Although hereditary folate malabsorption was first described 50 years ago, only recently has the molecular basis of this disease been determined. In this issue of Blood, Salojin et al describe the proton coupled folate transporter (PCFT) null mouse, whose phenotype resembles that of the human disease.1
Five decades ago Victor Herbert performed a self-experiment to demonstrate that a diet deficient in folates could cause megaloblastic anemia. For several months he ate overcooked food (eg, thrice-boiled rice) and an occasional deep-water lobster with low folate content.2 He did indeed develop early stages of megaloblastic anemia, which was quickly corrected by folic acid.3 Coincident with Herbert's self-induced acquired folate deficiency, the first cases of hereditary folate malabsorption (HFM) were described in infants presenting with megaloblastic anemia, failure to thrive, intracranial calcifications, peripheral neuropathies, recurrent infections, and developmental delay.4 Serum, red blood cells, and cerebrospinal fluid folates were low. Even after treatment with large oral doses of folic acid, or parenteral administration of folinic acid or folic acid, patients generally suffered with poor neurologic outcomes that included mental retardation, ataxia, and seizure.
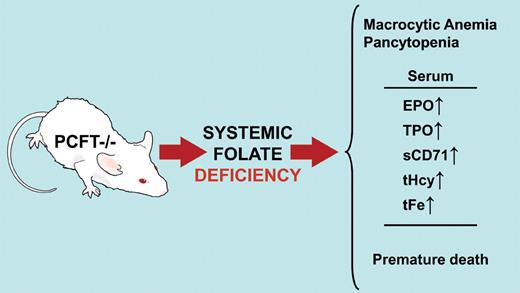
The proton-coupled folate transporter (PCFT) knockout mouse develops systemic folate deficiency after birth and, if untreated, succumbs within 10 to 12 weeks. Macrocytic anemia and pancytopenia with accumulation of immature erythroblasts in bone marrow and spleen are observed in these animals. Serum levels of erythropoietin (EPO), thrombopoietin (TPO), and soluble transferrin receptor (sCD71) are increased. Plasma total homocysteine (tHcy) and total iron (tFe) are also increased. PCFT−/− mice are a model for human hereditary folate malabsorption. Professional illustration by David Schumick.
The proton-coupled folate transporter (PCFT) knockout mouse develops systemic folate deficiency after birth and, if untreated, succumbs within 10 to 12 weeks. Macrocytic anemia and pancytopenia with accumulation of immature erythroblasts in bone marrow and spleen are observed in these animals. Serum levels of erythropoietin (EPO), thrombopoietin (TPO), and soluble transferrin receptor (sCD71) are increased. Plasma total homocysteine (tHcy) and total iron (tFe) are also increased. PCFT−/− mice are a model for human hereditary folate malabsorption. Professional illustration by David Schumick.
In 2006 Qiu et al reported that a loss-of-function mutation in the gene for the PCFT was the molecular basis of HFM.5 The 50-kDa PCFT membrane protein is localized in the apical brush borders of intestinal cells of the duodenum where the bulk of folate absorption occurs and, to a lesser extent, the jejunum. PCFT mRNA is also expressed in kidney, liver, placenta, small intestine, and spleen. The PCFT has a high affinity for folic acid and N5-methyl-tetrahydrofolate (Ki ∼ 0.6μM) at pH 5.5, which is close to the acidic microenvironment of the intestinal surface in the duodenum and jejunum.6 Additional homozygous and compound heterozygous mutations of the PCFT gene have now been identified in 19 different families with this rare autosomal recessive disorder.7,8
A logical extension of the work on HFM would be to create an animal model of the disease. Thus, Salojin et al, using targeted disruption of the first 3 coding exons of the mouse homolog of the PCFT, deleted the gene. Surprisingly, the PCFT−/− animals survive through embryonic development, suggesting that maternal sources of folate are adequate. However, by 4 weeks of age the pups have systemic folate deficiency with macrocytic normochronic anemia and pancytopenia. If left untreated, most of the PCFT null mice are dead within 10 to 12 weeks of age (see figure).1
The authors claim that the PCFT-deficient mouse recapitulates human HFM and folate deficiency anemia and is the “most accurate animal model of folate deficiency.”1page4895 Let's look at the evidence. At 4 to 6 weeks of age, the PCFT−/− mice had decreased levels of all forms of leukocytes, red blood cells, and platelets. Serum concentrations of erythropoietin and soluble transferrin receptor were elevated, indicative of ineffective erythropoiesis. In line with the severe thrombocytopenia observed, serum thrombopoietin levels were elevated. Circulating reticulocytes and the reticulocyte production index were lower in the PCFT−/− animals compared with wild-type mice. Multilobed polymorphonuclear neutrophils were observed in blood smears from the PCFT null mice. These observations are consistent with the hematologic profile seen in patients with folate deficiency anemia.
Histologic examination of bone marrow and spleen from the PCFT−/− mice revealed marked hyperproliferation of erythroid cells with excessive proerythroblasts and large basophilic erythroblasts. FACS analysis suggested that the erythroid cell differentiation process was blocked at the intermediate erythroblast stage. There was a significantly higher proportion of annexin V-positive apoptotic cells in the intermediate erythroblast population of the PCFT−/− bone marrow than in wild-type bone marrow. This suggests that survival of erythroblasts at the latter stages of erythroid maturation is decreased.
To obtain evidence for systemic folate deficiency in the PCFT heterozygous and null mice, serum and tissue folate levels were measured. Plasma folate was 40% lower and almost nondetectable in the PCFT+/− and the PCFT−/− animals, respectively. Liver and kidney tissue levels of folate were markedly decreased in the PCFT−/− mice. Patients who are folate deficient usually have elevated concentrations of plasma total homocysteine because of impairment of the remethylation pathway in the methionine cycle. The plasma total homocysteine concentration was approximately 3-fold higher in the PCFT−/− mice compared with wild-type animals.
The above observations suggest that the PCFT null mouse is a good model for impaired folate absorption and systemic folate deficiency. Furthermore, this work strongly supports the earlier observation that the PCFT is the primary transporter of dietary folates during absorption.5 Future studies will hopefully delineate the role of the PCFT in other tissues. The intriguing observation that patients with HFM have low concentrations of folate in their spinal fluid accompanied by severe neurologic complications suggests that the PCFT may be involved in delivery of folates to the brain. This animal model of HFM may be pivotal in understanding the mechanism of folate delivery to other tissues, both normal and abnormal, in the body.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■