In this issue of Blood, Arons and colleagues provide evidence of a nonrandom pattern of somatic hypermutation in the immunoglobulin heavy chain gene in hairy cell leukemia (HCL) and provide further insights into the biology of this disease.1
Since its original description as an entity in 1958, HCL has fascinated practicing hematologists and researchers.2 The characteristic morphology of small- to medium-sized lymphoid cells with abundant cytoplasm and circumferential “hairy” projections, and the specific immunophenotypic expression of CD19, CD20, CD22, DBA.44, CD25, CD11c, FMC7, ANXA1, CD123, and CD103, but not CD5, CD10, or CD23 has clearly distinguished this rare leukemia from the other indolent lymphoid neoplasms.3
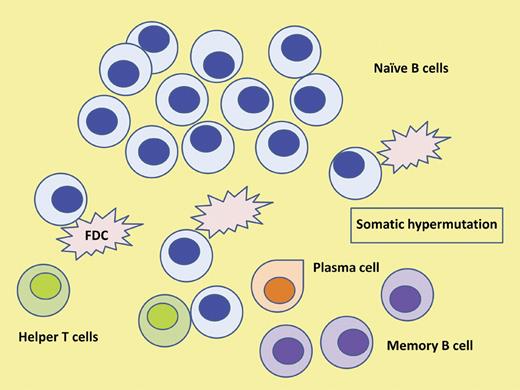
The cell of origin in HCL. Somatic hypermutation events in the GC of lymph nodes provide diversity to the B-cell receptor. In the majority of cases of HCL the malignant clone is derived from a post-GC memory B cell. Furthermore, evidence provided supports a canonical process suggestive of an antigen-driven process. FDC indicates follicular dendritic cell.
The cell of origin in HCL. Somatic hypermutation events in the GC of lymph nodes provide diversity to the B-cell receptor. In the majority of cases of HCL the malignant clone is derived from a post-GC memory B cell. Furthermore, evidence provided supports a canonical process suggestive of an antigen-driven process. FDC indicates follicular dendritic cell.
Over the past 2 decades there has been significant progress in the treatment of this disease with the introduction, first of interferon alfa, and then the nucleoside analogs, cladribine and pentostatin. The majority of patients treated with the latter drugs achieve long-lasting complete responses.4,5 This success has probably been the prime reason why further research into the biology and treatment of this rare disease has been limited in scope and conducted in a few, highly specialized centers with specific interest in HCL.
A number of studies have shed further light onto the cell of origin in HCL as well as predictors of response to treatment with the nucleoside analogs. Using gene-expression profiling, Basso and colleagues showed that HCL expressed a homogeneous pattern of genes clearly distinct from other lymphoid neoplasms and more related to the memory (postgerminal center [post-GC]) B cells than naive (pre-GC) B cells.6 Others have reported heterogeneity in the cell of origin in terms of mutational status with a few patients having unmutated immunoglobulin heavy chain variable (IGHV) region gene, indicative of a pre-GC cell of origin.7 Furthermore, the same group has reported that patients with unmutated IGHV were more likely to fail to respond to cladribine.8 In a previous study, Arons and colleagues also reported that patients with VH4-34 IGHV gene rearrangement were more frequently unmutated and had a greater white blood cell count at diagnosis, significantly lower response rate, and shorter progression-free and overall survival after initial therapy with cladribine.9
The ability of the immune system to recognize antigens depends on the immunoglobulins generated by the B cells and the antigen receptors on the surface of T cells. The significant diversity needed in antigen recognition is achieved by a number of well-characterized mechanisms such as the recombination of multiple V genes with D and J segments in the immunoglobulin heavy chain variable region (together with V and J recombination in the immunoglobulin light chains) that is largely responsible for the variability in the complimentary determining regions, an essential part of the antigen binding site. Further required diversity is provided by somatic mutations produced by single base pair changes within the variable regions of the immunoglobulin genes, providing further variations in antigen specificity. Membrane-bound immunoglobulin, or the B-cell receptor, then serves as the receptor for the antigens. Naive B cells that have not encountered antigen have unmutated variable region genes, while B cells that have entered the site of somatic mutation, considered to be the GC of lymph nodes, typically have acquired these somatic mutations (see figure). The origin of the neoplastic B cell reflects this mutational status and its clinical relevance has been clearly demonstrated in chronic lymphocytic leukemia (CLL) where the unmutated IGVH gene is clearly associated with a worse prognosis.10
The present study by Arons and colleagues provides further evidence that in the majority of patients with classic HCL (83% of 102 cases), the cell of origin is post-GC with mutated IGHV. This contrasted with their cohort of variant HCL where > half of the patients had unmutated IGHV and with historical data for patients with CLL where, again, ∼ half are unmutated. They also report higher usage of certain IGHV (and IGHD) gene families and a difference in mutational frequency among these genes. Furthermore, by demonstrating that the mutations fulfilled predefined characteristics of a canonical and nonrandom event, they provide further evidence suggestive of an antigen-driven process. As shown in studies reported earlier, the origin of the malignant cell in HCL is of clinical relevance in predicting response to treatment with cladribine (and perhaps, as the authors suggest, with CD22 directed therapy). Similarly, insights into the process of somatic hypermutation may provide more understanding of the potential triggers for the genesis of the disease.
Clearly, further research is needed into this and other aspects of the biology of the disease; for example, the interaction between the malignant cells and their microenvironment and the potential role of the B cell receptor in this process. The rarity of the disease and the success of treatment decelerated the pace of such research in the past. The recent development of the Hairy Cell Leukemia Consortium that may further stimulate such research is welcome news.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal