Abstract
We evaluated the role of granulocyte colony-stimulating factor (G-CSF) in patients with severe aplastic anemia (SAA) treated with antithymocyte globulin (ATG) and cyclosporine (CSA). Between January 2002 and July 2008, 192 patients with newly diagnosed SAA not eligible for transplantation were entered into this multicenter, randomized study to receive ATG/CSA with or without G-CSF. Overall survival (OS) at 6 years was 76% ± 4%, and event-free survival (EFS) was 42% ± 4%. No difference in OS/EFS was seen between patients randomly assigned to receive or not to receive G-CSF, neither for the entire cohort nor in subgroups stratified by age and disease severity. Patients treated with G-CSF had fewer infectious episodes (24%) and hospitalization days (82%) compared with patients without G-CSF (36%; P = .006; 87%; P = .0003). In a post hoc analysis of patients receiving G-CSF, the lack of a neutrophil response by day 30 was associated with significantly lower response rate (56% vs 81%; P = .048) and survival (65% vs 87%; P = .031). G-CSF added to standard ATG and CSA reduces the rate of early infectious episodes and days of hospitalization in very SAA patients and might allow early identification of nonresponders but has no effect on OS, EFS, remission, relapse rates, and mortality. This study was registered at www.clinicaltrials.gov as NCT01163942.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 4679.
Disclosures
Judith Marsh was a consultant for Genzyme Therapeutics from 2008-2009, and received research funding from Genzyme in 2010. The remaining authors; the Associate Editor Grover C. Bagby Jr; and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC; declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the effect of G-CSF on overall survival and event-free survival in patients with SAA treated with ATG and cyclosporine
Describe the effect of G-CSF on number of infectious episodes and hospitalization days in patients with very severe aplastic anemia treated with ATG and cyclosporine
Describe the predictive value of the lack of a neutrophil response to G-CSF by day +30 in terms of response rate and survival
Release date: April 28, 2011; Expiration date: April 28, 2012
Introduction
Aplastic anemia (AA) is a bone marrow failure disorder characterized by pancytopenia. Death occurs secondary to infection, bleeding, or complications of severe anemia. Hematopoietic stem cell transplantation (HSCT) can cure the disease, but only a minority of patients has a histocompatible sibling donor. Immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporine (CSA) is the treatment of choice for patients not eligible for HSCT.1-4 Overall survival (OS) at 10 years lies between 60% and 80%. However, immunosuppression remains a suboptimal option and usually does not result in cure. About 30% of patients fail to respond, and, even in responding patients, blood counts often remain subnormal, and relapse and late clonal complications such as myelodysplastic syndromes (MDSs) are frequent. Other immunosuppressive drugs or combinations thereof, as well as the use of high-dose cyclophosphamide, have been used. However, infectious complications remain the main cause of death, and these newer regimens failed to improve response and survival over the ATG plus CSA standard combination.5-7
Growth factors alone do not correct AA and may be harmful because of delay in starting definitive treatment.8 In contrast, the role of growth factors added to standard immunosuppressive therapy with ATG and CSA is still a matter of debate. Six previous small prospective randomized controlled trials were inconclusive, and none of them showed a survival advantage.9-14 Nevertheless, despite the lack of evidence for preemptive use, many centers add granulocyte colony-stimulating factor (G-CSF) to ATG plus CSA, particularly in pediatric patients or in patients with low neutrophil counts.11,15
Therefore, the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) conducted a randomized controlled study that compared ATG and CSA with or without G-CSF. We sought to define, in a large cohort of patients, the role of G-CSF in patients with newly diagnosed severe AA (SAA) when used with ATG and CSA (registered at www.clinicaltrials.gov as NCT01163942).
Methods
Design of the study
This prospective, open-label, multicenter randomized study was conducted by the Severe Aplastic Anemia Working Party of the EBMT in patients with newly diagnosed acquired SAA who were not eligible for HSCT. Disease severity was assessed with the use of standard criteria, into SAA or very SAA (VSAA), and patients were randomly assigned to receive ATG and CSA or ATG, CSA, and G-CSF. Randomization was stratified by center and disease severity. The study was approved by the ethical committees of all participating institutions.
The inclusion criteria comprised patients with SAA or VSAA; with disease duration of < 6 months; who had not received prior ATG at any time, CSA within 4 weeks, other growth factors within 4 weeks, or G-CSF within 2 weeks of enrolment. Patients of any age were included, except in Germany and the United Kingdom, where the ethical committees did not accept the inclusion of children < 18 years. Patients with congenital SAA, such as Fanconi anemia, as well as patients with MDS were excluded.
Definitions
The diagnosis of SAA required bone marrow cellularity of < 30% and ≥ 2 of the 3 following criteria from peripheral blood counts: platelet counts ≤ 20 × 109/L, neutrophil count ≤ 0.5 × 109/L, and reticulocyte count (performed by manual counting) ≤ 20 × 109/L.16 Patients with a neutrophil count < 0.2 × 109/L were classified as VSAA.17 Complete response was defined as transfusion independence associated with a hemoglobin level of ≥ 110 g/L, neutrophil count of ≥ 1.5 × 109/L, and a platelet count of ≥ 150 × 109/L. Partial response was defined as no longer meeting the criteria for SAA and no transfusion dependence for platelets or red blood cells. Continuous transfusion dependency was classified as no response. Relapse was defined as a decrease in blood counts to values either requiring transfusions or needing reinstitution of immunosuppressive therapy or HSCT.
