |
| miR-433 | 100 417 976 | 100 418 068 | NA | |
| miR-127 | 100 419 069 | 100 419 165 | .0001 | |
| miR-543 | 100 568 077 | 100 568 154 | NA | |
| miR-495 | 100 569 845 | 100 569 926 | NA | |
| miR-487b | 100 582 545 | 100 582 628 | NA | |
| miR-889 | 100 583 991 | 100 584 069 | NA | |
| Chr 15 (amplification) | miR-184 | 77 289 185 | 77 289 268 | .76 |
| miR-9–3 | 87 712 252 | 87 712 341 | NA | |
| Chr 17 (deletion) | miR-132 | 1 899 952 | 1 900 052 | .67 |
| miR-744 | 11 925 941 | 11 926 038 | NA | |
| Chr 18 (amplification) | miR-122 | 54 269 286 | 54 269 370 | .21 |
| Chr 19 (amplification) | miR-199a-1 | 10 789 102 | 10 789 172 | NA |
| miR-24–2 | 13 808 101 | 13 808 173 | NA | |
| miR-27a | 13 808 254 | 13 808 331 | NA | |
| miR-23a | 13 808 401 | 13 808 473 | NA | |
| miR-1270 | 20 371 080 | 20 371 162 | NA | |
| miR-330 | 50 834 092 | 50 834 185 | .013 | |
| miR-99b | 56 887 677 | 56 887 746 | NA | |
| let-7e | 56 887 851 | 56 887 929 | .52 | |
| miR-125a | 56 888 319 | 56 888 404 | .0001 | |
| miR-512-1 | 58 861 745 | 58 861 828 | NA | |
| miR-512-2 | 58 864 223 | 58 864 320 | NA | |
| miR-1323 | 58 867 034 | 58 867 106 | NA | |
| miR-520a | 58 885 947 | 58 886 031 | NA | |
| miR-518b | 58 897 803 | 58 897 885 | NA | |
| miR-517a | 58 907 334 | 58 907 420 | NA | |
| miR-517b | 58 916 142 | 58 916 208 | NA | |
| miR-516b-2 | 58 920 508 | 58 920 592 | NA | |
| miR-518a-1 | 58 926 072 | 58 926 156 | NA | |
| miR-518a-2 | 58 934 399 | 58 934 485 | NA | |
| miR-371 | 58 982 741 | 58 982 807 | .0001 | |
| Chr 20 (amplification) | miR-103-2 | 3 846 141 | 3 846 218 | .086 |
| miR-103-2-as | 3 846 149 | 3 846 210 | NA | |
| Chr 21 (amplification) | let-7c | 16 834 019 | 16 834 102 | .0015 |
| miR-125b-2 | 16 884 428 | 16 884 516 | .0001 | |
| miR-155 | 25 868 163 | 25 868 227 | .0001 | |
| Chr 22 (amplification) | miR-185 | 18 400 662 | 18 400 743 | .58 |
| miR-130b | 20 337 593 | 20 337 674 | .0001 | |
| chr X (deletion) | miR-221 | 45 490 529 | 45 490 638 | .02 |
| miR-222 | 45 491 365 | 45 491 474 | .0001 | |
| miR-98 | 53 599 909 | 53 600 027 | NA | |
| let-7f-2 | 53 600 878 | 53 600 960 | NA | |
| miR-1298 | 113 855 906 | 113 856 017 | NA | |
| miR-363 | 133 131 074 | 133 131 148 | NA | |
| miR-91a-2 | 133 131 234 | 133 131 308 | NA | |
| miR-19b-2 | 133 131 367 | 133 131 462 | NA | |
| miR-20b | 133 131 505 | 133 131 573 | NA |
| Chromosome . | miR . | Bp start . | Bp end . | P* . |
|---|---|---|---|---|
| Chr 1 (amplification) | miR-30e | 40 s683 565 | 40 683 656 | .08 |
| miR-30c-1 | 40 686 494 | 40 686 582 | NA | |
| miR-101-1 | 65 296 705 | 65 296 779 | .0008 | |
| miR-186 | 71 305 902 | 71 305 987 | .32 | |
| miR-9-1 | 154 656 757 | 154 656 845 | .26 | |
| miR-181b-1 | 197 094 625 | 197 094 734 | .0001 | |
| miR-181a-1 | 197 094 796 | 197 094 905 | .0084 | |
| miR-29c | 206 041 820 | 206 041 907 | NA | |
| Chr 3 (deletion) | miR-128-2 | 35 760 972 | 35 761 055 | .0001 |
| miR-26a-1 | 37 985 899 | 37 985 975 | .0001 | |
| miR-138-1 | 44 130 708 | 44 130 806 | .11 | |
| miR-425 | 49 032 585 | 49 032 671 | NA | |
| miR-191 | 49 033 055 | 49 033 146 | .95 | |
| let-7g | 52 277 334 | 52 277 417 | .0001 | |
| miR-15b | 161 605 070 | 161 605 167 | NA | |
| miR-16-2 | 161 605 227 | 161 605 307 | .042 | |
| miR-28 | 189 889 263 | 189 889 348 | NA | |
| Chr 5 (deletion) | miR-9-2 | 87 998 427 | 87 998 513 | NA |
| miR-886 | 135 444 076 | 135 444 196 | NA | |
| miR-143 | 148 788 674 | 148 788 779 | NA | |
| miR-378 | 149 092 581 | 149 092 646 | NA | |
| miR-146a | 159 844 937 | 159 845 035 | .0008 | |
| miR-103-1 | 167 920 487 | 167 920 548 | NA | |
| miR-340 | 179 374 909 | 179 375 003 | .0001 | |
| Chr 6 (amplification) | miR-30a | 72 169 975 | 72 170 045 | NA |
| Chr 7 (deletion) | miR-148a | 25 956 064 | 25 956 131 | .0001 |
| miR-25 | 99 529 119 | 99 529 202 | NA | |
| miR-93 | 99 529 327 | 99 529 406 | NA | |
| miR-106b | 99 529 552 | 99 529 633 | NA | |
| miR-129-1 | 127 635 161 | 127 635 232 | NA | |
| miR-182 | 129 197 459 | 129 197 568 | .0002 | |
| miR-183 | 129 201 981 | 129 202 090 | .0035 | |
| miR-29a | 130 212 046 | 130 212 109 | NA | |
| miR-26b-1 | 130 212 758 | 130 212 838 | NA | |
| Chr 8 (amplification) | miR-320a | 22 158 420 | 22 158 501 | NA |
| miR-486 | 41 637 116 | 41 637 183 | NA | |
| miR-30d | 135 886 301 | 135 886 370 | NA | |
| miR-151 | 141 811 845 | 141 811 934 | .057 | |
| Chr 9 | miR-101-2 | 4 840 297 | 4 840 375 | .54 |
| miR-199b | 130 046 821 | 130 046 930 | NA | |
| Chr 10 (deletion) | miR-146b | 104 186 259 | 104 186 331 | .0001 |
| miR-1307 | 105 144 000 | 105 144 148 | NA | |
| Chr 11 (amplification) | miR-130a | 57 165 247 | 57 165 335 | .0001 |
| miR-192 | 64 415 185 | 64 415 294 | 0.68 | |
| miR-34c | 110 889 374 | 110 889 450 | .0001 | |
| let-7a-2 | 121 522 440 | 121 522 511 | .0001 | |
