Abstract
Chronic lymphocytic leukemia (CLL) is characterized by an accumulation of mature CD19+CD5+CD20dim B lymphocytes that typically express the B-cell activation marker CD23. In the present study, we cloned and expressed in T lymphocytes a novel chimeric antigen receptor (CAR) targeting the CD23 antigen (CD23.CAR). CD23.CAR+ T cells showed specific cytotoxic activity against CD23+ tumor cell lines (average lysis 42%) and primary CD23+ CLL cells (average lysis 58%). This effect was obtained without significant toxicity against normal B lymphocytes, in contrast to CARs targeting CD19 or CD20 antigens, which are also expressed physiologically by normal B lymphocytes. Moreover, CLL-derived CD23.CAR+ T cells released inflammatory cytokines (1445-fold more TNF-β, 20-fold more TNF-α, and 4-fold more IFN-γ). IL-2 was also produced (average release 2681 pg/mL) and sustained the antigen-dependent proliferation of CD23.CAR+ T cells. Redirected T cells were also effective in vivo in a CLL Rag2−/−γc−/− xenograft mouse model. Compared with mice treated with control T cells, the infusion of CD23.CAR+ T cells resulted in a significant delay in the growth of the MEC-1 CLL cell line. These data suggest that CD23.CAR+ T cells represent a selective immunotherapy for the elimination of CD23+ leukemic cells in patients with CLL.
Introduction
Chronic lymphocytic leukemia (CLL), the most common form of leukemia in adults in Western countries,1 remains an incurable disease despite the development of new therapeutic regimens.2,3 Allogeneic hematopoietic stem cell transplantation can be curative, but its application is limited to young adults, who represent a small percentage of patients with CLL.2,4 Antibodies directed against different surface antigens are currently used in patients with CLL.3 Although anti-CD52 (Campath-1) antibodies rapidly reduce the leukemic burden in the peripheral blood, they have limited biodistribution to secondary lymphoid organs, where CLL cells tend to accumulate.5 In the case of anti-CD20 antibodies, the low levels of the antigen on leukemic B cells limit their use as a single agent in this disease. In addition, antibodies do not lead to long-term control of the disease because they do not establish an active memory immune response.6 CLL is also susceptible to cell-mediated immune control, as indicated by the graft-versus-leukemia effect associated with allogeneic hematopoietic stem cell transplantation,7 and by the immune responses elicited in patients receiving leukemia-tumor vaccines.8,9
Adoptive transfer of T lymphocytes genetically modified to express a chimeric antigen receptor (CAR) can combine the beneficial effects of both antibody- and T-cell–mediated immune responses. CARs are chimeric molecules that contain an extracellular binding moiety derived from an mAb (single-chain variable fragment [scFv]) coupled to an intracellular signaling moiety (usually the ζ chain of the T-cell receptor complex).6,10,11 When expressed by T lymphocytes, CARs can trigger T-cell activation and perforin/granzyme-B release12 upon binding with the antigen expressed by tumor cells in a non-MHC-restricted manner, thus avoiding an important mechanism of tumor immune escape represented by the down-regulation of MHC molecules by tumor cells.6 Adoptive transfer of CAR-transduced T lymphocytes may offer several advantages compared with the passive administration of antibodies, because T cells have enhanced tissue biodistribution and may establish a long-lasting antitumor immune response.6 CARs targeting either CD19 or CD20 antigens have been developed to treat human B-cell–derived malignancies,13-15 and clinical trials using these chimeric molecules are currently ongoing in several institutions. However, a potential major disadvantage of this strategy is that both CD19 and CD20 are expressed not only by leukemic cells, but also by normal B lymphocytes, and therefore the sustained elimination of these cells by CAR-modified T cells could result in a severe impairment of the humoral immunity, exacerbating the characteristic immunodeficiency present in patients with CLL.16 Because of the importance of preserving the normal B cell compartment, the generation of CARs targeting antigens with a more restricted expression in tumor cells may have clinical relevance.17 CD23 antigen represents an attractive alternative in CLL, because leukemic B cells typically overexpress CD23 compared with normal B lymphocytes.18,19 We have exploited this feature to selectively target malignant CLL cells while sparing the normal B cell compartment by developing a novel CAR against this antigen.
In the present study, we report that T cells engineered to express a CD23-specific CAR secrete immunostimulatory cytokines and have cytotoxic activity against CD23+ tumor cell lines and primary CLL cells in vitro while sparing normal B lymphocytes. Moreover, engineered T cells also provide significant control of leukemia growth in vivo when infused in mice engrafted with the MEC-1 CLL cell line, which closely reproduces human CLL disease and is therefore an optimal tool with which to test the efficacy of new therapeutic agents.20 In conclusion, our data suggest that the administration of CD23.CAR+ T cells may pave the way for a novel cell-based approach for the treatment of CLL.
Methods
Cell lines and CLL primary cells
The MEC-1 CLL cell line21 was obtained from the German Resource Center for Biologic Material (DSMZ). Wild-type BJAB was obtained from the American Type Culture Collection, and the Jeko-1 lymphoma B cell line was obtained from DMSZ. The latter 2 cell lines were genetically modified with a retroviral vector encoding the human CD23 molecule (PL-CD23-SP) to generate CD23+ BJAB and CD23+ Jeko-1 cells. Epstein-Barr virus–transformed (lymphoblastoid cell line [LCL]) cells were obtained from the peripheral blood of healthy donors, as described previously.22 All cell lines were maintained in culture with RPMI 1640 medium (Lonza) supplemented with 10% heat-inactivated FCS (Lonza), 2mM l-glutamine (Lonza), 25 IU/mL of penicillin, and 25 mg/mL of streptomycin (Lonza) (complete RPMI). B lymphocytes were isolated from fresh PBMCs of healthy donors using CD20 paramagnetic beads (Miltenyi Biotec) according to the manufacturer's instructions. Primary CLL cells were obtained from both fresh and frozen PBMCs of CLL patients after removal of CD3+ T cells using CD3 paramagnetic beads (Miltenyi Biotec) when T cells were > 10%. Informed consent was obtained from both healthy donors and CLL patients according to protocols approved by the local institutional review board following the Declaration of Helsinki.
Generation of activated T cells
PBMCs were isolated by Ficoll density gradient centrifugation (GE Healthcare) from the peripheral blood collected from healthy donors and CLL patients, and were activated with 1 μg/mL of anti-CD3 (OKT3; eBioscience) and 0.5 μg/mL of anti-CD28 (Becton Dickinson) antibodies with the addition of 100 U/mL of recombinant human IL-2 (Proleukin; Chiron Therapeutics) in non-tissue culture–treated 24-well plates (Becton Dickinson) at a concentration of 1 × 106 cells/well.