Treatment protocol
Horse ATG (Lymphoglobuline; Genzyme) was administered at a dose of 15 mg/kg body weight per day on 5 consecutive days. To prevent serum sickness, prednisolone 1 mg/kg/d was started from the first day of ATG and maintained over 14 days. Thereafter, the dose was tapered off over the subsequent 14 days. CSA, given orally at a dose of 5 mg/kg/d, was started on day 1 and administered for a minimum of 6 months and then tapered according to institution guidelines. In patients randomly assigned to receive G-CSF, glycosylated recombinant human G-CSF at a dose of 150 μg/m2/d was administered daily from day 8 as a subcutaneous injection. Treatment with G-CSF was continued through day 240 except for subjects who achieved a complete remission before.
Patients were also randomly assigned to receive or not to receive an early second course of immunosuppression after day 120 if a response was not achieved. Data on this second randomization are not sufficiently mature at this time, and the patient groups are small. Therefore, only a limited report is included here.
Statistical analysis
The study was powered to detect a 15% difference in event-free survival (EFS), with a baseline estimate at 5 years of 40%. Assuming a type II error of 20% and a type I error of 5%, 340 patients were to be enrolled, 170 patients into each study arm.18 Time to event analyses (OS and EFS) start on the day of randomization. For OS, patients were censored either at time of last follow-up or at time of transplantation, used as salvage therapy. For EFS analysis, events were defined as relapse of the disease, a newly diagnosed clonal complication (MDS, paroxysmal nocturnal hemoglobinuria [PNH]), nonresponse at day 120, allogeneic transplantation, and death, whichever came first. In 2008 the horse preparation of ATG (Lymphoglobuline, Genzyme GmbH) was no longer available in Europe, and as a consequence the patient accrual declined rapidly. It was therefore decided to close the study prematurely as of August 1, 2008, after enrolling 205 patients.
Primary outcome parameters analyzed were EFS and mortality. Secondary endpoints of the study were causes of death, response to immunosuppression (assessed at day 30, 60, 90, 120, 180, 270, 365), relapse in responders, late complications, number of infectious complications, and days of hospitalization, both by follow-up periods (from 0 to 30 days, from 30 to 60 days, and from 60 to 90 days after randomization). Causes of death were classified as related to aplastic anemia (infection, bleeding), secondary neoplasm (MDS, leukemia, solid tumor), transplantation related in patients who received HSCT for treatment failure, unrelated to aplastic anemia, or of unknown cause.
Group differences were analyzed with the use of the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. Survival probabilities were calculated with the use of the Kaplan-Meier estimator. Time at risk started at the date of randomization and ended at the date of death for OS, an event for EFS, or the date of last known assessment, whichever came first. The 95% confidence interval (CI) was calculated according to Rothman and Boice. To calculate the cumulative incidence of relapse in responding patients, and of secondary clonal complications, death from other causes was considered as a competing risk. Variables significantly associated with the risk of death were assessed by univariate and multivariate analyses. The log-rank test with 2-sided significance levels was used for comparisons in Kaplan-Meier estimates. Proportional hazards regression analysis was used to assess the effect of risk factors for the outcome. Variables considered were sex, age, disease severity, and treatment group (with or without G-CSF). A backward stepwise procedure was used to eliminate nonsignificant variables.
Results
From January 2002 to July 2008, 205 patients were randomly assigned to treatment; 13 were excluded from analysis (1 patient for incorrect diagnosis and 12 patients without follow-up information). In total, 192 patients were included in the analysis (95 with G-CSF, 97 without G-CSF). The median age of the patients was 46 years (range, 2-81 years), 94 (49%) were males and 70 (36%) had VSAA. There was no difference between treatment groups with respect to age, sex, severity of the AA, the presence of a PNH clone, number of platelet and red blood cell transfusions before treatment, or prior exposure to hepatitis A, B, or C virus infection (Table 1).
Characteristics of the patients
| . | All patients . | No G-CSF . | With G-CSF . | P . |
|---|---|---|---|---|
| No. of patients in the study | 192 | 95 | 97 | |
| Median age at random assignment, y (range) | 46 (2-81) | 44 (7-80) | 47 (2-81) | .279 |
| Age groups | ||||
| < 20 y, n (%) | 31 (16) | 15 (16) | 16 (16) | |
| 20-40 y, n (%) | 51 (27) | 27 (29) | 24 (25) | .362 |
| 40-60 y, n (%) | 51 (26) | 29 (30) | 22 (23) | |
| > 60 y, n (%) | 59 (31) | 24 (25) | 35 (36) | |
| Sex | ||||
| Male, n (%) | 94 (49) | 46 (48) | 48 (49) | .883 |
| Female, n (%) | 98 (51) | 49 (52) | 49 (51) | |
| Severity | ||||
| SAA, n (%) | 122 (64) | 56 (59) | 66 (68) | .191 |
| Very SAA, n (%) | 70 (36) | 39 (41) | 31 (32) | |
| PNH clone at diagnosis | ||||
| Yes, n (%) | 23 (12) | 14 (15) | 9 (9) | |
| No, n (%) | 116 (60) | 53 (55) | 63 (65) | .326 |
| Not done, n (%) | 53 (28) | 28 (30) | 25 (26) | |
| Last follow-up | ||||
| Alive, n (%) | 148 (77) | 72 (76) | 76 (78) | .673 |
| Dead, n (%) | 44 (23) | 23 (24) | 21 (22) |
| . | All patients . | No G-CSF . | With G-CSF . | P . |
|---|---|---|---|---|
| No. of patients in the study | 192 | 95 | 97 | |
| Median age at random assignment, y (range) | 46 (2-81) | 44 (7-80) | 47 (2-81) | .279 |
| Age groups | ||||
| < 20 y, n (%) | 31 (16) | 15 (16) | 16 (16) | |
| 20-40 y, n (%) | 51 (27) | 27 (29) | 24 (25) | .362 |
| 40-60 y, n (%) | 51 (26) | 29 (30) | 22 (23) | |
| > 60 y, n (%) | 59 (31) | 24 (25) | 35 (36) | |
| Sex | ||||
| Male, n (%) | 94 (49) | 46 (48) | 48 (49) | .883 |
| Female, n (%) | 98 (51) | 49 (52) | 49 (51) | |
| Severity | ||||
| SAA, n (%) | 122 (64) | 56 (59) | 66 (68) | .191 |
| Very SAA, n (%) | 70 (36) | 39 (41) | 31 (32) | |
| PNH clone at diagnosis | ||||
| Yes, n (%) | 23 (12) | 14 (15) | 9 (9) | |
| No, n (%) | 116 (60) | 53 (55) | 63 (65) | .326 |
| Not done, n (%) | 53 (28) | 28 (30) | 25 (26) | |
| Last follow-up | ||||
| Alive, n (%) | 148 (77) | 72 (76) | 76 (78) | .673 |
| Dead, n (%) | 44 (23) | 23 (24) | 21 (22) |
General results of the study
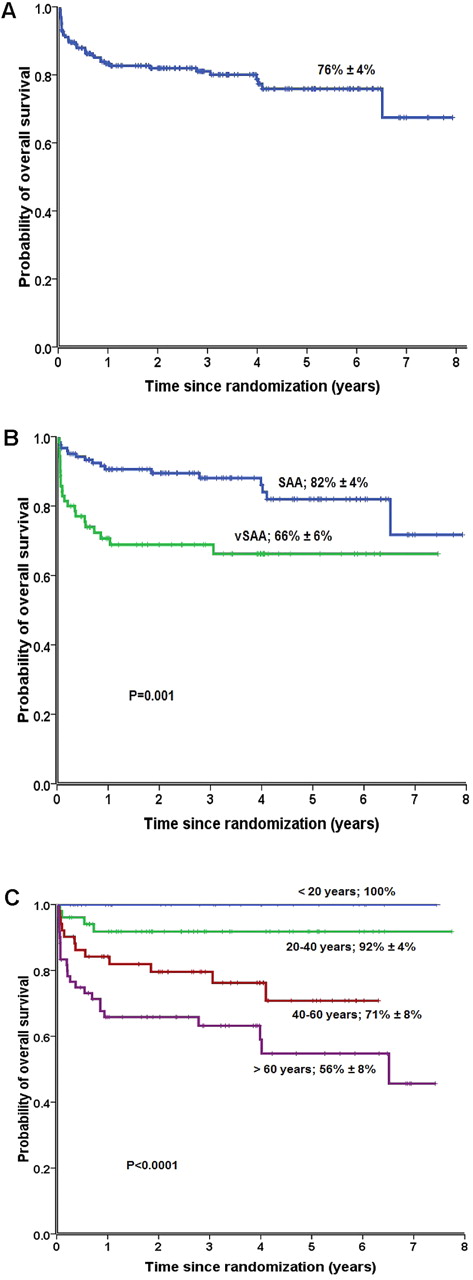
At a median follow-up of 41 months (range, 1-96 months) for surviving patients, 148 patients (77%) were alive. The OS and the EFS at 6 years of all patients were 76% ± 4% (Figure 1A) and 42% ± 4%, respectively. The overall response rate was 70% (134 of 192 patients). Complete response was observed in 21 (11%) and partial response in 113 (59%) patients. Fifty-eight patients (30%) showed no response. Nineteen patients (10%) who did not respond subsequently underwent allogeneic HSCT from an unrelated donor (Table 2). During the study period, 44 patients died. The median time from randomization to death was 135 days (range, 15-2378 days). Bacterial and fungal infections were by far the most common causes of death, accounting for 55% of all deaths (24 of 44 deaths). Other causes of death were noninfectious SAA-related deaths (bleeding, disease progression) in 8, secondary MDS or acute myeloid leukemia (AML) in 2, solid tumor in 1, cardiovascular complications in 4, transplantation-related death in 4, and unknown in 1 patient. Fourteen of 44 deaths (32%) occurred within the first 30 days from randomization, 12 of them because of infectious complications.
Six-year OS rate in patients with SAA treated with ATG/CSA with or without G-CSF. (A) OS of all 192 patients; (B) OS according to disease severity, comparing patients with SAA (blue), and very SAA (green); (C) OS according to age groups: patients < 20 years (blue), patients aged 20-40 years (green), patients aged 40-60 years (red), and patients aged > 60 years (lilac).