| Chr 12 (deletion) | miR-200c | 6 943 123 | 6 943 190 | .027 |
| miR-26a-2 | 56 504 659 | 56 504 742 | .0025 | |
| let-7i | 61 283 733 | 61 283 816 | .045 | |
| Chr 14 (amplification) | miR-342 | 99 645 745 | 99 645 843 | .0024 |
| miR-493 | 100 405 150 | 100 405 238 | NA | |
| miR-433 | 100 417 976 | 100 418 068 | NA | |
| miR-127 | 100 419 069 | 100 419 165 | .0001 | |
| miR-543 | 100 568 077 | 100 568 154 | NA | |
| miR-495 | 100 569 845 | 100 569 926 | NA | |
| miR-487b | 100 582 545 | 100 582 628 | NA | |
| miR-889 | 100 583 991 | 100 584 069 | NA | |
| Chr 15 (amplification) | miR-184 | 77 289 185 | 77 289 268 | .76 |
| miR-9–3 | 87 712 252 | 87 712 341 | NA | |
| Chr 17 (deletion) | miR-132 | 1 899 952 | 1 900 052 | .67 |
| miR-744 | 11 925 941 | 11 926 038 | NA | |
| Chr 18 (amplification) | miR-122 | 54 269 286 | 54 269 370 | .21 |
| Chr 19 (amplification) | miR-199a-1 | 10 789 102 | 10 789 172 | NA |
| miR-24–2 | 13 808 101 | 13 808 173 | NA | |
| miR-27a | 13 808 254 | 13 808 331 | NA | |
| miR-23a | 13 808 401 | 13 808 473 | NA | |
| miR-1270 | 20 371 080 | 20 371 162 | NA | |
| miR-330 | 50 834 092 | 50 834 185 | .013 | |
| miR-99b | 56 887 677 | 56 887 746 | NA | |
| let-7e | 56 887 851 | 56 887 929 | .52 | |
| miR-125a | 56 888 319 | 56 888 404 | .0001 | |
| miR-512-1 | 58 861 745 | 58 861 828 | NA | |
| miR-512-2 | 58 864 223 | 58 864 320 | NA | |
| miR-1323 | 58 867 034 | 58 867 106 | NA | |
| miR-520a | 58 885 947 | 58 886 031 | NA | |
| miR-518b | 58 897 803 | 58 897 885 | NA | |
| miR-517a | 58 907 334 | 58 907 420 | NA | |
| miR-517b | 58 916 142 | 58 916 208 | NA | |
| miR-516b-2 | 58 920 508 | 58 920 592 | NA | |
| miR-518a-1 | 58 926 072 | 58 926 156 | NA | |
| miR-518a-2 | 58 934 399 | 58 934 485 | NA | |
| miR-371 | 58 982 741 | 58 982 807 | .0001 | |
| Chr 20 (amplification) | miR-103-2 | 3 846 141 | 3 846 218 | .086 |
| miR-103-2-as | 3 846 149 | 3 846 210 | NA | |
| Chr 21 (amplification) | let-7c | 16 834 019 | 16 834 102 | .0015 |
| miR-125b-2 | 16 884 428 | 16 884 516 | .0001 | |
| miR-155 | 25 868 163 | 25 868 227 | .0001 | |
| Chr 22 (amplification) | miR-185 | 18 400 662 | 18 400 743 | .58 |
| miR-130b | 20 337 593 | 20 337 674 | .0001 | |
| chr X (deletion) | miR-221 | 45 490 529 | 45 490 638 | .02 |
| miR-222 | 45 491 365 | 45 491 474 | .0001 | |
| miR-98 | 53 599 909 | 53 600 027 | NA | |
| let-7f-2 | 53 600 878 | 53 600 960 | NA | |
| miR-1298 | 113 855 906 | 113 856 017 | NA | |
| miR-363 | 133 131 074 | 133 131 148 | NA | |
| miR-91a-2 | 133 131 234 | 133 131 308 | NA | |
| miR-19b-2 | 133 131 367 | 133 131 462 | NA | |
| miR-20b | 133 131 505 | 133 131 573 | NA |
Bp indicates base pair; as, antisense; and NA, not available.
Value was generated from microarray data when comparing miRNA expression changes between AML patient (n = 58) and control CD34+ samples (n =11).
It is possible that tumor suppressor miRNAs may be transcriptionally silenced by mechanisms other than genomic deletions and unintentionally omitted in our analysis above. Therefore, we evaluated miRNAs within deleted regions in the leukemic cell lines and compared their expression levels with nonleukemic human CD34+ cells. We used a recently published sequencing library of small RNAs generated from human CD34+ bone marrow cells12 (supplemental Table 4). Of the miRNAs mapping to deleted regions, 29 are expressed in human CD34+ cells (Figure 4) and were further analyzed. We compared the expression of these 29 miRNAs between normal CD34+ cells and each leukemic cell line and found that 65%-86% of miRNAs that map to leukemia-associated deleted regions were expressed lower in the cell lines compared with CD34+ cells. A similar pattern of miRNA repression was observed in primary AML samples (n = 58) compared with control CD34+ cells (n = 11) (Figure 4). Collectively, these findings suggest that a subset of miRNAs within deleted regions in leukemic cells correlates with reduced expression (Figure 4). Interestingly, a few miRNAs (eg, miR-378, miR-103-1, miR-25, and let-7f-2) are consistently overexpressed in the leukemic cell lines despite residing within a hemizygous deletion (Figure 4), potentially representing genes essential to cell maintenance.
miRNA expression comparison to normal human CD34+ cells. Tag counts of miRNAs that encoded from deleted regions for each cell line are compared with normal CD34+ cells. Shown are the log expression levels for individual cell lines relative to CD34+ cells. The sequencing library of small RNAs generated from human CD34+ bone marrow cells was obtained from a recently published analysis.12 miRNA expression levels from AML patient samples are shown from CD34+ marrow cells (n = 58) and shown relative to CD34+ cells evaluated from nondiseased persons (n = 11). Data are adapted from published microarray data (E-TABM-970 and E-TABM-405).
miRNA expression comparison to normal human CD34+ cells. Tag counts of miRNAs that encoded from deleted regions for each cell line are compared with normal CD34+ cells. Shown are the log expression levels for individual cell lines relative to CD34+ cells. The sequencing library of small RNAs generated from human CD34+ bone marrow cells was obtained from a recently published analysis.12 miRNA expression levels from AML patient samples are shown from CD34+ marrow cells (n = 58) and shown relative to CD34+ cells evaluated from nondiseased persons (n = 11). Data are adapted from published microarray data (E-TABM-970 and E-TABM-405).
miRNAs on chromosome 5q may be important in AML
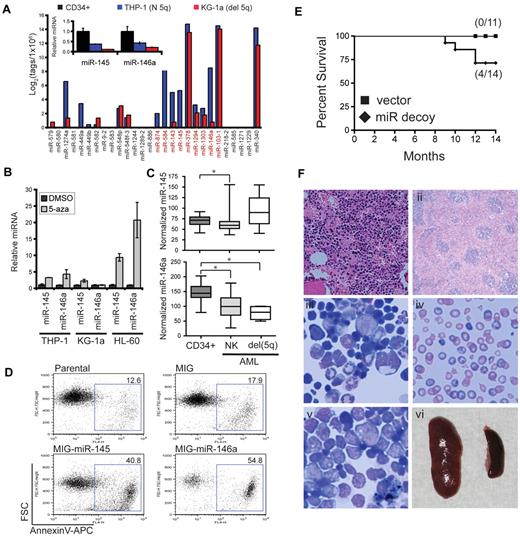
To further explore the role of miRNAs within deleted regions in myeloid malignancies we focused on miRNAs on chromosome 5 (supplemental Figure 1). Deletion of chromosome 5q is the most common cytogenetic alteration in AML and myelodysplastic syndrome (MDS; Figure 1).20 To evaluate the potential effect of del (5q) on miRNA expression, we compared the relative tag counts for 2 leukemic cell lines, one with a deletion of chromosome 5q (KG-1a) and one diploid at chromosome 5q (THP-1). Effect of the chromosome 5q deletion on miRNA expression is evident by the lower expression levels of miRNAs in KG-1a compared with THP-1 (Figure 5A). As an example, miR-145 and miR-146a, 2 miRNAs within a commonly deleted region in del (5q) myeloid malignancies (supplemental Figure 1), are expressed lower in cell lines with the chromosome 5q deletion or diploid at this locus compared with CD34+ cells (Figures 4 and 5A). To independently confirm this observation, we performed quantitative PCR to access miR-145 and miR-146a expression levels in CD34+ (n = 3), THP-1, and KG-1a cells. As shown in Figure 5A (inset), miR-145 and miR-146a levels are highest in CD34+ cells and lower in leukemic cell lines with del (5q) or diploid (5q). Further, miR-145 and miR-146a are expressed significantly lower in diploid chromosome 5q leukemic cell lines compared with CD34+ cells (P = .009 and P = .02, respectively; Figure 5A inset). This suggests that miR-145 or miR-146a or both may be silenced in leukemia with a diploid chromosome 5q and further depressed in leukemias with del (5q). To investigate a potential epigenetic suppression (such as by DNA methylation) of miR-145 and miR-146a in AML, we treated 3 AML cell lines with a demethylating agent (5-aza) and examined miRNA expression. As shown in Figure 5B, expression of miR-145 and miR-146a increase ≥ 3-fold after 5-aza treatment of THP-1 and HL-60 but not in KG-1a. To further confirm the importance of these miRNAs in primary AML patient samples, we evaluated the expression of miR-145 and miR-146a in CD34+ cells isolated from patients with AML with a normal karyotype (NK; n = 54) and with del(5q) (n = 4) and controls (n = 11) from previously published expression array data4 (Figure 5C). Expression of miR-145 is significantly reduced in patients with AML with a normal karyotype compared with controls (P = .019) but did not reach significance in the group with del(5q) (P = .14). Expression of miR-146a is significantly reduced in patients with AML with a NK (P = .0006) and further reduced in patients with del(5q) (P = .0055) compared with control samples (Figure 5C). That we observe reduced expression of miRNAs that are associated with deleted regions in AML, even in cell lines or patient samples that may not harbor the specific deletion, suggests that this subset of miRNAs may play a leukemia suppressor role.
Reduced expression of miR-145 and miR-146a results in myelodysplastic/myeloproliferative features. (A) miRNA tag counts (log2) are shown for 2 cell lines, one with a chromosome 5q deletion (KG-1a) and one diploid at chromosome 5q (THP-1). (Inset) Relative levels of miR-145 and miR-146a were determined by quantitative PCR in human CD34+ cells (n = 3), KG-1a, and THP-1 cells. (B) KG-1a, THP-1, and HL-60 cells were treated with 10μM 5-azacytadine (5-aza) or dimethyl sulfoxide (DMSO) for 48 hours. Expression of miR-145 and miR-146a was evaluated by quantitative reverse transcription PCR and shown relative to DMSO after normalization to 5S. (C) miR-145 (top) and miR-146a (bottom) expression levels are shown for CD34+ marrow cells isolated from patients with AML with a NK (n = 54) or deletion of chr 5q (del 5q; n = 4). As controls, CD34+ cells were evaluated from nondiseased persons (n = 11). Data are adapted from published microarray data (E-TABM-970 and E-TABM-405). Normalized expression levels are visualized as box-and-whisker plots. (D) HL-60 cells were retrovirally transduced with empty vector (MIG), miR-145, or miR-146a. Transduced cells were isolated by fluorescence-activated cell sorting and analyzed for Annexin V binding after 1 week in culture. Shown is a representative analysis from 2 independent transductions. Gating strategy is provided in supplemental Figure 2. (E) Kaplan-Meier survival curves for mice reconstituted with marrow transduced with vector (n = 11) or miR-145/miR-146a decoy (n = 15) from 3 independent transplants. (F) Hematoxylin and eosin–stained femur (i) and spleen (ii) sections from a myeloproliferative-like diseased mouse. Wright-Giemsa–stained bone marrow cytospins (iii-v) and blood smears (iv) from a bone marrow failure mouse (iii-iv) and a leukemic mouse (v). Spleen image was obtained at time of death from a leukemic mouse (587 mg) and compared with a spleen from a control mouse (100 mg; vi).