Cloning of the CD23-specific CAR, generation of the retroviral supernatant, and T-cell transduction
The sequence of the anti-human CD23 mAb has been reported previously (accession numbers BD232452.1 and BD232451.1).23 Based on this sequence, we generated an optimized single-chain antibody (scFv) encoding the variable regions of the heavy chain and light chain of the mAb using synthetic DNA technology (Assembly PCR Oligo Maker).24 The scFv sequence was then cloned in-frame with the human IgG1-CH2CH3 endodomain, the CD28-costimulatory endodomain, and the ζ chain of the TCR/CD3 complex into the SFG retroviral backbone to generate the CD23.CAR vector, as described previously.17 We used a CAR targeting the CD19 molecule and also containing the CD28 endodomain (CD19.CAR)25 as a positive control vector. To produce the retroviral supernatant, 293T cells were cotransfected with CAR-containing retroviral vectors, Peq-Pam-e plasmid encoding the MoMLV gag-pol, and the RDF plasmid encoding the RD114 envelope, as described previously.17 Activated T lymphocytes were then transduced with the retroviral supernatants using retronectin-coated plates (Takara; Shuzo).17 After removal from the retronectin plates, T-cell lines were maintained in RPMI complete medium in a humidified atmosphere containing 5% CO2 at 37°C with the addition of IL-2 (50 U/mL) every 3 days.
Immunophenotyping
PE-conjugated CD8, FITC-conjugated CD4, PE-conjugated CD56, and peridinin chlorophyll protein–conjugated CD3 mAbs were used to stain T lymphocytes, and allophycocyanin-conjugated CD5, FITC-conjugated CD19, and PE-conjugated CD23 mAbs were used to stain tumor cell lines and primary CLL cells. All antibodies except CD8 and CD4 (from Tema Ricerca) were purchased from Becton Dickinson. To detect the expression of CD19.CAR and CD23.CAR, T lymphocytes were stained with the Fc-specific cyanine-Cy5-conjugated (Fc-γCy5) antibody (Jackson ImmunoResearch), which recognizes the IgG1-CH2CH3 component of the CARs.17 We evaluated the presence of regulatory T cells using peridinin chlorophyll protein–conjugated CD4 (Becton Dickinson), allophycocyanin-conjugated CD25, and PE-conjugated FOXP3 (BioLegend), and analyzed cells by fluorescence-activated cell sorting with a FACScan flow cytometer (Becton Dickinson).
Cytotoxicity assay
Cytotoxic activity of control nontransduced (NT) T cells and CAR+ T cells was measured using a standard 51Cr-release assay after a 4-hour incubation. The targets tested included Jeko-1, BJAB, and LCL cells labeled with 25μCi of 51Cr (Perkin-Elmer) for 45 minutes, as well as primary CLL cells and normal B lymphocytes, each labeled with 50 μCi of 51Cr for 1 hour. Target cells (5 × 103) were then cocultured in triplicate with T cells at effector:target (E:T) ratios of 40:1, 20:1, 10:1, and 5:1, in complete medium alone or in 1% SDS (Sigma-Aldrich) to determine spontaneous and maximum 51Cr release, respectively. Samples were collected and the mean specific lysis was calculated as described previously.17,26 Whereas LCL, CD23+ Jeko-1, and CD23+ BJAB cells maintained surface CD23 expression when the cells were kept in culture, in primary CLL cells, CD23 was cleaved off after few hours of in vitro culture, largely because of the lack of specific plasmatic metalloprotease inhibitors in the cell culture medium.27 Therefore, to evaluate the CD23.CAR-specific killing of primary CLL cells, we preincubated leukemic lymphocytes with the metalloprotease-inhibitor imidazole (Sigma-Aldrich) at a concentration of 10mM for 2 hours at 37°C before performing the assay.27 This approach stabilized the expression of CD23 on the cell surface of CLL cells for at least 24 hours (data not shown), allowing us to perform the experiments. To adapt the 51Cr-release assay to smaller target cells such as resting B cells and CLL cells, we optimized assay conditions by labeling resting B cells and CLL with 50 μCi of 51Cr instead of 25 μCi for 1 hour instead of 45 minutes. We also increased the time of coculture from 4 to 6 hours and reduced the final reaction volume from 200 to 100μL, thereby increasing the sensitivity of 51Cr detection.
Short-term coculture experiments
The cytotoxic activity of NT and CAR+ T cells toward autologous CLL cells and normal donor–derived B cells was also evaluated in a 24-hour coculture assay using target cells labeled with CFSE (eBioscience), as described previously.17 In these experiments as described for the 51Cr-release assay, CLL cells were preincubated with the metalloprotease-inhibitor imidazole. We cocultured NT and CAR+ T cells (9 × 105 cells/well) with 3 × 105 CFSE-stained cells/well at an E:T ratio of 3:1 for 24 hours at 37°C. Cells were then collected, stained with CD3 to detect T lymphocytes, and analyzed by flow cytometry to quantify residual normal B or tumor cells as CFSE-positive cells.
Long-term coculture experiments
To evaluate whether the soluble form of CD23 could affect the cytotoxic activity of CD23.CAR+ T lymphocytes, we cocultured NT and CAR+ T cells (1 × 106 cells/well) with viable LCL cells (E:T ratio, 1:1) in the presence or in the absence of serial dilutions of plasma enriched in soluble CD23 obtained from CLL patients. After 4 days of coculture, cells were collected, stained with CD3 and CD19 mAbs to detect T lymphocytes and tumor cells, respectively, and analyzed by flow cytometry to quantify residual tumor cells.
Cell expansion
To evaluate the expansion of T lymphocytes in response to CD23+ cell lines (LCL and MEC-1 cells) and autologous CLL cells, NT and CD23.CAR+ T cells (1 × 106) obtained from healthy donors and CLL patients were stimulated once a week with allogeneic, γ-irradiated (30 rads) LCL cells (E:T ratio, 1:1), γ-irradiated (100 rads) MEC-1 cells (E:T ratio, 1:1), or nonirradiated autologous CLL cells (E:T ratio, 1:1) preincubated with imidazole without the addition of exogenous cytokines. T lymphocytes were counted with Trypan blue (Sigma-Aldrich) to assess the increase at different time points.
Cytokine release
NT and CAR+ T lymphocytes (2 × 105 or 1 × 106) were cocultured with γ-irradiated allogeneic LCL or MEC-1 cells or nonirradiated autologous CLL cells (E:T ratio, 1:1) and culture supernatants were collected after 24 or 48 hours. IFN-γ, TNF-α, and TNF-β were measured with the Flow Cytomix Assay (Bender MedSystems), and IL-2 production was measured using a specific ELISA (Peprotech). Our data were normalized by subtracting the spontaneous release of cytokines from tumor cell lines or autologous CLL cells.
Soluble CD23 antigen analysis
Plasma samples from healthy donors and CLL patients were obtained by centrifugation (10 minutes at 1000g) of fresh peripheral blood. The plasma fractions were then stored at −20°C until soluble CD23 quantification was performed. Soluble CD23 levels of healthy donors and CLL patient–derived plasma samples were detected using a human CD23 ELISA kit (Bender MedSystems) according to the manufacturer's instructions.
In vivo studies
Eight-week-old Rag2−/−γc−/− female mice were injected subcutaneously in the left flank with 10 × 106 MEC-1 cells in 0.1 mL of saline. Ten days later, mice bearing subcutaneous tumors were adoptively transferred with a single IV infusion of either 10 × 106 CD23.CAR+ T cells or NT T cells obtained from healthy donors. Animals were then monitored twice a week for weight and tumor growth (measuring 3 perpendicular diameters), and killed when the mean tumor volume reached a dimension of ≥ 1000 mm3 before the animals exhibited clinical signs and symptoms to avoid unnecessary pain and discomfort according to standard ethical animal guidelines. Two separate experiments were performed with 3 mice per group each (manipulated and control, nonmanipulated T cells).
Statistical analysis
The data are reported as means ± SD. The paired Student t test was used to determine the statistical significance of differences between samples. P < .05 was considered statistically significant.
Results
Functional CD23.CAR can be expressed by activated T lymphocytes
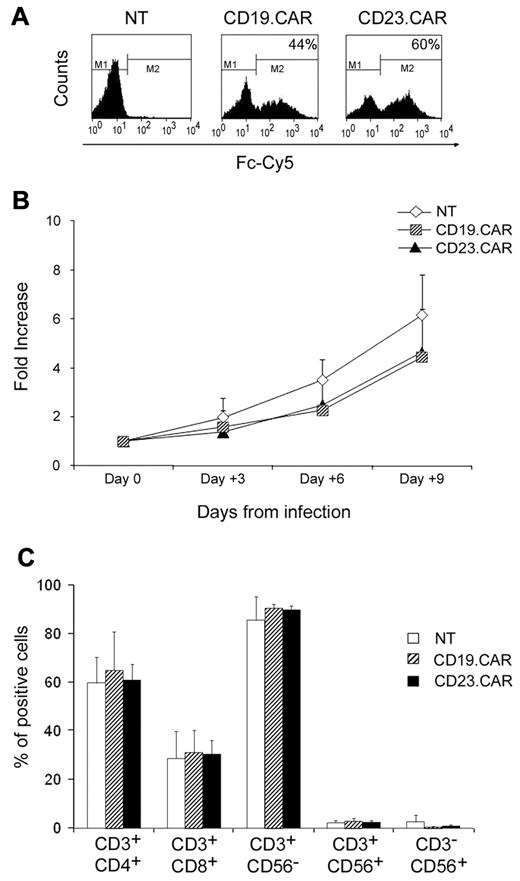
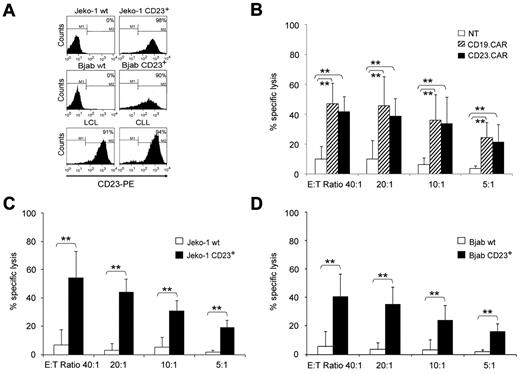
We first evaluated whether CD23.CAR could be expressed by activated T lymphocytes and if these cells had cytotoxic activity against CD23+ target cells. T lymphocytes expressing CD19.CAR, previously largely validated,25 were used as a positive control. Activated T lymphocytes obtained from the peripheral blood of healthy donors were transduced either with CD19.CAR or CD23.CAR retroviral supernatants. As shown in Figure 1A, both CD19.CAR and CD23.CAR were efficiently expressed by T lymphocytes (average 58%, range 23%-82%, and 58%, range 18%-81%, respectively; n = 10). Both CD4+ and CD8+ T lymphocytes were transduced with similar efficiency, and the expression of the transgene was maintained in cells cultured for more than 2 weeks (data not shown). After transduction, both CD19.CAR+ and CD23.CAR+ cells expanded equally well in vitro in the presence of IL-2 (n = 8; Figure 1B). Phenotypic analysis performed 3 days after gene transfer showed that T-cell lines were a mixture of CD4+ cells (average 64%, range 54%-73%, for CD23.CAR+ cells, and 68%, range 40%-88%, for CD19.CAR+ cells; n = 6) and CD8+ cells (average 32%, range 24%-40%, for CD23.CAR+ cells, and 33%, range 21%-44%, for CD19.CAR+ cells; n = 6). Less than 2% of CAR+ cells were CD3−CD56+ natural killer cells (Figure 1C). To evaluate the cytotoxic activity of NT and CAR+ cells, we used a 4-hour standard 51Cr-release assay. Target cells were allogeneic Epstein-Barr virus–positive LCL cells, which naturally express both CD23 and CD19 molecules, and 2 CD19+ tumor cell line gene modified to express comparable levels of CD23 (CD23+ Jeko-1 and CD23+ BJAB) (Figure 2A). CD23.CAR+ T cells had a cytotoxic activity comparable to CD19.CAR+ T cells against allogeneic LCL cells (average lysis 42%, range 27%-54%, at an E:T ratio of 40:1, and average lysis 47%, range 30%-72% at an E:T ratio of 40:1, respectively; n = 7, P ≤ .005) (Figure 2B). Less than 10% lysis was observed for NT T lymphocytes (Figure 2B). CD23.CAR+ cells also acquired specific cytotoxic activity against CD23+ Jeko-1 (n = 6; Figure 2C) and CD23+ BJAB cells (n = 6; Figure 2D), whereas these cells had negligible cytotoxic activity against wild-type Jeko-1 (Figure 2C) and BJAB (Figure 2D) cells.
Activated T lymphocytes can efficiently express either CD23.CAR or CD19.CAR. (A) CAR expression in CD19.CAR- and CD23.CAR-redirected T lymphocytes for one representative experiment of 10. Control NT T cells were also included. (B) Median expansion (-fold) of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from healthy donors and cultured for 9 days with IL-2 on retroviral-mediated gene transfer. Mean and SDs are shown for 8 different T-cell lines. (C) Phenotype of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from healthy donors and stained for 3 days on retroviral gene transfer. Data represent the means ± SD for 6 different T-cell lines.
Activated T lymphocytes can efficiently express either CD23.CAR or CD19.CAR. (A) CAR expression in CD19.CAR- and CD23.CAR-redirected T lymphocytes for one representative experiment of 10. Control NT T cells were also included. (B) Median expansion (-fold) of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from healthy donors and cultured for 9 days with IL-2 on retroviral-mediated gene transfer. Mean and SDs are shown for 8 different T-cell lines. (C) Phenotype of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from healthy donors and stained for 3 days on retroviral gene transfer. Data represent the means ± SD for 6 different T-cell lines.
CD23.CAR+ and CD19.CAR+ T lymphocytes have equal cytotoxic activity against CD19+CD23+ tumor cells. (A) Expression of CD23 antigen on target cells used in the cytotoxicity assay. (B) Cytotoxic activity of CD19.CAR+ and CD23.CAR+ T lymphocytes obtained from healthy donors against CD23+ LCL targets. Cytotoxic activity was evaluated in a standard 4-hour 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD of 7 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells. The difference between the cytotoxic activity of CD19.CAR+ and CD23.CAR+ T lymphocytes was not statistically significant at any given E:T ratio. (C) Cytotoxic activity of CD23.CAR+ T lymphocytes generated from healthy donors against the wild-type Jeko-1 cell line or CD23+ cells. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 6 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells. (D) Cytotoxic activity of CD23.CAR+ T lymphocytes generated from healthy donors against the wild-type BJAB cell line or CD23+ cells. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 6 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells.
CD23.CAR+ and CD19.CAR+ T lymphocytes have equal cytotoxic activity against CD19+CD23+ tumor cells. (A) Expression of CD23 antigen on target cells used in the cytotoxicity assay. (B) Cytotoxic activity of CD19.CAR+ and CD23.CAR+ T lymphocytes obtained from healthy donors against CD23+ LCL targets. Cytotoxic activity was evaluated in a standard 4-hour 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD of 7 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells. The difference between the cytotoxic activity of CD19.CAR+ and CD23.CAR+ T lymphocytes was not statistically significant at any given E:T ratio. (C) Cytotoxic activity of CD23.CAR+ T lymphocytes generated from healthy donors against the wild-type Jeko-1 cell line or CD23+ cells. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 6 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells. (D) Cytotoxic activity of CD23.CAR+ T lymphocytes generated from healthy donors against the wild-type BJAB cell line or CD23+ cells. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 6 different T-cell lines. **P ≤ .005 comparing NT and CAR+ T cells.
CD23.CAR+ T lymphocytes do not show cytotoxic activity against normal B lymphocytes
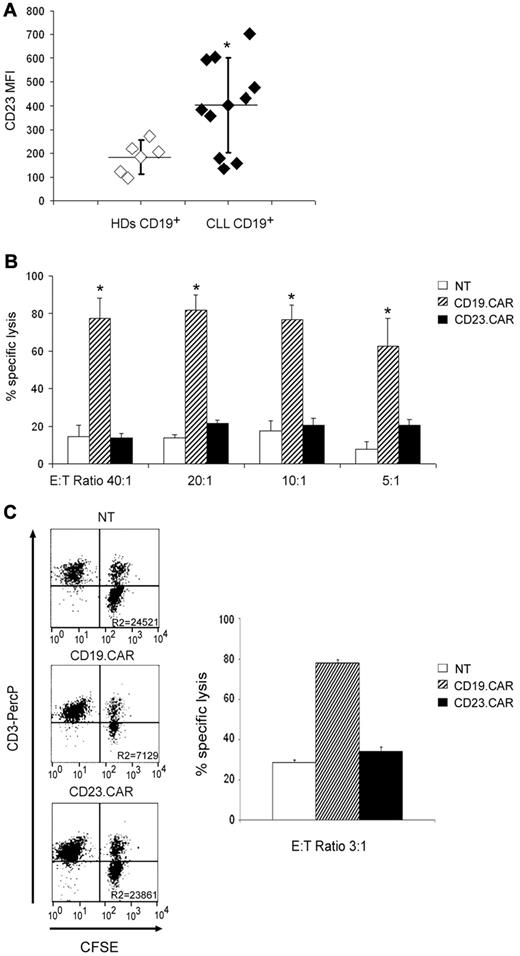
Because one potential concern associated with the adoptive transfer of CD19.CAR+ T cells in patients with B-cell malignancies is the elimination of both leukemic and nonmalignant B lymphocytes, we investigated whether CD23.CAR+ T cells could spare normal B lymphocytes because they express lower levels of the CD23 antigen.28 Whereas the percentages of double-positive CD19/CD23 cells were comparable in healthy donors and CLL patients at levels ≥ 90% (n = 5 and n = 7, respectively; data not shown), as expected,28,29 the level of expression of CD23 antigen was higher in CD19+ CLL cells, when assessed as mean fluorescence intensity (MFI) (average MFI, 507, range 356-704; n = 7, for CD19+ CLL cells; average MFI, 183, range 97-273; n = 5, for normal CD19+ B lymphocytes; P ≤ .005; Figure 3A). We then analyzed the cytotoxic effects of either CD23.CAR+ or CD19.CAR+ T lymphocytes against normal B lymphocytes. As shown in Figure 3B, lysis of normal B lymphocytes by CD23.CAR+ cells (average lysis 13%, range 7%-17%, at an E:T ratio of 40:1; n = 3) was significantly lower compared with lysis by CD19.CAR+ T cells (average lysis 77%, range 57%-100%, at an E:T ratio of 40:1; n = 3, P ≤ .05) and, similar to that of NT T cells, that was less than 15%. We also evaluated the individual cytotoxic effects of CD23.CAR+ and CD19.CAR+ T lymphocytes against normal B lymphocytes in 24-hour coculture experiments using CFSE-labeled target cells. As shown in Figure 3C, the 24-hour coculture experiments confirmed that CD23.CAR+ CLL–derived T cells had only a marginal cytotoxic effect against normal B lymphocytes compared with CD19.CAR+ cells.
CD23.CAR+ T lymphocytes lack cytotoxic activity against normal B lymphocytes. (A) MFI of CD23 antigen on B cells isolated from the peripheral blood of healthy donors and CLL patients is shown. Percentages of double-positive CD19/CD23 cells were comparable in healthy donors and CLL patients at levels ≥ 90% (data not shown), as expected. **P ≤ .005 comparing healthy donors and CLL-derived B cells. (B) Cytotoxic activity of control NT, CD19.CAR+, and CD23.CAR+ T lymphocytes obtained from healthy donors against purified CD20+ B cells obtained from the peripheral blood of healthy donors. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing CAR+ with NT T lymphocytes. (C) Cytotoxic activity of control NT, CD19.CAR+, and CD23.CAR+ T lymphocytes obtained from CLL patients against purified CD20+ B cells labeled with CFSE. Cytotoxic activity was evaluated in a 24-hour coculture experiment, and results are shown at an E:T ratio of 3:1. The experiment shown is representative of 3 different experiments.
CD23.CAR+ T lymphocytes lack cytotoxic activity against normal B lymphocytes. (A) MFI of CD23 antigen on B cells isolated from the peripheral blood of healthy donors and CLL patients is shown. Percentages of double-positive CD19/CD23 cells were comparable in healthy donors and CLL patients at levels ≥ 90% (data not shown), as expected. **P ≤ .005 comparing healthy donors and CLL-derived B cells. (B) Cytotoxic activity of control NT, CD19.CAR+, and CD23.CAR+ T lymphocytes obtained from healthy donors against purified CD20+ B cells obtained from the peripheral blood of healthy donors. Cytotoxic activity was evaluated in a 51Cr-release assay, and results are shown at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing CAR+ with NT T lymphocytes. (C) Cytotoxic activity of control NT, CD19.CAR+, and CD23.CAR+ T lymphocytes obtained from CLL patients against purified CD20+ B cells labeled with CFSE. Cytotoxic activity was evaluated in a 24-hour coculture experiment, and results are shown at an E:T ratio of 3:1. The experiment shown is representative of 3 different experiments.
CD23.CAR+ T cells generated from CLL patients have cytotoxic activity against CLL cells
As described for T-cell lines generated from healthy donors, we efficiently generated CD23.CAR+ T lymphocytes from samples collected from CD23+ CLL patients. The expression of CD23.CAR and CD19.CAR was 71% (range, 45%-90%) and 68% (range, 47%-92%; n = 10), respectively. The phenotypic profile and expansion rate of these T-cell lines (data not shown) were similar to those observed for T-cell lines generated from healthy donors (Figure 1A-B). Moreover, because regulatory T cells may be abundant in the peripheral blood of CLL patients,8,30,31 we measured the presence of these cells using phenotypic analysis before and 14 days after CAR gene transfer. The percentages of CD4+CD25+FoxP3+ cells before and 14 days after gene transfer were similar (1.2%, range 0.9%-1.6%, and 0.8%, range 0.2%-1.9%, respectively; n = 5).
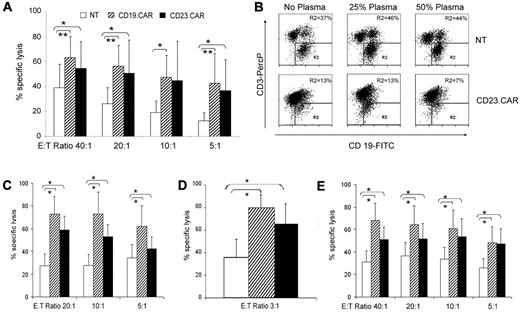
As illustrated in Figure 4A, CD23.CAR+ T cells showed efficient cytotoxic activity against LCL cells, as assessed by the 51Cr-release assay (average lysis 54%, range 24%-70%, at an E:T ratio of 40:1; n = 5, P ≤ 0.05 compared with NT T lymphocytes), which was comparable to the cytotoxic effects of CD19.CAR+ T cells (average lysis 63%, range 40%-75%, at an E:T ratio of 40:1; n = 5, P ≤ .005 compared with NT T lymphocytes). The antitumor effect of CD23.CAR+ T cells was not inhibited by the addition of human plasma enriched in soluble CD23 (2000 U/mL), as assessed in coculture experiments (n = 3; Figure 4B). The average percentage of residual CD19+CD23+ tumor cells in the presence of CD23.CAR+ T cells was 15% (range 13%-17%) in the absence of plasma, 17% (range 13%-20%) in the presence of 25% plasma, and 12% (range 7%-18%) in the presence of 50% plasma (n = 3 CLL donors). In contrast, the average percentage of residual CD19+CD23+ tumor cells was 44% (range 31%-63%), 51% (range 39%-68%), and 47% (range 30%-66%), respectively, when control NT T lymphocytes were used regardless of the addition of soluble CD23 (n = 3). We then evaluated the capacity of CAR-redirected T cells to specifically target autologous and allogeneic CD23+ CLL cells. CD23.CAR+ T cells efficiently lysed both autologous CD23+ leukemic cells (average lysis 58%, range 26%-84%, at an E:T ratio of 20:1; n = 3; Figure 4C) and allogeneic CD23+ leukemic cells (average lysis 51%, range 13%-76%, at an E:T ratio of 20:1; n = 3) using a 6-hour 51Cr-release assay (Figure 4E). Coculture experiments using CFSE-labeled target cells also showed substantial elimination of autologous CD23+ leukemic cells within 24 hours (average lysis 66%, range 30%-68%, at an E:T ratio of 3:1; n = 4; Figure 4D). These effects were comparable to those obtained using CD19.CAR+ cells using autologous CLL cells in a 51Cr-release assay (average lysis of autologous CLL cells, 72%, range 18%-100%, at an E:T ratio of 20:1; n = 3), in a 24-hour coculture CFSE assay (average lysis of autologous CLL cells, 78%, range 69%-88%, at an E:T ratio of 3:1; n = 2), and using allogeneic CLL cells in a 51Cr-release assay (average lysis of allogeneic CLL, 64%, range 18%-100%; n = 3).
Activated T lymphocytes generated from CLL patients are efficiently transduced with CAR-encoding retroviral vectors and have cytotoxic activity against CD23+ tumor cells. (A) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL donors and tested against allogeneic LCL cells. Data represent the means ± SD for 5 different T-cell lines. *P ≤ .05 and **P ≤ .005 comparing CAR+ with NT T lymphocytes. (B) Dot-plot analysis of the coculture experiments in which CD23.CAR+ T cells derived from CLL patients were cocultured with allogeneic LCL cells in the absence or in the presence of different percentages of plasma enriched in soluble CD23. One representative experiment is shown. The numbers represent the percentage of residual CD19+ LCL cells (tumor cell line) enumerated by FACS analysis after 4 days of coculture. The experiment shown is representative of 3 different experiments. (C) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against autologous CD23+ CLL cells. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing transduced and NT T lymphocytes. (D) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against autologous CD23+ CLL cells using CFSE staining and cocultured for 24 hours. Data represent the means ± SD for 4 different T-cell lines. *P ≤ .05 comparing CAR+ transduced with NT T lymphocytes. (E) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against allogeneic CD23+ CLL cells. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing transduced with NT T lymphocytes.
Activated T lymphocytes generated from CLL patients are efficiently transduced with CAR-encoding retroviral vectors and have cytotoxic activity against CD23+ tumor cells. (A) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL donors and tested against allogeneic LCL cells. Data represent the means ± SD for 5 different T-cell lines. *P ≤ .05 and **P ≤ .005 comparing CAR+ with NT T lymphocytes. (B) Dot-plot analysis of the coculture experiments in which CD23.CAR+ T cells derived from CLL patients were cocultured with allogeneic LCL cells in the absence or in the presence of different percentages of plasma enriched in soluble CD23. One representative experiment is shown. The numbers represent the percentage of residual CD19+ LCL cells (tumor cell line) enumerated by FACS analysis after 4 days of coculture. The experiment shown is representative of 3 different experiments. (C) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against autologous CD23+ CLL cells. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing transduced and NT T lymphocytes. (D) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against autologous CD23+ CLL cells using CFSE staining and cocultured for 24 hours. Data represent the means ± SD for 4 different T-cell lines. *P ≤ .05 comparing CAR+ transduced with NT T lymphocytes. (E) Cytotoxic activity of NT, CD19.CAR+, and CD23.CAR+ T lymphocytes generated from CLL patients and tested against allogeneic CD23+ CLL cells. Data represent the means ± SD for 3 different T-cell lines. *P ≤ .05 comparing transduced with NT T lymphocytes.
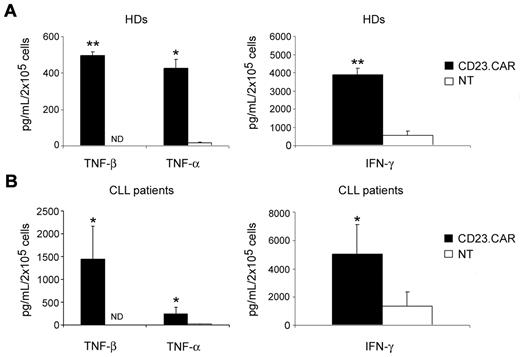
CD23.CAR+ T lymphocytes generated from both healthy donors and CLL patients produce immunostimulatory cytokines in response to CD23+ tumor cells
NT and CD23.CAR+ T cells generated from either healthy donors or CLL patients were cocultured with γ-irradiated LCL cells (E:T ratio, 1:1). As shown in Figure 5A and B, in contrast to NT T lymphocytes, CD23.CAR+ T cells derived from healthy donors secreted TNF-β (497 pg/mL, range 460-529 pg/mL; n = 3, P ≤ .005). In addition, transduced T cells secreted > 25-fold more TNF-α (426 pg/mL, range 325-483 pg/mL vs 16 pg/mL, range 10-20 pg/mL; n = 3, P ≤ .05) and approximately 7-fold more IFN-γ (3878 pg/mL, range 3393-4539 pg/mL vs 561 pg/mL, range 184-928 pg/mL; n = 3, P ≤ .005) compared with NT T cells. Similarly, CD23.CAR+ T cells generated from CLL patients secreted TNF-β (1445 pg/mL, range 610-1990 pg/mL; n = 4, P ≤ .05) that was not produced by NT T cells. In addition, transduced T cells secreted 4-fold more IFN-γ (5023 pg/mL, range 2860-9235 pg/mL vs 1340 pg/mL, range 265-3347 pg/mL; n = 4, P ≤ .05) and 20-fold more TNF-α (245 pg/mL, range 77-435 pg/mL vs 12 pg/mL, range 0-25 pg/mL; n = 4, P ≤ .05), compared with NT T lymphocytes. No major differences in cytokine secretion were detected between CD23.CAR+ and CD19.CAR+ T cells (data not shown). As illustrated in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), CD23.CAR+ T cells also produced inflammatory cytokines against autologous CLL cells and the MEC-1 cell line.
CD23.CAR+ T lymphocytes generated from healthy donors and CLL patients produce inflammatory cytokines in response to CD23+ tumor cells. CD23.CAR+ T cells generated from healthy donors (A) and CLL patients (B) produced higher levels of TNF-β, TNF-α, and IFN-γ in response to allogeneic LCL cells compared with NT T cells. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 and **P ≤ .005 comparing CAR+-transduced with NT T lymphocytes.
CD23.CAR+ T lymphocytes generated from healthy donors and CLL patients produce inflammatory cytokines in response to CD23+ tumor cells. CD23.CAR+ T cells generated from healthy donors (A) and CLL patients (B) produced higher levels of TNF-β, TNF-α, and IFN-γ in response to allogeneic LCL cells compared with NT T cells. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 and **P ≤ .005 comparing CAR+-transduced with NT T lymphocytes.
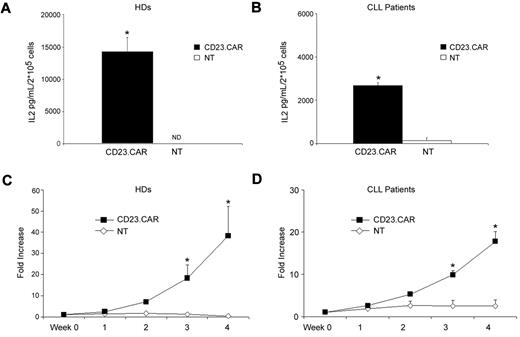
CD23.CAR+ T cells produce IL-2 and proliferate in response to CD23+ target cells
Because our novel CAR included the CD28 costimulatory endodomain, we evaluated whether CD23.CAR+ T cells released IL-2 and proliferated in response to CD23+ target cells (LCL and MEC-1 cells) and autologous CLL cells, as described for other CARs incorporating the same costimulatory endodomain.17,25,30 As shown in Figure 6A, CD23.CAR+ T cells generated from healthy donors released IL-2 after stimulation with γ-irradiated allogeneic LCL cells (E:T ratio, 1:1; average 14 229 pg/mL, range 9262-19 539 pg/mL; n = 4), whereas no IL-2 was produced by NT T lymphocytes (0 pg/mL, P ≤ .05). Similarly, CD23.CAR+ T cells generated from CLL samples produced IL-2 in response to CD23+ target cells (LCL cells; average 2681 pg/mL, range 2411-2833 pg/mL; n = 3; Figure 6B). As shown in Figure 6C-D, CD23.CAR+ T cells proliferated in response to the CD23+ LCL cells without the addition of exogenous IL-2. After 4 weeks of culture, CD23.CAR+ T cells showed an average increase of 38-fold (range 20-65; n = 3) and 18-fold (range 14-24; n = 4) when generated from healthy donors and CLL samples, respectively. In contrast, control NT T lymphocytes did not proliferate in response to CD23+ targets: the average increase was less than 2-fold for both healthy donors and CLL samples. As shown in supplemental Figure 1, CD23.CAR+-modified T cells also proliferated in response to either the MEC-1 cell line or autologous CLL cells.
CD23.CAR+ T lymphocytes generated from healthy donors and CLL patients produce IL-2 and proliferate in response to CD23+ tumor cells. (A-B) CD23.CAR+ T cells generated from healthy donors and CLL patients produced IL-2 when stimulated with allogeneic LCL cells (E:T ratio, 1:1) compared with NT T lymphocytes. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 comparing transduced with NT T lymphocytes. (C-D) Expansion of CD23.CAR+ T cells derived from healthy donors and CLL patients in response to allogeneic LCL cells compared with NT T lymphocytes. Cells were stimulated once a week without the addition of exogenous cytokines. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 comparing transduced and NT T lymphocytes.
CD23.CAR+ T lymphocytes generated from healthy donors and CLL patients produce IL-2 and proliferate in response to CD23+ tumor cells. (A-B) CD23.CAR+ T cells generated from healthy donors and CLL patients produced IL-2 when stimulated with allogeneic LCL cells (E:T ratio, 1:1) compared with NT T lymphocytes. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 comparing transduced with NT T lymphocytes. (C-D) Expansion of CD23.CAR+ T cells derived from healthy donors and CLL patients in response to allogeneic LCL cells compared with NT T lymphocytes. Cells were stimulated once a week without the addition of exogenous cytokines. Data represent the means ± SD for 3 and 4 different T-cell lines generated from healthy donors and CLL patients, respectively. *P ≤ .05 comparing transduced and NT T lymphocytes.
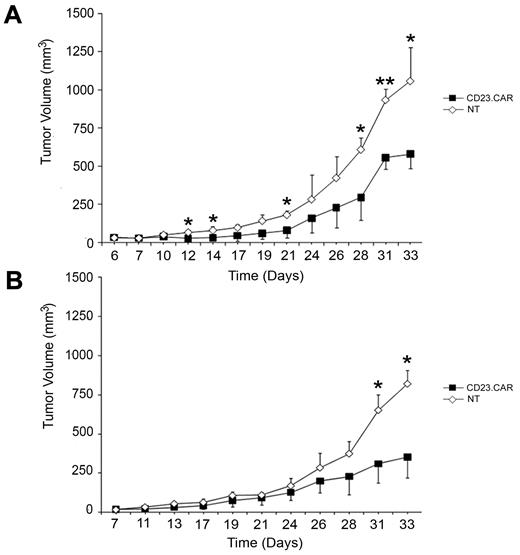
CD23.CAR+ T cells provide in vivo antitumor effect in the Rag2−/−γc−/− xenograft model of CLL
To investigate the antitumor activity of CD23.CAR+ T lymphocytes in vivo, MEC-1 cells were injected subcutaneously into the left flank of Rag2−/−γc−/− female mice. Ten days later, when tumors had reached a mean volume of 44 ± 8.3 mm3, either CD23.CAR+ or NT T lymphocytes were injected IV into the animals, which were then monitored for tumor growth. The follow-up was interrupted at day 33, when the mean tumor volume reached 1056 mm3 in the group with NT T cells. Adoptive cellular transfer did not modify the animal weight compared with the controls (NT-cell–injected animals, data not shown); however, by day 12, mice receiving CD23.CAR+ T cells showed a significant decrease in MEC-1 tumor growth compared with control mice, as shown in Figure 7A (CD23.CAR+ T cells vs NT T cells: day 12, P ≤ .05; day 14, P ≤ .05; day 21, P ≤ .05; day 28, P ≤ .05; day 31, P ≤ .005; and day 33, P ≤ .05; n = 3). These in vivo results were confirmed when repeated in a second independent experiment in which MEC-1–transplanted Rag2−/−γc−/− mice were injected with either CD23.CAR+ T cells or NT T cells obtained from a different donor, as shown in Figure 7B (CD23.CAR+ T cells vs NT T cells: day 31, P ≤ .05 and day 33, P ≤ .05; n = 3).
CD23.CAR+ T cells control tumor growth in a CLL xenograft model. (A-B) 10 × 106 MEC-1 tumor cells were inoculated subcutaneously in the left flank of Rag2−/−γc−/− male mice. To test the therapeutic efficacy of CD23.CAR+ T cells, mice bearing an established tumor were inoculated IV with 10 × 106 NT or CD23.CAR+ T cells derived from different donors for each experiment. Tumor growth was then monitored as tumor diameter per day. Data represent the means ± SD of 3 mice for each panel presented. *P ≤ .05 and **P < .005, respectively, comparing CD23.CAR+ and NT T lymphocytes.
CD23.CAR+ T cells control tumor growth in a CLL xenograft model. (A-B) 10 × 106 MEC-1 tumor cells were inoculated subcutaneously in the left flank of Rag2−/−γc−/− male mice. To test the therapeutic efficacy of CD23.CAR+ T cells, mice bearing an established tumor were inoculated IV with 10 × 106 NT or CD23.CAR+ T cells derived from different donors for each experiment. Tumor growth was then monitored as tumor diameter per day. Data represent the means ± SD of 3 mice for each panel presented. *P ≤ .05 and **P < .005, respectively, comparing CD23.CAR+ and NT T lymphocytes.
Discussion
Because of its chronic clinical course and the need to reduce treatment-related toxicity, CLL is suited to immunotherapy-based approaches. Adoptive transfer of T lymphocytes genetically modified to express CARs conveniently combines both antibody-mediated specificity and T cell–effector functions.17,32 Because the ideal tumor-associated antigen should be expressed by tumor cells but virtually absent in normal tissues, we took advantage of the selective higher expression of CD23 by CLL cells to target malignant cells while sparing normal B cells. We cloned a fully humanized CAR specific for the CD23 antigen, which is highly expressed by CLL cells, and showed that T lymphocytes carrying this receptor efficiently eliminated CD23+ tumor cell lines and primary CLL cells while sparing normal B cells. Being able to preserve the normal B-cell compartment in CLL patients represents an important improvement because it would preserve their humoral immunity, which is often already impaired.
When targeting CD23, our primary concern was to show a lack of activity toward normal B cells. CD23 has been implicated in normal B-cell proliferation and survival,33 and although CD23-deficient mice have normal B-cell development, indicating that the elimination of CD23+ cells may not compromise the normal B-cell compartment,34 we carefully evaluated the effects of CD23.CAR-specific T cells on normal B lymphocytes. These cells can indeed express CD23,28 especially as IgM- and IgD-positive B cells in the mantle zone of the secondary follicle, and, as illustrated in our experiments, they can circulate in the peripheral blood of healthy individuals. However, their expression of CD23 is low and, accordingly, we did not detect significant CD23.CAR-mediated cytolytic activity against these cells. Because normal B lymphocytes are not intrinsically resistant to the cytotoxic effects of CAR-redirected T cells, as illustrated by their elimination by CD19.CAR-specific T cells, we suggest that the lack of cytotoxicity by CD23.CAR+ T cells on these cells is likely the result of both the low absolute number of CD23 molecules per cells (as indicated by the low MFI of the membrane-bound CD23) and/or a suboptimal affinity of the single chain cloned from the CD23 antibody, even if the native antibody may have high affinity. Indeed, the cross-linking of CAR molecules, which is required for dimerization of the ζ chains and activation of CAR-redirect T cells, is influenced by both the antigen density on target cells and the affinity of the single-chain antibody. As a consequence, if T cells express a CAR with relatively low affinity, successful activation of T lymphocytes to eliminate target cells can only occur when the antigen is highly expressed like for the high-proliferating subset of CD23bright CLL cells35 but not on the CD23dim-expressing normal B cells.
Therefore, CD23 seems to be a good target for CLL because it is highly expressed on leukemic cells, contributes in sustaining their viability,36,37 and appears to be essential for maintaining their aggressive neoplastic phenotype.38 CD23 can be cleaved from CLL cells, with high levels of the soluble form correlating with disease activity39 and poor clinical outcome.40,41 Although soluble antigens may reduce the bioavailability of mAbs and impair their antitumor effects,42 likely accounting for the limited efficacy of the CD23-specific lumiliximab as single agent,43 compared with a combined treatment,44 we showed that CD23.CAR+ T lymphocytes do not suffer from these competitive effects. This was not surprising, because similar results were previously observed for CARs targeting other antigens also present in a soluble form, such as GD2,45 CD30,46 CEA,47 and the light immunoglobulin chains.17 The observed lack of cytolytic activity against normal B lymphocytes was not due to a rapid loss of membrane-bound CD23 by normal B cells in culture, because cytofluorimetric analysis showed stable CD23 expression by normal B lymphocytes for at least 24 hours in culture, even in the absence of metalloprotease inhibitors (data not shown).
Previous studies have shown that CD23 can be processed and presented in the context of MHC molecules and that HLA-A2–restricted cytotoxic T lymphocytes (CTLs) specific for CD23-derived peptides can be elicited from the peripheral blood of CLL patients and recognize CD23+ CLL cells.48 Although the generation of CTLs targeting tumor-associated antigens using the native αβT-cell receptor is a feasible approach, the large-scale production of these cells remains challenging.49 The lack or limited availability of professional antigen-presenting cells for ex vivo T-cell expansion, the paucity of tumor-associated antigen–specific CTL precursors, and their anergy or hyporesponsiveness often observed in cancer patients (including CLL patients) represent major limitations to the success of this strategy.50 In contrast, a CAR-based approach allows the rapid generation of a large number of tumor-specific T cells that function in a non-MHC–restricted fashion, and thus is applicable to all CLL patients.
Two more advantages can be envisioned for CAR-based strategies, particularly those targeting CLL. First, this approach overcomes the anergy often observed for CLL-derived T cells, because after CAR gene transfer, T-cell lines generated from CLL patients release immunostimulatory cytokines such as IFN-γ, TNF-α, and TNF-β in response to CD23+ cell targets. Second, the lack of costimulatory molecules by CLL cells8 does not impair effective T-cell activation and persistence. Indeed, the incorporation of the CD28 costimulatory endodomain within the CD23.CAR construct17,25 allows the production of IL-2, which sustains their expansion in response to primary tumors, suggesting enhanced activity in vivo after adoptive transfer. The CD28 endodomain should also help in sustaining the cytotoxic activity and proliferative response of CAR-redirected T cells in the presence of regulatory T cells,30 which are particularly abundant in CLL patients.30,31
The therapeutic potential of our CD23.CAR construct was finally validated using a recently published murine xenograft model of human CLL.20 This model, based on either subcutaneous or IV injection of the CLL-derived cell line MEC-1 in an immunodeficient Rag2−/−γc−/− mouse model, resembles the aggressive form of human CLL in terms of disease development, tissue distribution, and organ involvement. Furthermore, because these mice lack B, T, and natural killer cells, it reveals a therapeutic effect exclusively mediated by the adoptively transferred T cells. Our in vivo experiments showed that CD23.CAR+ T cells were able to significantly control the growth of CD23+ tumor cells for more than 3 weeks after one single IV dose of T cells without any systemic addition of exogenous cytokines.
In conclusion, CD23.CAR-redirected T cells provide cytotoxic activity against CD23+ CLL cells both in vitro and in vivo while sparing normal B lymphocytes, unlike other available CARs targeting pan-B-cell antigens such as CD19. The selection of this antigen offers advantages because the most aggressive fraction of CLL cells, which are present within the proliferation centers and express higher levels of the CD23 antigen,35 are efficiently targeted. Ultimately, clinical studies will be required to determine whether targeting the CD23 molecule offers a significant benefit over targeting a pan-B-cell antigen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Fausto Rossini (Department of Adult Hematology, San Gerardo Hospital, Monza, Italy) provided CLL samples; Malika Brandi (Centro di Ricerca Matilde Tettamanti, Department of Pediatrics, University of Milano-Bicocca, San Gerardo Hospital, Monza, Milan, Italy) contributed to setting up the initial experiments of CLL-derived T cells and to isolation of CLL cells; and Elisabetta Cribioli (Centro di Ricerca Matilde Tettamanti, Department of Pediatrics, University of Milano-Bicocca, San Gerardo Hospital, Monza, Milan, Italy) contributed to setting up CLL-derived T cell–proliferation assays and to cytokine production analysis.
This work was supported in part by a Leukemia & Lymphoma Society Specialized Center of Research (SCOR) grant (no. 7018), by the National Institutes of Health (grants P01CA94237 and P50CA126752), by a Leukemia & Lymphoma Society Translational Research Grant, and by a Doris Duke Charitable Foundation/Clinical Scientist Development Award and Associazione Italiana per la Ricerca sul cancro (AIRC) molecular clinical oncology 5 per mille, “Innate immunity in cancer. Molecular targeting and cellular therapy” (no. 9962). E.B. was supported by AIRC Milano (AIRC 2007, 4069, and 4636), “Redirecting T lymphocytes to leukemia antigens by Chimeric T-cell Receptors: targeted therapy of leukemias” and “Progetto Integrato Oncologia 2006,” Ministero della Salute Direzione Generale della Ricerca Scientifica e Tecnologica. A.B. was supported by AIRC “Childhood ALL: from clinical studies to research questions to understand molecular history and pathogenesis.” P.G. was supported by AIRC Program Grant 5 per mille (programma no. 9965). G.M.P.G.A. is a fellow of the Vita-Salute San Raffaele University doctoral program in molecular medicine. M.T.S.B. is supported by a fellowship with the Ferrero Foundation (Alba, Italy). V.M. and I.P. are supported by a grant from STREP2006 (6th framework; LSHC-ct-2006–037381) “Chimeric T cells for the treatment of pediatric cancers (Childhope).”
National Institutes of Health
Authorship
Contribution: G.M.P.G.A., V.M., P.G., G.D., and E.B. designed the research, critically analyzed the data, and wrote the paper; G.M.P.G.A., V.H., B.S., I.P., S.T., and V.A. performed the in vitro experiments; G.M.P.G.A. and M.T.S.B. performed the in vivo animal studies; M. Parma and A.B. analyzed data and wrote the paper; and M. Ponzoni set up the animal studies and evaluated the in vivo response.
Conflict-of-interest declaration: The authors declare no competing financial interests.
Correspondence: Ettore Biagi, MD, Centro di Ricerca Matilde Tettamanti, Department of Pediatrics, University of Milano-Bicocca, San Gerardo Hospital, via Pergolesi 33, 20052, Monza, Milan, Italy; e.biagi@hsgerardo.org; or Gianpietro Dotti, MD, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St, MC 3-3320, Houston, TX 77030; e-mail: gdotti@bcm.tmc.edu.
References
Author notes
V.M. and V.H. contributed equally to this study.
G.D. and E.B. contributed equally to this study.