Six-year OS rate in patients with SAA treated with ATG/CSA with or without G-CSF. (A) OS of all 192 patients; (B) OS according to disease severity, comparing patients with SAA (blue), and very SAA (green); (C) OS according to age groups: patients < 20 years (blue), patients aged 20-40 years (green), patients aged 40-60 years (red), and patients aged > 60 years (lilac).
Response rates, infections, hospitalization days, relapses, late complications, and deaths
| . | All patients . | No G-CSF . | With G-CSF . | P . |
|---|---|---|---|---|
| Best response ever reached | ||||
| Complete response, n (%) | 21 (11) | 9 (9) | 12 (12) | .535 |
| Partial response, n (%) | 113 (59) | 54 (57) | 59 (61) | |
| No response, n (%) | 58 (30) | 32 (34) | 26 (27) | |
| Best response ever reached for very SAA | ||||
| Complete response, n (%) | 6 (8) | 2 (5) | 4 (13) | |
| Partial response, n (%) | 33 (47) | 19 (49) | 14 (45) | .513 |
| No response, n (%) | 31 (44) | 18 (46) | 13 (42) | |
| Best response ever reached for SAA | ||||
| Complete response, n (%) | 15 (12) | 7 (12) | 8 (12) | |
| Partial response, n (%) | 80 (66) | 35 (63) | 45 (68) | .764 |
| No response, n (%) | 27 (22) | 14 (25) | 13 (20) | |
| No. of infectious episodes per month at risk during the first 90 days | ||||
| All patients, n/N (%) | 137/462 (30) | 81/228 (36) | 56/234 (24) | .006 |
| Patients with SAA, n/N (%) | 67/302 (22) | 33/136 (24) | 34/16 (20) | .431 |
| Patients with very SAA, n/N (%) | 70/160 (44) | 48/92 (52) | 22/68 (32) | .014 |
| Days spent in the hospital during the first 30 days, n/N (%) | 3008/3551 (84) | 1612/1858 (87) | 1396/1693 (82) | .0003 |
| No. of patients with first relapse (%) | 38 (20) | 19 (20) | 19 (20) | .679 |
| No. of HSCT as second- or third-line therapy (%) | 19 (10) | 11 (11) | 9 (9) | .570 |
| Secondary malignant neoplasm, n (%) | 7 (3.6) | 4 (4) | 3 (3) | .488 |
| PNH | ||||
| At diagnosis, n (%) | 23 (12) | 14 15) | 9 (9) | .350 |
| Since first IS treatment, n (%) | 14 (7) | 8 (8) | 6 (6) | .590 |
| Number of deaths (%) | 44 (23) | 23 (24) | 21 (22) | .673 |
| Cumulative incidence of a complications at 6 years (95% CI) | ||||
| Relapse | 33 (24-46) | 33 (21-53) | 32 (21-51) | .792 |
| Malignant neoplasm | 4 (2-10) | 6 (2-16) | 3 (1-11) | .537 |
| PNH clone | 19 (14-27) | 22 (15-33) | 16 (9-27) | .067 |
| . | All patients . | No G-CSF . | With G-CSF . | P . |
|---|---|---|---|---|
| Best response ever reached | ||||
| Complete response, n (%) | 21 (11) | 9 (9) | 12 (12) | .535 |
| Partial response, n (%) | 113 (59) | 54 (57) | 59 (61) | |
| No response, n (%) | 58 (30) | 32 (34) | 26 (27) | |
| Best response ever reached for very SAA | ||||
| Complete response, n (%) | 6 (8) | 2 (5) | 4 (13) | |
| Partial response, n (%) | 33 (47) | 19 (49) | 14 (45) | .513 |
| No response, n (%) | 31 (44) | 18 (46) | 13 (42) | |
| Best response ever reached for SAA | ||||
| Complete response, n (%) | 15 (12) | 7 (12) | 8 (12) | |
| Partial response, n (%) | 80 (66) | 35 (63) | 45 (68) | .764 |
| No response, n (%) | 27 (22) | 14 (25) | 13 (20) | |
| No. of infectious episodes per month at risk during the first 90 days | ||||
| All patients, n/N (%) | 137/462 (30) | 81/228 (36) | 56/234 (24) | .006 |
| Patients with SAA, n/N (%) | 67/302 (22) | 33/136 (24) | 34/16 (20) | .431 |
| Patients with very SAA, n/N (%) | 70/160 (44) | 48/92 (52) | 22/68 (32) | .014 |
| Days spent in the hospital during the first 30 days, n/N (%) | 3008/3551 (84) | 1612/1858 (87) | 1396/1693 (82) | .0003 |
| No. of patients with first relapse (%) | 38 (20) | 19 (20) | 19 (20) | .679 |
| No. of HSCT as second- or third-line therapy (%) | 19 (10) | 11 (11) | 9 (9) | .570 |
| Secondary malignant neoplasm, n (%) | 7 (3.6) | 4 (4) | 3 (3) | .488 |
| PNH | ||||
| At diagnosis, n (%) | 23 (12) | 14 15) | 9 (9) | .350 |
| Since first IS treatment, n (%) | 14 (7) | 8 (8) | 6 (6) | .590 |
| Number of deaths (%) | 44 (23) | 23 (24) | 21 (22) | .673 |
| Cumulative incidence of a complications at 6 years (95% CI) | ||||
| Relapse | 33 (24-46) | 33 (21-53) | 32 (21-51) | .792 |
| Malignant neoplasm | 4 (2-10) | 6 (2-16) | 3 (1-11) | .537 |
| PNH clone | 19 (14-27) | 22 (15-33) | 16 (9-27) | .067 |
IS indicates immunosuppression.