Reduced expression of miR-145 and miR-146a results in myelodysplastic/myeloproliferative features. (A) miRNA tag counts (log2) are shown for 2 cell lines, one with a chromosome 5q deletion (KG-1a) and one diploid at chromosome 5q (THP-1). (Inset) Relative levels of miR-145 and miR-146a were determined by quantitative PCR in human CD34+ cells (n = 3), KG-1a, and THP-1 cells. (B) KG-1a, THP-1, and HL-60 cells were treated with 10μM 5-azacytadine (5-aza) or dimethyl sulfoxide (DMSO) for 48 hours. Expression of miR-145 and miR-146a was evaluated by quantitative reverse transcription PCR and shown relative to DMSO after normalization to 5S. (C) miR-145 (top) and miR-146a (bottom) expression levels are shown for CD34+ marrow cells isolated from patients with AML with a NK (n = 54) or deletion of chr 5q (del 5q; n = 4). As controls, CD34+ cells were evaluated from nondiseased persons (n = 11). Data are adapted from published microarray data (E-TABM-970 and E-TABM-405). Normalized expression levels are visualized as box-and-whisker plots. (D) HL-60 cells were retrovirally transduced with empty vector (MIG), miR-145, or miR-146a. Transduced cells were isolated by fluorescence-activated cell sorting and analyzed for Annexin V binding after 1 week in culture. Shown is a representative analysis from 2 independent transductions. Gating strategy is provided in supplemental Figure 2. (E) Kaplan-Meier survival curves for mice reconstituted with marrow transduced with vector (n = 11) or miR-145/miR-146a decoy (n = 15) from 3 independent transplants. (F) Hematoxylin and eosin–stained femur (i) and spleen (ii) sections from a myeloproliferative-like diseased mouse. Wright-Giemsa–stained bone marrow cytospins (iii-v) and blood smears (iv) from a bone marrow failure mouse (iii-iv) and a leukemic mouse (v). Spleen image was obtained at time of death from a leukemic mouse (587 mg) and compared with a spleen from a control mouse (100 mg; vi).
Reduced expression of miR-145 and miR-146 results in AML in vivo, whereas re-expression of miR-145 or miR-146a suppresses AML cells in vitro
To determine whether miR-145 and miR-146a function as leukemic suppressors, restoration of their expression in AML cells harboring a del(5q) should therefore negatively effect survival or proliferation or both. We overexpressed miR-145 or miR-146a in HL-60 cells with the use of a retroviral vector coexpressing GFP as a marker. HL-60 cells were chosen because miR-145 and miR-146a are both expressed at low levels (Figure 4) and are also potentially suppressed by epigenetic mechanisms (Figure 5B). After infection of HL-60 cells and sorting for the transduced population, a noticeable impairment in growth and loss of transduced cells (GFP+) was observed in HL-60 cells expressing miR-145 or miR-146a (supplemental Figure 2; data not shown). To determine whether miR-145 and miR-146a effect the survival of HL-60 cells, we measured annexin V staining. As shown in Figure 5C, ∼ 40% and 50% of HL-60 cells transduced with miR-145 or miR-146a, respectively, stained positively for annexin V. In contrast, vector control cells or nontransduced parental cells grew robustly after infection and exhibited ∼ 10% annexin V staining (Figure 5C). Re-expression of miR-145 or miR-146a inhibits survival and growth of leukemic cells, therefore implicating them in the pathogenesis of AML.
In a recent study, we showed the knockdown of miR-145 and miR-146a results in nonfatal myelodysplastic syndrome in vivo.14 Because MDS often precedes evolution to leukemia, we wanted to determine whether miR-145 and miR-146a contribute to an eventual AML by extending the analysis of mice for ≤ 14 months. Stably knocked down miR-145 and miR-146a in mouse hematopoietic stem/progenitor cells was achieved with the use of retroviral-mediated overexpression of miR-145 and miR-146 target sequences (miR decoy) engineered into the 3′-UTR of YFP.14 Lethally irradiated C57Bl/6 mice received a transplant with 7.5 × 105 marrow cells transduced with the miR decoy construct or vector alone, along with 2 × 105 wild-type competitor marrow cells. We found that starting at 8 months, ∼ 30% (4 of 14) of the miR decoy mice began to succumb to hematologic myeloid malignancies (P = .049; Figure 5E). Diseased mice displayed features of bone marrow failure, myeloproliferation, or acute leukemia, as supported by histologic findings and blood counts (Figure 5D; data not shown). Depending on the diseased mouse, evidence of hypercellular marrows, dyshematopoiesis, anemia, immature blastlike cells, and splenomegaly was observed (Figure 5F). To investigate previously defined targets of miR-145 and miR-146a in the mice, we measured protein expression of interleukin-1 receptor–associated kinase 1 (IRAK1; miR-146a target), tumor necrosis factor receptor–associated factor 6 (TRAF6; miR-146a target), and Mal/toll–interleukin-1 receptor domain–containing adaptor protein (TIRAP; miR-145 target). We confirmed by Western blot analysis that IRAK1, TRAF6, and TIRAP were, generally, increased in miR decoy marrow cells before transplantation, after transplantation, and in a leukemic miR-decoy mouse (supplemental Figure 3). Reduced expression of miR-145 and miR-146a, 2 leukemia-associated miRNAs identified in our analysis, results in myeloid diseases preceded by an MDS-like phenotype in vivo.
Novel miRNAs mapping to leukemia-associated genomic alterations
Unclassified sequence reads represented 10%-60% of the filtered libraries (supplemental Table 3). To identify novel candidate miRNAs among the unclassified reads, we used available algorithms.11 Novel miRNAs from the 6 cell line libraries include 28 unique miRNA sequences (Table 2; supplemental Table 5). Five novel miRNAs were expressed at levels near the background cutoff (> 0.001), including miR-478 and miR-486 (supplemental Table 5). Because we are most interested in miRNAs that map to CNAs, we determined which of the novel miRNA sequences are encoded from leukemia-associated alterations in the 6 cell lines. Eighteen (of 28) of the novel miRNAs are encoded from genomic regions of CNAs, distributed evenly between deletions and amplifications (Figure 2D; Table 2; supplemental Table 5). For example, miR-468 and miR-484 both map to a region of chromosome 11q, a recurring region of amplification in our cell lines (Figure 1; Table 2).
Novel miRNAs mapping to copy number alterations
| miRNA name . | Chromosome . | Bp start . | Bp end . | Deletion/amplification . | Mature miRNA sequence . | Target genes* . |
|---|---|---|---|---|---|---|
| miR-472 | 10 | 115 041 356 | 115 041 465 | Del | AAATGAATCATGTTGGGCCTGT | ADIPOQ |
| miR-463 | 10 | 103 351 146 | 103 351 255 | Del | AAGGGCTTCCTCTCTGCAGGAC | SYNGR1 |
| miR-431 | 13 | 49 468 528 | 49 468 637 | N | ACAAAAAAAAAAGCCCAACCCT | FOXK1, RELB, NF1 |
| miR-443 | 16 | 14 902 849 | 14 902 958 | N | AGAAGGGGTGAAATTTAAACGT | SYNGAP1 |
| miR-474 | 1 | 159 683 046 | 159 683 155 | Amp | AGCGGAACTTGAGGAGCCGAG | MEX3A |
| miR-460 | 4 | 153 629 916 | 153 630 025 | Del | AGCTTTTGGGAATTCAGGTAG | PTPRT |
| miR-468 | 11 | 61 032 625 | 61 032 734 | Amp | AGGGGGCGGGCTCCGGCG | ZNF385A, AAK1, NFIX |
| miR-478 | 2 | 96 827 730 | 96 827 839 | N | ATCAGGGCTTGTGGAATGGGAAG | ADAM11 |
| miR-432 | 19 | 6 367 420 | 6 367 529 | Amp | CAGCCCGGATCCCAGCCCACTTA | FBXL19 |
| miR-457 | 16 | 2 260 666 | 2 260 775 | N | CTCGTGGGCTCTGGCCACGGC | MN1 |
| miR-442 | 3 | 69 180 778 | 69 180 887 | Del | CTGACTGAATAGGTAGGGTCAT | CADM2 |
| miR-422 | 15 | 63 798 621 | 63 798 730 | Amp | GAAGAACTGTTGCATTTGCCC | MSL2L1, ERG, MPL, TRAF7 |
| miR-425 | 22 | 18 331 270 | 18 331 379 | Amp | GAGGGCATGCGCACTTTGTCC | RC3H1 |
| miR-427 | 3 | 50 687 498 | 50 687 607 | Del | GATGCGCCGCCCACTGCCCCGC | IMPAD1 |
| miR-437 | 2 | 207 356 179 | 207 356 288 | N | GCTGCACCGGAGACTGGGTAA | SP8, MAF, TET3 |
| miR-486 | 22 | 29 886 043 | 29 886 152 | Amp | GGAGGAACCTTGGAGCTTCGGC | SPTBN1, NFIX, NF2, ADAR |
| miR-418 | 19 | 12 675 394 | 12 675 503 | Amp | GTATTCGTACTGTCTGATGGG | |
| miR-416 | 2 | 12 256 683 | 12 256 792 | Del | TAGTGGATGATGCACTCTGTGC | ABCA5, JAK1 |
| miR-428 | 8 | 96 154 304 | 96 154 413 | Amp | TGAGGAGATCGTCGAGGTTGGC | LUZP1 |
| miR-419 | 16 | 1 724 971 | 1 725 080 | N | TGCACGGCACTGGGGACACGT | RELT |
| miR-404 | 16 | 14 912 572 | 14 912 681 | N | TGGGGCGGAGCTTCCGGAGGCC | TGRB1, NFIX |
| miR-408 | 16 | 84 505 724 | 84 505 833 | Del | TGGTGTGGAAGTCTAGGCCTG | FBXO41 |
| miR-481 | 7 | 29 686 866 | 29 686 975 | Del | TGTCTTACTCCCTCAGGCACAT | CUGBP2, MN1 |
| miR-450 | 9 | 136 881 134 | 136 881 243 | N | TGTGATATCATGGTTCCTGGGA | NLGN4X, ROCK1, ITCH |
| miR-402 | 9 | 96 888 098 | 96 888 207 | N | TGTTCCTGCTGAACTGAGCCAG | KLHL9, src |
| miR-421 | 19 | 12 912 277 | 12 912 386 | Amp | TTGGAGGGTGTGGAAGACATC | ACT1, SPARC |
| miR-414 | 19 | 764 560 | 764 669 | N | TTGGCCATGGGGCTGCGCGGGGC | KRAS |
| miRNA name . | Chromosome . | Bp start . | Bp end . | Deletion/amplification . | Mature miRNA sequence . | Target genes* . |
|---|---|---|---|---|---|---|
| miR-472 | 10 | 115 041 356 | 115 041 465 | Del | AAATGAATCATGTTGGGCCTGT | ADIPOQ |
| miR-463 | 10 | 103 351 146 | 103 351 255 | Del | AAGGGCTTCCTCTCTGCAGGAC | SYNGR1 |
| miR-431 | 13 | 49 468 528 | 49 468 637 | N | ACAAAAAAAAAAGCCCAACCCT | FOXK1, RELB, NF1 |
| miR-443 | 16 | 14 902 849 | 14 902 958 | N | AGAAGGGGTGAAATTTAAACGT | SYNGAP1 |
| miR-474 | 1 | 159 683 046 | 159 683 155 | Amp | AGCGGAACTTGAGGAGCCGAG | MEX3A |
| miR-460 | 4 | 153 629 916 | 153 630 025 | Del | AGCTTTTGGGAATTCAGGTAG | PTPRT |
| miR-468 | 11 | 61 032 625 | 61 032 734 | Amp | AGGGGGCGGGCTCCGGCG | ZNF385A, AAK1, NFIX |
| miR-478 | 2 | 96 827 730 | 96 827 839 | N | ATCAGGGCTTGTGGAATGGGAAG | ADAM11 |
| miR-432 | 19 | 6 367 420 | 6 367 529 | Amp | CAGCCCGGATCCCAGCCCACTTA | FBXL19 |
| miR-457 | 16 | 2 260 666 | 2 260 775 | N | CTCGTGGGCTCTGGCCACGGC | MN1 |
| miR-442 | 3 | 69 180 778 | 69 180 887 | Del | CTGACTGAATAGGTAGGGTCAT | CADM2 |
| miR-422 | 15 | 63 798 621 | 63 798 730 | Amp | GAAGAACTGTTGCATTTGCCC | MSL2L1, ERG, MPL, TRAF7 |
| miR-425 | 22 | 18 331 270 | 18 331 379 | Amp | GAGGGCATGCGCACTTTGTCC | RC3H1 |
| miR-427 | 3 | 50 687 498 | 50 687 607 | Del | GATGCGCCGCCCACTGCCCCGC | IMPAD1 |
| miR-437 | 2 | 207 356 179 | 207 356 288 | N | GCTGCACCGGAGACTGGGTAA | SP8, MAF, TET3 |
| miR-486 | 22 | 29 886 043 | 29 886 152 | Amp | GGAGGAACCTTGGAGCTTCGGC | SPTBN1, NFIX, NF2, ADAR |
| miR-418 | 19 | 12 675 394 | 12 675 503 | Amp | GTATTCGTACTGTCTGATGGG | |
| miR-416 | 2 | 12 256 683 | 12 256 792 | Del | TAGTGGATGATGCACTCTGTGC | ABCA5, JAK1 |
| miR-428 | 8 | 96 154 304 | 96 154 413 | Amp | TGAGGAGATCGTCGAGGTTGGC | LUZP1 |
| miR-419 | 16 | 1 724 971 | 1 725 080 | N | TGCACGGCACTGGGGACACGT | RELT |
| miR-404 | 16 | 14 912 572 | 14 912 681 | N | TGGGGCGGAGCTTCCGGAGGCC | TGRB1, NFIX |
| miR-408 | 16 | 84 505 724 | 84 505 833 | Del | TGGTGTGGAAGTCTAGGCCTG | FBXO41 |
| miR-481 | 7 | 29 686 866 | 29 686 975 | Del | TGTCTTACTCCCTCAGGCACAT | CUGBP2, MN1 |
| miR-450 | 9 | 136 881 134 | 136 881 243 | N | TGTGATATCATGGTTCCTGGGA | NLGN4X, ROCK1, ITCH |
| miR-402 | 9 | 96 888 098 | 96 888 207 | N | TGTTCCTGCTGAACTGAGCCAG | KLHL9, src |
| miR-421 | 19 | 12 912 277 | 12 912 386 | Amp | TTGGAGGGTGTGGAAGACATC | ACT1, SPARC |
| miR-414 | 19 | 764 560 | 764 669 | N | TTGGCCATGGGGCTGCGCGGGGC | KRAS |
Bp indicates base pair; Del, deletion; Amp, amplification; and N, copy number neutral.
Target Scan v5.1 was used to identify predicted target genes.
Identification of miRNA variants and mature mRNA variants
Use of small RNA sequencing approaches allowed identification of miRNAs that exhibit variations in nucleotides compared with their miRBase reference sequence. In the 6 libraries, mature mRNA variants (isomiRs) were found more abundant compared with the miRBase reference sequence in some instances. For example, the most abundant sequence of miR-140-3p in the HL-60 library did not match the miRBase reference sequence. The miRBase sequence (TACCACAGGGTAGAACCACGG) was expressed at low levels in this library, but instead an isomiR of miR-140-3p (ACCACAGGGTAGAACCACGGAC) with a 5′ nucleotide deletion and 3′ nucleotide additions (underlined) was abundantly detected (913 vs 3 reads). Examination of the libraries revealed that 18 highly expressed miRNAs are overrepresented by an isomiR rather than the reference miRNA sequence (Table 3). Modifications among the isomiRs included nucleotide 5′ and 3′ additions or deletions. The functional effect of the various miRNA modifications has not been studied in depth, but theoretically it could affect the function of miRNAs as well as detection by standard real-time PCR approaches.
Abundantly expressed and overrepresented isomiRNAs
| Cell line . | miRNA name . | miRBase counts . | miRBase sequence* . | isomiR sequence* . | isomiR counts . | Ratio (isomR:miRbase) . |
|---|---|---|---|---|---|---|
| HL60 | miR-107 | 1136 | AGCAGCATTGTACAGGGCTATCA | AGCAGCATTGTACAGGGCTAT | 3915 | 3.4 |
| miR-181a | 840 | AACATTCAACGCTGTCGGTGAGT | AACATTCAACGCTGTCGGTGAGTTT | 3481 | 4.1 | |
| miR-199a-3p | 0 | ACAGTAGTCTGCACATTGGTTA | ACAGTAGTCTGCACATTGGTT | 2896 | 2896 | |
| miR-181b | 296 | AACATTCATTGCTGTCGGTGGGT | AACATTCATTGCTGTCGGTGGGTT | 2455 | 8.3 | |
| miR-140-3p | 3 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 913 | 304.3 | |
| miR-101 | 74 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 624 | 8.4 | |
| miR-222 | 24 | AGCTACATCTGGCTACTGGGT | AGCTACATCTGGCTACTGGGTCTC | 391 | 16.3 | |
| miR-23a | 79 | ATCACATTGCCAGGGATTTCC | ATCACATTGCCAGGGATTTCCA | 295 | 3.7 | |
| miR-30d | 7 | TGTAAACATCCCCGACTGGAAG | TGTAAACATCCCCGACTGGAAGCT | 193 | 27.6 | |
| THP1 | miR-101 | 1859 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 7710 | 4.1 |
| miR-140-3p | 249 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 6943 | 27.9 | |
| UT-7 | miR-140-3p | 15 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 642 | 42.8 |
| miR-101 | 10 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 72 | 7.2 | |
| miR-142-5p | 23 | CATAAAGTAGAAAGCACTACT | ATAAAGTAGAAAGCACTACTAA | 68 | 3.0 | |
| MDSL | miR-183 | 133 | TATGGCACTGGTAGAATTCACT | ATGGCACTGGTAGAATTCACTG | 367 | 2.8 |
| miR-140-3p | 0 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 148 | 148 | |
| KG-1 | miR-101 | 877 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 2001 | 2.3 |
| KG-1a | miR-101 | 671 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 1573 | 2.3 |
| Cell line . | miRNA name . | miRBase counts . | miRBase sequence* . | isomiR sequence* . | isomiR counts . | Ratio (isomR:miRbase) . |
|---|---|---|---|---|---|---|
| HL60 | miR-107 | 1136 | AGCAGCATTGTACAGGGCTATCA | AGCAGCATTGTACAGGGCTAT | 3915 | 3.4 |
| miR-181a | 840 | AACATTCAACGCTGTCGGTGAGT | AACATTCAACGCTGTCGGTGAGTTT | 3481 | 4.1 | |
| miR-199a-3p | 0 | ACAGTAGTCTGCACATTGGTTA | ACAGTAGTCTGCACATTGGTT | 2896 | 2896 | |
| miR-181b | 296 | AACATTCATTGCTGTCGGTGGGT | AACATTCATTGCTGTCGGTGGGTT | 2455 | 8.3 | |
| miR-140-3p | 3 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 913 | 304.3 | |
| miR-101 | 74 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 624 | 8.4 | |
| miR-222 | 24 | AGCTACATCTGGCTACTGGGT | AGCTACATCTGGCTACTGGGTCTC | 391 | 16.3 | |
| miR-23a | 79 | ATCACATTGCCAGGGATTTCC | ATCACATTGCCAGGGATTTCCA | 295 | 3.7 | |
| miR-30d | 7 | TGTAAACATCCCCGACTGGAAG | TGTAAACATCCCCGACTGGAAGCT | 193 | 27.6 | |
| THP1 | miR-101 | 1859 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 7710 | 4.1 |
| miR-140-3p | 249 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 6943 | 27.9 | |
| UT-7 | miR-140-3p | 15 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 642 | 42.8 |
| miR-101 | 10 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 72 | 7.2 | |
| miR-142-5p | 23 | CATAAAGTAGAAAGCACTACT | ATAAAGTAGAAAGCACTACTAA | 68 | 3.0 | |
| MDSL | miR-183 | 133 | TATGGCACTGGTAGAATTCACT | ATGGCACTGGTAGAATTCACTG | 367 | 2.8 |
| miR-140-3p | 0 | TACCACAGGGTAGAACCACGG | ACCACAGGGTAGAACCACGGAC | 148 | 148 | |
| KG-1 | miR-101 | 877 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 2001 | 2.3 |
| KG-1a | miR-101 | 671 | TACAGTACTGTGATAACTGAA | GTACAGTACTGTGATAACTGAA | 1573 | 2.3 |
Bold letters in sequences indicate nucleotides that differ between the miRBase and isomiR sequence.
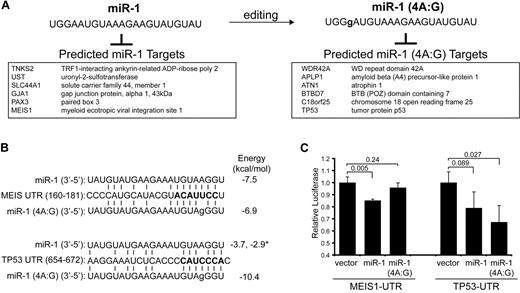
Another less-frequent subset of miRNAs are ones that show nucleotide discrepancies of the mature miRNA, other than single nucleotide extensions or eliminations. These nucleotide discrepancies are inconsistent with sequencing errors, but instead probably represent posttranscriptional RNA editing of miRNA mature sequences. We detected 7 miRNAs undergoing adenine-to-guanine (A:G) substitutions with a frequency > 1% of the respective isomiR reads. A:G substitutions are the most commonly identified nucleotide substitutions in our libraries, potentially explained by spontaneous or enzymatic deamination of adenine to guanine. Of the A:G edits, miR-423-5p represented 6% of all miR-423 isomiRs (Table 4). Six other miRNAs (miR-1, miR-30d, let-7d, miR-103, miR-25*, and miR-24) contained edits in > 1% of their respective isomiR pools (Table 4). Most edits occurred between positions 17 and 20. However, editing events observed in miR-1 were in the fourth position, which includes the seed region (2-8 nucleotides) (Figure 6A). As shown in Figure 6A, a substitution of A:G (position 4) changed the repertoire of predicted miRNA targets.
miRNA editing of adenine to guanine (A:G)
| miRNA . | Cell line . | Position . | Count . | Total . | Ratio* . |
|---|---|---|---|---|---|
| miR-1 | UT7 | 4 | 23 | 2306 | 0.0100 |
| miR-30d | KG1a | 20 | 11 | 1077 | 0.0102 |
| let-7d | UT7 | 1 | 12 | 999 | 0.0120 |
| miR-103 | UT7 | 20 | 15 | 1160 | 0.0129 |
| miR-25* | KG1a | 17 | 13 | 953 | 0.0136 |
| miR-24 | KG1a | 18 | 32 | 1774 | 0.0180 |
| miR-423-5p | KG1a | 19 | 770 | 11628 | 0.0662 |
| miRNA . | Cell line . | Position . | Count . | Total . | Ratio* . |
|---|---|---|---|---|---|
| miR-1 | UT7 | 4 | 23 | 2306 | 0.0100 |
| miR-30d | KG1a | 20 | 11 | 1077 | 0.0102 |
| let-7d | UT7 | 1 | 12 | 999 | 0.0120 |
| miR-103 | UT7 | 20 | 15 | 1160 | 0.0129 |
| miR-25* | KG1a | 17 | 13 | 953 | 0.0136 |
| miR-24 | KG1a | 18 | 32 | 1774 | 0.0180 |
| miR-423-5p | KG1a | 19 | 770 | 11628 | 0.0662 |
A:G edits occurring in > 1% of reads.
Ratio was calculated from the edited miRNA per all of the miRNA reads.
Editing of miR-1 occurs within the seed region and affects binding to mRNA targets. (A) Nucleotide modification at position 4 is shown for miR-1. An adenine to guanine (A:G) change was observed in UT-7, representing 23 of 2306 total miR-1 reads (Table 4). The effect of A:G edit at position 4 (4A:G) on mRNA target specificity is illustrated by comparing select miR-1 and miR-1 (4A:G) targets. The predicted targets for miR-1 (4A:G) are entirely different compared with the unedited miR-1. (B) Shown is a sequence alignment of miR-1 (wild-type and edited) and its binding sites in the 3′-UTR of MEIS1 (position 160-181) and TP53 (position 654-672). The miRNA-mRNA binding energy score (in kcal/mol) was determined for each pairing and shown on the right. The * indicates the binding energy score for a second miR-1 binding site in the TP53 UTR (position 689-698). (C) Luciferase activity was measured for MEIS1-UTR and TP53-UTR luciferase reporters in the presence of miR-1, miR-1 (4A:G), or vector control. Shown are relative values of 2 independent experiments performed in triplicate.
Editing of miR-1 occurs within the seed region and affects binding to mRNA targets. (A) Nucleotide modification at position 4 is shown for miR-1. An adenine to guanine (A:G) change was observed in UT-7, representing 23 of 2306 total miR-1 reads (Table 4). The effect of A:G edit at position 4 (4A:G) on mRNA target specificity is illustrated by comparing select miR-1 and miR-1 (4A:G) targets. The predicted targets for miR-1 (4A:G) are entirely different compared with the unedited miR-1. (B) Shown is a sequence alignment of miR-1 (wild-type and edited) and its binding sites in the 3′-UTR of MEIS1 (position 160-181) and TP53 (position 654-672). The miRNA-mRNA binding energy score (in kcal/mol) was determined for each pairing and shown on the right. The * indicates the binding energy score for a second miR-1 binding site in the TP53 UTR (position 689-698). (C) Luciferase activity was measured for MEIS1-UTR and TP53-UTR luciferase reporters in the presence of miR-1, miR-1 (4A:G), or vector control. Shown are relative values of 2 independent experiments performed in triplicate.
To demonstrate that the editing event in the seed region of miR-1 (4A:G) alters the binding properties of the miR-1, we measured miRNA binding with the use of 3′-UTR sequence fragments containing the predicted targets of miR-1 and miR-1 (4A:G) inserted downstream of a luciferase reporter. MEIS1, an AML-associated cofactor,21 is predicted to be a target of miR-1 but not of miR-1 (4A:G) (Figure 6B). In contrast, a tumor suppressor associated with AML,22,23 TP53, is predicted to be a target of miR-1 (4A:G) but not of miR-1 (Figure 6B). As expected, transient cotransfection of miR-1 or miR-1 (4A:G) and MEIS1 3′-UTR into HEK293 cells yielded a 20% decrease in reporter activity by miR-1 but not miR-1 (4A:G) (Figure 6C). Transient cotransfection of miR-1 (4A:G) and TP53 3′-UTR into HEK293 cells yielded a 40% decrease (P = .027) in reporter activity, but, surprisingly, miR-1 expression also suppressed luciferase activity of TP53 3′-UTR reporter but to a lesser extent (20%; P = .89). Further evaluation of the miRNA binding sites revealed that miR-1 is predicted to bind to 2 sites in the TP53 UTR, albeit weakly (−3.7 and −2.9 kcal/mol); however, editing of A to G at position 4 increases the binding affinity of miR-1 to one these sites (−3.7 vs −10.4 kcal/mol) (Figure 6B).
Discussion
Cytogenetic abnormalities represent a large proportion of patients with de novo and therapy-related AML. Identification of AML-relevant genes within cytogenetic alterations is the foremost objective and a daunting challenge to better understand AML. Previous work has shown that miRNAs are frequently located at cancer-associated fragile sites and firmly implicated them as drivers of leukemogenesis. We performed an integrative analysis to identify relevant miRNAs located in leukemia-associated cytogenetic changes. We further corroborated our observations by examining relative expression of the leukemia-associated miRNAs in AML patient samples from published gene expression data (Table 1; Figure 4). Collectively, our finding suggests that, although many miRNAs are located in regions of leukemia-associated cytogenetic changes (∼ 70%), only a subset (∼ 20%) of these miRNAs are expressed and ae probably relevant myeloid malignancies.
To determine whether our subset of miRNAs have been previously implicated in leukemogenesis or hematopoiesis, we searched PubMed for relevant findings. A search showed that miR-143, miR-145, miR-146a, miR-155, miR-181, miR-221, and miR-222 are implicated in cellular processes relevant to AML and thus are consistent with our conclusion that the refined subset of leukemia-associated miRNAs are potentially important2 (Table 1). miRNAs within the commonly deleted region on chromosome 5q in MDS have recently been evaluated by our group.14 Previously, we have shown that deletion of miR-145 and miR-146a disrupts hematopoiesis, resulting in a phenotype similar to del (5q) MDS (eg, 5q− syndrome) in mice.14 Depletion of miR-145 and miR-146a also results in a clonal advantage and enhanced survival of hematopoietic stem/progenitor cells.14 Further analysis revealed that a subset of these mice go onto develop myeloid diseases consistent with leukemia (Figure 5); therefore, deletion of miR-145 and miR-146a, 2 miRNAs identified in our analysis as potentially relevant to human leukemia, results in a long-latency myeloid disease in mice. In addition, reintroduction of miR-145 or iR-146a into AML cells significantly induced cell death (Figure 5D) and prevented growth in vitro (supplemental Figure 2). We also confirmed that miR-145 and miR-146a are down-regulated in AML patient samples and are potentially suppressed by epigenetic mechanisms (Figure 5B-C). Although miR-146a was down-regulated in patients with a NK and further suppressed in patients with del(5q), surprisingly miR-145 was significantly down-regulated only in patients with AML with a NK, but not in patients with AML with del(5q) (Figure 5C). This discrepancy may be explained by the relatively small cohort of patients with del(5q) or that low levels of miR-145 may be less critical for maintaining the AML phenotype and more important for initiation of a premalignant state conducive to development of AML. In support of the latter hypothesis, we previously reported that miR-145 is significantly down-regulated in patients with MDS with del(5q).14
Use of a massively parallel sequencing platform revealed a proportion of alternative miRNA species. Identification of miRNA sequence variants, miRNA*, and posttranscriptional editing adds to the complexity of the miRNA transcriptome. The relevance of alternative miRNA species is not precisely known but has also been recently described in a leukemia progression mouse model.19 Our best understanding of the effect of nucleotide modifications comes from studies on single nucleotide polymorphisms within miRNAs. These single nucleotide polymorphisms have been identified and are associated with altered miRNA biogenesis, stem-loop formation, and strand preference.24 The functional consequence of nucleotide deletions and additions remains to be known. Although rare occurrences in our libraries, we did identify miRNAs that exhibited editing within sequences of the miRNA:mRNA duplex. miRNA editing has also been reported in mouse embryo, Oryza sativa, and Arabidopsis thaliana libraries.25,26 We have shown that an edit within the seed region of miR-1 (4A:G) may alter binding to mRNA targets. In our example, we specifically found that wild-type miR-1, but not the edited version, can bind and repress the 3′-UTR of MEIS1 (Figure 6B-C). This observation is particularly relevant to AML because MEIS1 is a key cofactor of the Hox cluster and critical to leukemogenesis in mice and humans.21 Not only did we show that the edited version of miR-1 does not bind MEIS1, but it now binds and suppresses the 3′-UTR of TP53, a gene that is mutated in human AML and its loss induces AML in mice.22,23 Therefore, it is compelling to speculate that editing of miR-1 (4A:G) makes miR-1 proleukemic by de-repressing MEIS1 and simultaneously suppressing TP53.
Massively parallel sequencing also facilitated identification of novel miRNAs. Because this is the first reported attempt to perform massively parallel sequencing on human leukemia cells, we identified 28 novel miRNAs. Approximately 65% of the novel miRNAs are located in leukemia-associated genomic alterations. Notably, miR-481, which is within a deleted region on chromosome 7q, is predicted to target meningioma 1 (Mn1). Elevated expression of Mn1 is a predictor of poor outcome in patients with AML, and expression of a Mn1 transgene in mouse hematopoietic cells results in aggressive and rapid leukemia.27,28 As such, deletion of miR-481 provides a compelling mechanism to increase expression of Mn1 in AML. Collectively, we have delineated the genomic alterations and identified leukemia-associated miRNAs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Megan Fuller for animal husbandry.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR; MOP 89 976) and the Leukemia & Lymphoma Society of Canada (A.K.), grants from Genome Canada and CIHR (W.L.L.), and fellowships from CIHR and the Michael Smith Foundation for Health Research (MSFHR; D.T.S.). A.K. is a Senior Scholar of the MSFHR.
Authorship
Contribution: D.T.S. and A.K. participated in designing the research and drafting the manuscript; D.T.S., J.L., and J.W. performed experiments; F.K. and R.K.H. provided technical assistance and assisted in the analysis of data; W.L.L. and R.C. provided valuable reagents and expertise in array CGH; R.M., a.m., M.H., and M.M. performed analysis and provided expertise in small RNA sequencing on the Illumina platform; and K.T. provided the MDS-L cell line.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aly Karsan, British Columbia Cancer Agency Research Centre, 675 West 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: akarsan@bccrc.ca.