During the first 30 days, 53% of the patients had an infection (91 patients with infection of 171 reported patients) (Table 2). Between 30 and 60 days 23% (34 of 148) and between 60 and 90 days 8% (12 of 143) presented with an infectious complication. Thirty-eight of responding patients (20%) relapsed at a median of 12 months (range, 1-70 months) after randomization. The cumulative incidence of relapse at 6 years from randomization was 33% (95% CI, 24%-46%). Seven patients (3.6%) developed a secondary malignant neoplasm, 6 presented with MDS or AML, and 1 with a solid tumor. The cumulative incidence at 6 years of developing a clonal complication was 4% (95% CI, 2%-10%). Twenty-three patients (12%) had a PNH clone at time of diagnosis, and 14 patients (7%) developed a new PNH clone during follow-up after immunosuppressive treatment. The cumulative incidence of developing a PNH clone at 6 years was 19% (95% CI, 14%-27%).
Primary and secondary endpoints of first randomization
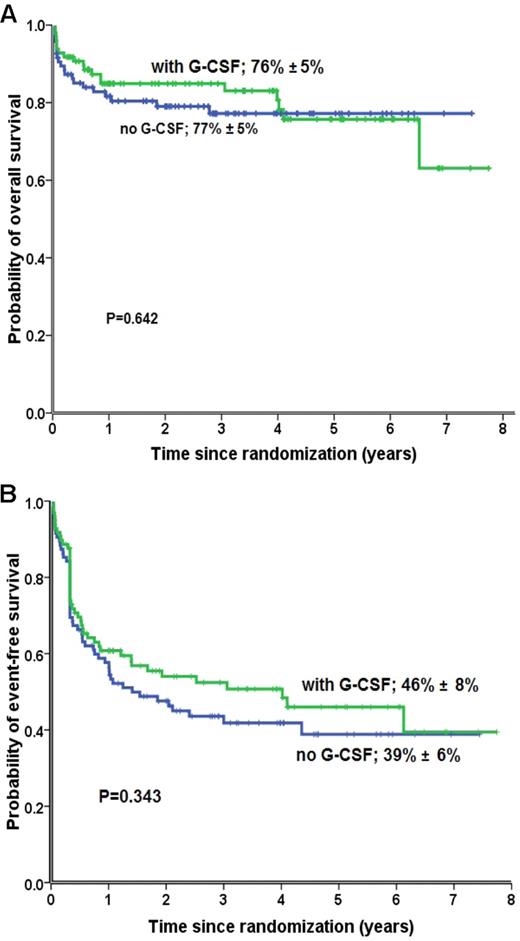
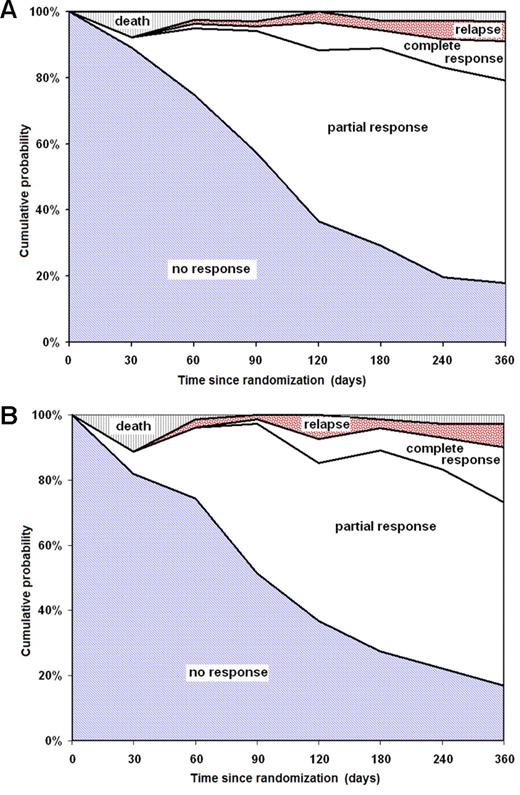
No difference was observed in OS (P = .64; Figure 2A) and in EFS (P = .343; Figure 2B) at 6 years between patients randomly assigned to receive or not to receive G-CSF. There was neither a difference in overall trilineage hematologic response between patients treated with or without G-CSF (Table 2). In total, 73% of patients who received G-CSF and 66% who did not receive G-CSF responded to immunosuppression (P = .535). The response rates increased progressively over time, but they were similar between both randomization groups (Figure 3A-B). There was no difference in death rates between patients randomly assigned to receive G-CSF or no G-CSF. In patients treated without G-CSF, 23 of 95 patients (24%) died, compared with 21 of 97 patients (22%) in the G-CSF group (P = .673; Table 2). The causes of death, and particularly deaths because of infections, were not different between patients treated without and with G-CSF.
OS and EFS rates. Six-year OS rate (A) and EFS (B) in patients with SAA treated with ATG/CSA with (green) or without (blue) G-CSF.
OS and EFS rates. Six-year OS rate (A) and EFS (B) in patients with SAA treated with ATG/CSA with (green) or without (blue) G-CSF.
Response rates according to the time since randomization. (A) Patients randomly assigned to ATG/CSA without G-CSF; (B) patients randomly assigned to ATG/CSA with G-CSF.
Response rates according to the time since randomization. (A) Patients randomly assigned to ATG/CSA without G-CSF; (B) patients randomly assigned to ATG/CSA with G-CSF.
Patients treated with G-CSF had fewer episodes of infection (56 of 234; 24%) than patients who were randomly assigned not to receive G-CSF (81 of 228; 36%; P = .006; Table 2). Overall, there were 3008 hospitalization days for a total period of 3551 observation days (results available from 120 patients) (Table 2). There were fewer hospitalization days in patients treated with G-CSF (82%) than in patients not receiving G-CSF (87%; P = .0003). Randomization with or without G-CSF had no effect on the need for a subsequent HSCT, the prevalence or cumulative incidence of relapse, the development of a secondary malignant neoplasm, or a PNH clone (Table 2).
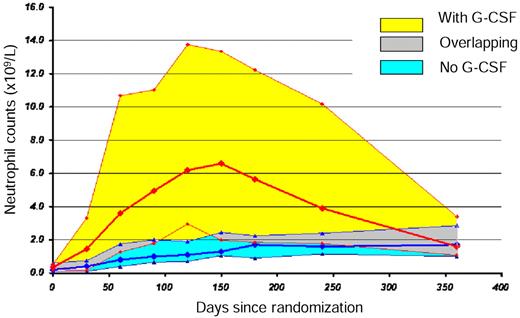
The median neutrophil count was significantly higher from day 30 to day 240 in the G-CSF arm, but this difference did not persist to day 360, at a time when most patients randomly assigned to receive G-CSF no longer received the drug (Figure 4).
Evolution of neutrophil counts during the first year in patients treated with ATG and CSA with and without G-CSF. The bold red line represents the median, and the yellow surface represents the 75% CI of the neutrophil counts in patients treated with G-CSF; the bold blue line represents the median, and the blue surface represents the 75% CI of the neutrophil counts in patients treated without G-CSF; the gray surface represents the overlapping surface of 75% CI of patients treated with and without G-CSF.
Evolution of neutrophil counts during the first year in patients treated with ATG and CSA with and without G-CSF. The bold red line represents the median, and the yellow surface represents the 75% CI of the neutrophil counts in patients treated with G-CSF; the bold blue line represents the median, and the blue surface represents the 75% CI of the neutrophil counts in patients treated without G-CSF; the gray surface represents the overlapping surface of 75% CI of patients treated with and without G-CSF.
In the stepwise multivariate Cox regression analysis, age at time of randomization as a continuous variable (relative risk [RR], 1.043; 95% CI, 1.019-1.067; P < .001) and severity of the disease (RR, 2.935; 95% CI, 1.109-7.766; P = .030) were statistically associated with overall survival. The use of G-CSF compared with no G-CSF (RR = 1) was not significant (RR, 1.00; 95% CI, 0.416-12.403; P = .999).
Second randomization
On day 120, 62 of 146 evaluable patients had not achieved a response to immunosuppression and were therefore eligible for early retreatment. Thirty-eight patients (17 with G-CSF, 21 without G-CSF) were randomly assigned to receive a second ATG course, and 24 patients (14 with G-CSF, 10 without G-CSF) were randomly assigned to continued care. In an intention-to-treat analysis, there was no difference in OS between treatment groups: The OS at 6 years was 83% ± 7% for the early retreatment arm and 77% ± 10% for the continued care arm (P = .441). There were many study violations because only 20 of 38 patients randomly assigned to early retreatment actually received an early second treatment, whereas 7 of 24 patients randomly assigned to continued care received a second ATG between day 120 and day 180 for various reasons (4 of these because of disease relapse).
Post hoc, secondary analyses
We further evaluated the effect of severity of the disease and age of the patients at random assignment on outcomes of all patients as well as on patients randomly assigned to receive or not G-CSF. OS was 82% ± 4% for patients with SAA and 66% ± 6% for patients with VSAA (P = .001; Figure 1B). Survival was better for young patients aged < 20 or 20-40 years than for older patients (P < .001; Figure 1C). EFS was 44% ± 5% for patients with SAA and 39% ± 6% for patients with VSAA (P = .013). EFS of patients < 20 years (58% ± 9%) and patients aged between 20 and 40 years (49% ± 9%) was significantly higher than patients > 60 years (29% ± 6%; P = .035 and 0.006, respectively). When patients were stratified according to age and severity of the disease, there was no difference in OS, EFS, and in response rates between patients randomly assigned to receive or not to receive G-CSF. The number of nonresponders was higher in patients with VSAA (31 of 70; 44%) than in patients with SAA (27 of 122; 22%; P = .006). Patients ≥ 40 years of age were more often nonresponders (44 of 110; 40%) than younger patients (14 of 82; 17%; P = .0012), because of higher death rates before treatment response in older patients.
Patients with VSAA had higher infectious rates during 3 periods than patients with SAA [first 30 days, VSAA = 65% vs SAA = 47% (P = .015); between 30 and 60 days, VSAA = 41% vs SAA = 14% (P < .0001); between 60 and 90 days, VSAA = 17% vs SAA = 5% (P = .021)]. The difference observed in episodes of infection between patients treated or not with G-CSF was mainly because of the excess of infections in patients with VSAA (P = .014) compared with patients with SAA (P = .431; Table 2). Furthermore, there were more hospitalization days in patients with VSAA (1244 of 1329; 94%) than in patients with SAA (1764 of 2222; 79%; P = .0001).
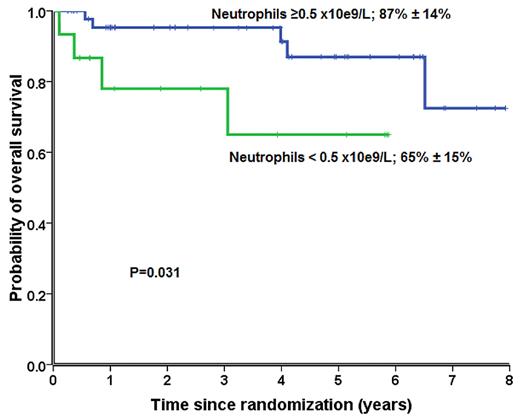
We further assessed the effect of neutrophil counts (< 0.5 vs ≥ 0.5 × 109/L) on day 30 after randomization on prediction for response and survival. We therefore compared response and survival according to neutrophil counts at day 30 in a post hoc analysis. For this analysis, only patients who survived > 30 days after random assignment were included. Patients randomly assigned to receive G-CSF with neutrophil counts ≥ 0.5 × 109/L had significant better overall response rates (38 of 47; 81%) and better survival at 6 years (87% ± 14%) than patients with lower neutrophil counts (response rates: 10 of 18, 56%, P = .048; survival: 65% ± 15%; P = .031). This difference was not observed among patients not receiving G-CSF (response rate, P = .350; survival, P = .247) (Figure 5).
Survival of patients randomly assigned to receive G-CSF with neutrophil counts ≥ 0.50 × 109/L (blue) at day 30 compared with patients with neutrophil counts < 0.50 × 109/L (green).
Survival of patients randomly assigned to receive G-CSF with neutrophil counts ≥ 0.50 × 109/L (blue) at day 30 compared with patients with neutrophil counts < 0.50 × 109/L (green).
Discussion
This is the largest prospective randomized trial on the addition of G-CSF to standard immunosuppression with ATG/CSA, and we show that G-CSF had no significant effect on OS, EFS, or on remission, mortality, and relapse rates. We could solely demonstrate in G-CSF–treated patients a reduced rate of early infection episodes and reduced days of hospitalization in VSAA patients. Despite there being no survival advantage by adding G-CSF to ATG/CSA treatment, some findings may be relevant for the early management of patients with SAA. Shorter hospitalization and fewer infections have clinical implications in the daily care in SAA, particularly in high-risk patients. Furthermore, the detection of an early factor that might predict response and detect patients who are likely to fail to immunosuppressive treatment is of major importance. This finding has to be interpreted with great caution because it is the result of a post hoc analysis and requires independent confirmation. We show in a subgroup analysis that the neutrophil response to G-CSF at 1 month predicted subsequent clinical outcome. Patients with neutrophil counts ≥ 0.5 × 109/L at day 30 had a significantly better response rate and survival than patients with lower values. The relevance of early neutrophil response to G-CSF as an outcome indicator has already been supported in a previous study, using a different cutoff for neutrophil count and time of evaluation.19 Patients who are refractory to conventional immunosuppression represent difficult management problems. For these patients, new approaches, including early HSCT with an alternative donor, are being evaluated.20,21 Therefore, early signs of response to treatment with G-CSF, as well as other predictive factors,4 could help to identify early nonresponders and justify exploring these novel treatment approaches.22
Our other results corroborate those of previous, smaller randomized studies on the use of hematopoietic growth factors with respect to OS. No study has ever shown a survival advantage attributable to the use of G-CSF or granulocyte-macrophage CSF. However, there are differences in other endpoints. In a Japanese study of 101 adults, there were significantly better response rates at 6 months but not at 3 months or at 1 year after immunosuppressive therapy in patients receiving G-CSF.13 This improved response rate at 6 months was restricted to patients with VSAA. We looked for response rates at 6 different time points but could not confirm such results. Teramura et al13 also showed differences in the probability of relapse. Patients treated with G-CSF had a significantly lower cumulative incidence of relapse. We and other groups were not able to show a similar effect of G-CSF on relapse rates.11 Only one other randomized trial showed a reduction in severe infectious episodes in patients treated with G-CSF.9 Ethnic differences as well as differences in study designs could explain some of the discrepancies between trials. In a pediatric Japanese trial, all patients with VSAA received G-CSF, and randomization applied only to patients with higher neutrophil counts.11 We found a reduction in the number of infectious episodes but only in patients with VSAA. In a meta-analysis on the use of growth factors in patients with aplastic anemia, including 6 randomized trials, the addition of hematopoietic growth factors did not affect mortality, response rate, or infectious complications.23
This prospective trial documents other new information. In contrast to what has been claimed recently,15,24 the severity of the disease is still the most important factor affecting overall survival. Excellent OS of children and young adults does not comprehensively reflect long-term outcome. Indeed, EFS in the younger cohort of the patients decreases over time as it does in the older age groups, because nonresponse, relapse, PNH, or clonal malignant transformation occur in patients at any age. Therefore, survival is no longer the major endpoint to be evaluated in SAA. This is of particular importance, in younger, nonresponding patients for whom unrelated HSCT should be considered early.
One of the strengths of this trial is that it included an unselected cohort of patients with SAA. Inclusion criteria were not restrictive and permitted recruitment of any patient with newly, diagnosed, untreated acquired SAA. Furthermore, the study was not restricted to specialized centers. In total, 54 European centers from 8 countries participated in this study, and 36 of the 54 participating centers included only 1 or 2 patients (supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Therefore, we considered the cohort of the patients to be representative of what is observed in daily practice.
Our study has some limitations. The randomized study, designed to detect a statistical difference for EFS of 15%, planned to enroll a total of 340 patients. Unfortunately, because of slow accrual for this rare disease and the withdrawal of horse ATG in Europe, the EBMT was forced to close the study early. Despite this, this is still the largest trial ever done comparing in patients with SAA treated with immunosuppression with and without G-CSF. Most of the previous studies included < 50 patients in each arm.10-14 In view of the data presented here, it is unlikely that a larger number of patients would have changed the conclusions. It is also unlikely that another trial of G-CSF with a larger number of patients will ever be performed. Furthermore, the definition of infectious episodes and the decision on the duration of hospitalization was not standardized and may be unequal, given the large number of participating centers. Some institutions may have hospital discharge protocols that require a given level of neutrophils. This could bias the number of hospitalized days to be in favor of the growth factor arm. To minimize a center effect randomization was stratified for centers. We furthermore performed an analysis to evaluate the center effect in patients who were randomly assigned with or without G-CSF on the number of infections and days of hospitalization. Therefore, we compared the results between large centers (≥ 4 patients included into the study) and small centers (< 4 patients included). There was no difference for each randomization arm (with or without G-CSF) between large and small centers. The difference between both arms (G-CSF, no G-CSF) remained similar for the number of infections and the number of hospitalization days for large centers. However, for the small centers, despite that a similar trend existed, the difference was no longer significant between patients randomly assigned to G-CSF and no G-CSF. Therefore, we cannot exclude a bias for the small centers with respect to number of infections and hospitalization days. Early retreatment with ATG in patients not responding at 3 months does not appear to be of benefit in terms of OS. However, these data have to be interpreted cautiously, given the low number of patients in each arm and the many protocol violations. Survival of patients eligible for early retreatment is good, (83% and 77%), but this only includes patients alive without response at one time point, day 120.
The issue about the risk of secondary MDS/AML related to the use of G-CSF in SAA is still unresolved. We did not demonstrate an excess risk of a clonal disorder with the use of G-CSF. Despite that the follow-up time is still too short for a definitive statement, most other studies did not show more clonal diseases with G-CSF. The Italian Aplastic Anemia Study Group also showed no excess risk of developing clonal disorders even when larger G-CSF doses were used over a 6-month period.25 Furthermore, neither the meta-analysis nor any of the randomized trials referred to previously showed an increased risk of a clonal disorder associated with the use of G-CSF.9-14,23 In contrast, in a large registry-based retrospective study of the EBMT, the use of G-CSF was associated with a higher hazard of MDS/AML in patients with AA treated with immunosuppression.26 An increased risk of MDS/AML has also been described in patients from Japan treated with immunosuppression and G-CSF, mainly in nonresponse patients.27 In conclusion, G-CSF added to standard immunosuppression with ATG and CSA reduces the rate of early infectious episodes and days of hospitalization in VSAA patients and might allow early identification of nonresponders, but it has no effect on OS, EFS, remission, relapse rates, and mortality.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all patients who accepted to enter the study and the treating centers for including patients into the study (see the supplemental Appendix).
This study was supported by an unrestricted grant from Chugai-Aventis, France, for data acquisition in the amount of 50 000 EU.
Authorship
Contribution: A.T. served as the principal investigator for this study and wrote the paper; J.R.P., G.S., H.S., A. Bacigalupo, and J.M. contributed to data analysis and writing the paper; H.S., G.S., J.M., A. Bacigalupo, U.D., A.F., M.H., E.T., M.W., B.H., and A.T. contributed to patient recruitment; A.T., H.S., G.S., J.M., and J.R.P. served as investigators in this study; A. Barrois, K.C., and B.H. contributed to the data collection; A.T., H.S., G.S., J.M., and J.R.P. contributed to the study design; and J.P. and A.T. contributed to the statistical analysis of the study.
Conflict-of-interest disclosure: J.M. was a consultant for Genzyme Therapeutics from 2008-2009, and received research funding from Genzyme in 2010. The remaining authors declare no competing financial interests.
Correspondence: André Tichelli, Hematology, University Hospitals, Petersgraben 4, 4031 Basel, Switzerland; e-mail: tichelli@datacomm.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal