Abstract
T-cell therapy with genetically modified T cells targeting CD19 or CD20 holds promise for the immunotherapy of hematologic malignancies. These targets, however, are only present on B cell–derived malignancies, and because they are broadly expressed in the hematopoietic system, their targeting may have unwanted consequences. To expand T-cell therapies to hematologic malignancies that are not B cell–derived, we determined whether T cells can be redirected to CD70, an antigen expressed by limited subsets of normal lymphocytes and dendritic cells, but aberrantly expressed by a broad range of hematologic malignancies and some solid tumors. To generate CD70-specific T cells, we constructed a chimeric antigen receptor (CAR) consisting of the CD70 receptor (CD27) fused to the CD3-ζ chain. Stimulation of T cells expressing CD70-specific CARs resulted in CD27 costimulation and recognition of CD70-positive tumor cell lines and primary tumor cells, as shown by IFN-γ and IL-2 secretion and by tumor cell killing. Adoptively transferred CD70-specific T cells induced sustained regression of established murine xenografts. Therefore, CD70-specific T cells may be a promising immunotherapeutic approach for CD70-positive malignancies.
Introduction
Immunotherapy with antigen-specific T cells has shown promise in the treatment of hematologic malignancies in preclinical models and in phase 1/2 clinical studies.1-3 One attractive strategy to generate tumor-specific T cells is by genetic modification with chimeric antigen receptors (CARs), which consist of an extracellular antigen-recognition domain, a transmembrane domain, and an intracellular signaling domain derived from the TCR CD3-ζ chain often linked to costimulatory molecule endodomains.4,5 CARs targeting CD19 and CD20 antigens for the treatment of hematologic malignancies have been explored extensively, but this approach is limited to B cell–derived malignancies and may produce prolonged impairment of humoral immunity because of the potentially long life span of T cells.6,7 It is therefore desirable to prepare CARs directed against alternative antigens that could broaden the spectrum of potentially treatable tumors and/or potentially reduce damage to normal cells.
CD70 is the membrane-bound ligand of the CD27 receptor, which belongs to the tumor necrosis factor receptor superfamily.8,9 CD70 is expressed by diffuse large B-cell and follicular lymphoma and also by the malignant cells of Hodgkin lymphoma, Waldenström macroglobulinemia, and multiple myeloma, and by human T-lymphotropic virus type 1– and EBV-associated malignancies.10-14 In addition, CD70 is expressed by nonhematologic malignancies such as renal cell carcinoma and glioblastoma.15,16 Physiologically, CD70 expression is transient and restricted to a subset of highly activated T, B, and dendritic cells. Whereas CD70/CD27 costimulation plays a role in T-cell activation, CD70/CD27 signaling is not essential for the development and maintenance of a functional immune system, because CD27-knockout mice have no overt immunodeficiency and recover from influenza virus infection within the same time frame as wild-type mice.17,18
Targeting CD70-positive malignancies with CD70-specific monoclonal antibodies has shown promise in preclinical animal models,14,19,20 and we have now evaluated whether T cells can be redirected to CD70 by forced expression of the appropriate CAR. Because CARs consist of an extracellular antigen-recognition domain derived from murine monoclonal antibodies, they may induce human anti–mouse antibody on infusion unless fully humanized.21,22 One potential strategy to overcome this limitation is to engineer the antigen-recognition domain using endogenous protein ligands or receptors rather than monoclonal antibodies.23,24 To target CD70 with T cells, we took advantage of the physiologic CD70/CD27 interaction and generated a CD70-specific CAR, which consists of full-length CD27 as the antigen-recognition domain fused to the intracellular domain of the CD3-ζ chain. Engagement of chimeric CD27-ζ by tumor targets expressing the CD70 ligand resulted in T-cell activation and CD27 costimulation, which was dependent on the presence of the TRAF2-binding site within the cytoplasmic tail of CD27. CD70-specific T cells killed CD70-positive tumor cell lines and primary tumors and had antitumor activity in a murine SCID xenograft model.
Methods
Cell lines and tumor cells
Protocols to obtain blood samples or primary tumor cells were approved by the institutional review board of Baylor College of Medicine. The cell lines 293T, Daudi, Raji, CCL-120, U266, and K562 were obtained from ATCC. K562 cells expressing CD70 (K562.70) were generated by transducing K562 cells with a self-inactivating lentiviral vector encoding human CD70 and green fluorescent protein (GFP). The cell line L1236 was obtained from DSMZ. SNK6 and SNT16 were kindly provided by Dr Norio Shimizu (Tokyo Medical and Dental University, Tokyo, Japan).25 Primary B-cell non-Hodgkin lymphoma samples, which had been cryopreserved without in vitro culture, were provided by Dr Stephen Ansell (Mayo Clinic, Rochester, MN).
Generation of the CD70-specific CAR construct
Full-length human CD27 (CD70 receptor) was fused in-frame to the signaling domain (amino acids 52-164) of TCR-ζ using overlap PCR; pORF.CD27 (Invitrogen) and pSFG.FRP5ζ26 served as PCR templates. Primers were modified to create 5′-NcoI and 3′-SphI restriction sites, and the CD27 TCR-ζ fusion gene (CD70-CAR) was subcloned into the SFG retroviral vector. To facilitate unequivocal detection of transduced T cells, an internal ribosomal entry sequence (IRES)–truncated CD19 (tCD19)27 expression cassette (IRES-tCD19) was created by overlap PCR and subcloned 3′ of the CD27 TCR-ζ fusion gene into 5′-SphI and 3′-AccIII restriction sites of the SFG retroviral vector (pSFG.CD70-CAR-IRES-tCD19; Figure 1A). In addition, a retroviral vector was created containing a CD70-CAR-IRES-DsRed expression cassette or ΔCD70-CAR-IRES-DsRed expression cassette in which the 23–amino acid TRAF2-binding site of CD27 was deleted (residues 238-26028 ; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Retrovirus production and transduction of T lymphocytes
RD114 pseudotyped retroviral particles were generated by transient transfection of 293T cells with the CD70-CAR SFG retroviral vector, Peg-Pam-e plasmid containing the sequence for Moloney Murine Leukemia Virus gag-pol, and the RDF plasmid containing the RD114 envelope,29 using GeneJuice transfection reagent (Novagen).26 Supernatant containing the retrovirus was collected 48-72 hours later. For retroviral transduction, non-tissue culture–treated 24-well plates were treated overnight with OKT3 (Ortho Biotech) and CD28 (Becton Dickinson) antibodies. The following day, 0.5 × 106 PBMCs were added to each well and cultured in RPMI 1640 complete medium (GIBCO-BRL) containing 10% heat-inactivated fetal calf serum and 1% GlutaMax (GIBCO-BRL). Recombinant human IL-2 (rhIL-2 [Proleukin]; Prometheus) was added to cultures on day 3. Viral supernatant was added to 24-well plates that had been pretreated with RetroNectin (Takara Shuzo), and the cultured OKT3/CD28–stimulated cells were added to each well (5 × 105 cells/well). Cells were spun and incubated at 37°C in 5% CO2. CAR expression on T cells was measured 72 hours later, and the cells were maintained in culture in complete medium with the addition of rhIL-2 (50-100 U/mL) every 3 days. Nontransduced T cells, used as controls, were activated with OKT3/CD28 and expanded in the presence of 50-100 U/mL of IL-2 for 10-15 days before use.
Flow cytometry
A FACSCalibur flow cytometer (BD Biosciences) was used to acquire immunofluorescence data, which were analyzed with FCS Express software Version 3 (De Novo). All antibodies for surface staining were purchased from BD Biosciences. Isotype controls were IgG1-FITC, IgG1-PE, IgG1–peridinin chlorophyll protein (IgG1-PerCP), and IgG1-allophycocyanin (IgG1-APC). Forward- and side-scatter gating were used to discriminate live cells from dead cells. CD70-CAR expression was analyzed on 293T cells using CD27-FITC and CD19-PE, and on human CD3/CD28–stimulated T cells using CD19-PE, CD3-FITC, CD4-PerCP, and CD8-APC. CD70 expression on tumor cells was determined using CD70-PE. For intracellular staining, cells were fixed with 4% paraformaldehyde (BD Biosciences) and permeabilized with 1% saponin (Sigma-Aldrich). A mouse monoclonal antibody to Bcl-xl (Santa Cruz Biotechnology) was used for primary staining, and goat anti–mouse allophycocyanin (BD Biosciences) was used for secondary staining. Isotype controls were cells incubated with goat anti–mouse allophycocyanin alone.
Analysis of cytokine production
CD70-specific or nontransduced T cells from healthy donors were cocultured with CD70-positive cell lines or primary CD70-positive lymphomas at a 2:1 effector-to-target ratio in a 48-well plate. After 24 hours of incubation, culture supernatants were harvested and IFN-γ and IL-2 were measured by ELISA as per the manufacturer's instructions (R&D Systems).
IFN-γ ELISPOT assay
We used ELISPOT assays, as described previously,30 to determine the frequency of IFN-γ–secreting T cells. CD70-CAR or nontransduced T cells were plated at 1 × 105 and incubated for 18 hours with the appropriate stimulus. Plates were then developed, dried overnight, and sent to ZellNet Consulting (New York, NY) for quantification.
Coimmunoprecipitation
293T cells stably expressing CD70-CAR or ΔCD70-CAR were generated by retroviral transduction. Cells expressing CARs were transfected with 2 μg of FLAG-tagged TRAF2, kindly provided by Dr Jinhua Yang (Baylor College of Medicine) using GeneJuice transfection reagent (Novagen). Twenty-four hours after transfection, the cells were cocultured with K562.70 cells at a ratio of 1:1 to cross-link the receptor. After 12 hours, cells were washed with ice-cold PBS and the nonadherent K562.70 cells were aspirated from the culture. The remaining 293T cells were lysed and proteins were precipitated with anti-FLAG M2 antibody (Sigma-Aldrich) using μMACS Protein G MicroBeads and a μColumn (Miltenyi Biotec). The immunoprecipitate was separated by SDS-PAGE and blotted with a CD3-ζ antibody (Santa Cruz Biotechnology).
Chromium-release assay
Standard chromium-release assays were performed in triplicate, as described previously.30 Briefly, 1 × 106 target cells were labeled with 0.1 mCi (3.7 MBq) 51Cr and mixed with decreasing numbers of effector cells to give effector-to-target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 4 hours, supernatants were collected and radioactivity was measured in a gamma counter (Cobra Quantum; PerkinElmer). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: [test release − spontaneous release] / [maximal release − spontaneous release] × 100.
CFSE proliferation and long-term killing assay
To measure T-cell proliferation and long-term killing, we incubated 1 × 107 T cells for 10 minutes at room temperature with 1.5μM CFSE (Molecular Probes). We cultured CFSE-labeled T cells in the absence of exogenous IL-2 with the appropriate CD70-postive or CD70-negative tumor cells at a 2:1 effector-to-target ratio. After 5-7 days of coculture, cells were collected, stained with CD3, and analyzed for CFSE dilution by FACS analysis. Positive and negative controls for proliferation experiments were T cells cultured in the presence of 100 U/mL of rhIL-2 and T cells alone with no cytokine, respectively. For long-term killing experiments, FACS analysis was performed using forward- and side-scatter gating to determine viable cells, whereas CFSE staining and CD3 positivity were used to distinguish CD70-specific or nontransduced T cells from CD3-negative, unlabeled tumor cells.
Xenograft models and bioluminescence imaging
All animal experiments were conducted under a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. To assess the antitumor effect of CD70-specific T cells in vivo, we used 2 SCID mouse models and an IVIS (Caliper Life Sciences) in vivo imaging system.26 Eight- to 10-week-old SCID mice (IcrTac:ICR-Prkdcscid; Taconic) were sublethally irradiated (2.5 Gy) and 2 days later, 5 × 105 Daudi cells expressing an enhanced GFP (eGFP)–firefly luciferase (eGFP-FFLuc) fusion gene, suspended in Matrigel (BD Biosciences) were injected IP. To monitor tumor growth, isoflurane-anesthetized animals were injected IP with D-luciferin (150 mg/kg), and a bioluminescence image was obtained and analyzed after 10 minutes using Living Image software Version 4.0 (Caliper Life Sciences). A constant region of interest was drawn over the tumor region and the intensity of the signal measured as total photons per second per square centimeter per steradian (p/s/cm2/sr) was obtained. After 10 days, when the tumor signal was consistently increasing, mice were treated with CD70-specific or nontransduced T cells. Three IP injections of 1 × 107 T cells were given on days 10, 11, and 17, followed by 1500 U of rhIL-2 (R&D Systems) also given IP. Mice were imaged before each T-cell injection and 3 times weekly thereafter. We used a Raji SCID xenograft to evaluate the antitumor activity of CD70-specific T cells in a systemic non-Hodgkin lymphoma model.31-33 Briefly, 2 × 105 Raji.FFluc cells were injected IV into sublethally irradiated (2.5 Gy) SCID mice, which were treated 4 days later by IV administration of 1 × 107 CD70-specific or nontransduced T cells. We gave 3 doses of T cells (day 4, 5, and 11) with 1500 U of rhIL-2. We quantified metastatic tumors using bioluminescence imaging. For survival analysis, mice were euthanized at the first sign of hind-limb paralysis, identified as one or both limbs dragging while walking.
Statistical analysis
Comparisons of IFN-γ and IL-2 secretion between CD70-specific and nontransduced T cells were performed using the Wilcoxon signed-rank test. Tumor volume data were log-transformed, and changes from initial T-cell injection to posttreatment measurements were calculated. Pairwise comparisons were used to identify any statistically significant difference in light intensity between the 2 T-cell groups. P < .05 was considered statistically significant. The survival curves were constructed using the Kaplan-Meier method and compared using the weighted log-rank test.
Results
Generation of CD70-specific T cells
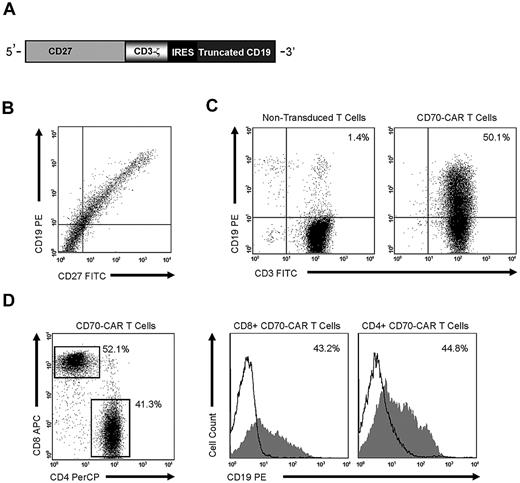
We constructed an SFG retroviral vector that encoded the CD70 receptor CD27 fused to the signaling domain of the TCR-ζ chain (CD70-CAR). Because most naive and memory T cells endogenously express low levels of CD27, an IRES-tCD19 expression cassette was also included in the retroviral vector to allow for unequivocal detection of transduced cells (Figure 1A). CD27 and tCD19 displayed a linear coexpression pattern, indicating that tCD19 is a suitable marker for CD70-CAR expression (Figure 1B). CD3/CD28–activated T cells were transduced with RD114-pseudotyped retroviral particles encoding CD70-CAR-IRES-tCD19, and the expression of tCD19 was determined by FACS analysis 10-14 days after transduction. A mean of 45% (± 6; n = 5) of T cells expressed tCD19, and both CD4- and CD8-positive cells were transduced (Figure 1C-D).
CD70-CAR generation, cell-surface expression, and transduction of human T cells. (A) CD70-CAR was generated by fusing full-length CD27 to the signaling domain of the CD3-ζ chain, an IRES sequence, and tCD19 was included for the detection of genetically modified T cells. (B) 293T cells transfected with CD70-CAR constructs express both CD27 and the marker gene tCD19. (C) CD70-CAR expression on transduced human T cells was 45% ± 6%, as determined by staining tCD19. (D) Both CD4 and CD8 T cells were genetically modified.
CD70-CAR generation, cell-surface expression, and transduction of human T cells. (A) CD70-CAR was generated by fusing full-length CD27 to the signaling domain of the CD3-ζ chain, an IRES sequence, and tCD19 was included for the detection of genetically modified T cells. (B) 293T cells transfected with CD70-CAR constructs express both CD27 and the marker gene tCD19. (C) CD70-CAR expression on transduced human T cells was 45% ± 6%, as determined by staining tCD19. (D) Both CD4 and CD8 T cells were genetically modified.
CD70-specific T cells secrete immunostimulatory cytokines and proliferate after exposure to CD70-positive tumor cells
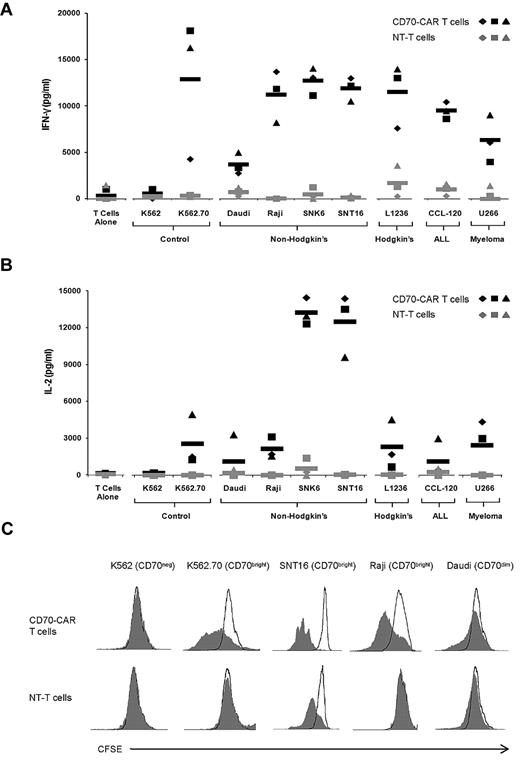
To detect recognition of CD70 by transgenic T cells, we initially used CD70-negative K562 cells and CD70-transgenic K562 cells (Figure 2). CD70-specific T cells and nontransduced T cells of 3 donors were stimulated with K562 or K562.CD70, and after 48 hours IFN-γ and IL-2 release were measured (Figures 3A-B). CD70-specific T cells produced significant amounts of IFN-γ (P = .03) and IL-2 (P = .02) after exposure to K562.CD70 compared with nontransduced T cells. In addition, CD70-negative K562 cells did not activate CD70-specific T cells, indicating that cytokine production requires both the expression of CD70 on target cells and the presence of the CD70-CAR on T cells. We observed a similar outcome when we compared T-cell proliferation in each of these culture combinations (Figure 3C).
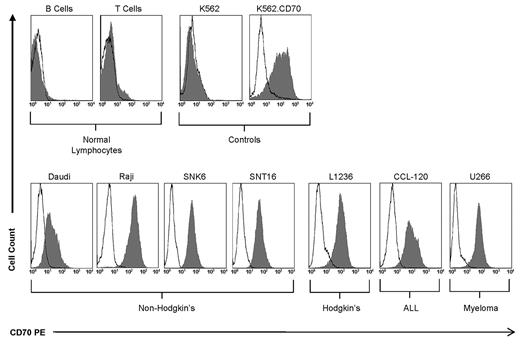
CD70 is overexpressed on several tumor cell lines but not on normal lymphocytes. Less than 5% of B and T lymphocytes from the peripheral blood of healthy donors express CD70. K562 and K562.70 served as negative and positive controls, respectively. CD70 overexpression was observed on non-Hodgkin (Daudi, Raji, SNK6, and SNT16), Hodgkin (L1236), ALL (CCL-120), and multiple myeloma (U266) cells.
CD70 is overexpressed on several tumor cell lines but not on normal lymphocytes. Less than 5% of B and T lymphocytes from the peripheral blood of healthy donors express CD70. K562 and K562.70 served as negative and positive controls, respectively. CD70 overexpression was observed on non-Hodgkin (Daudi, Raji, SNK6, and SNT16), Hodgkin (L1236), ALL (CCL-120), and multiple myeloma (U266) cells.
CD70-specific T cells release IFN-γ and IL-2 and proliferate in response to CD70-positive target cells. (A) T cells from 3 donors were transduced with CD70-CAR (black) or nontransduced (gray) and cocultured with K562.70 and K562 and various CD70-expressing tumor cell lines for 48 hours before performing IFN-γ ELISA. Black and gray rectangles represent mean IFN-γ release of CD70-CAR transduced and nontransduced T cells, respectively. CD70-CAR T cells were specific for CD70, because significantly more (P < .03) IFN-γ was released in the presence of K562.70 compared with K562 cells. CD70-CAR T cells also released significantly more (P < .0001) IFN-γ than nontransduced T cells when cocultured with CD70-expressing tumor cell lines. (B) Same coculture experiments but assayed for the presence of IL-2. CD70-CAR T cells released significantly more (P < .0001) IL-2 than nontransduced T cells in the presence of CD70-expressing tumors. (C) T cells were labeled with CFSE and cocultured for 5 days with K562, K562.70, SNT16, Raji, or Daudi in the absence of exogenous IL-2, and CFSE dilution was analyzed by flow cytometry. CD70-CAR T cells proliferated when cocultured with the CD70-overexpressing targets K562.70, SNT16, and Raji, but not CD70-dim Daudi cells or CD70-negative K562 cells.
CD70-specific T cells release IFN-γ and IL-2 and proliferate in response to CD70-positive target cells. (A) T cells from 3 donors were transduced with CD70-CAR (black) or nontransduced (gray) and cocultured with K562.70 and K562 and various CD70-expressing tumor cell lines for 48 hours before performing IFN-γ ELISA. Black and gray rectangles represent mean IFN-γ release of CD70-CAR transduced and nontransduced T cells, respectively. CD70-CAR T cells were specific for CD70, because significantly more (P < .03) IFN-γ was released in the presence of K562.70 compared with K562 cells. CD70-CAR T cells also released significantly more (P < .0001) IFN-γ than nontransduced T cells when cocultured with CD70-expressing tumor cell lines. (B) Same coculture experiments but assayed for the presence of IL-2. CD70-CAR T cells released significantly more (P < .0001) IL-2 than nontransduced T cells in the presence of CD70-expressing tumors. (C) T cells were labeled with CFSE and cocultured for 5 days with K562, K562.70, SNT16, Raji, or Daudi in the absence of exogenous IL-2, and CFSE dilution was analyzed by flow cytometry. CD70-CAR T cells proliferated when cocultured with the CD70-overexpressing targets K562.70, SNT16, and Raji, but not CD70-dim Daudi cells or CD70-negative K562 cells.
We confirmed the above findings using tumor cells in which CD70 expression was naturally present but at variable levels. We used a panel of CD70-positive tumor cell lines representing non-Hodgkin lymphoma (Daudi, SNK6, and SNT16), Hodgkin lymphoma (L1236), leukemia (CCL-120), and multiple myeloma (U266; Figure 2). CD70-specific T cells secreted significantly more IFN-γ (P < .0001) and IL-2 (P < .0001) than nontransduced T cells (Figure 3A-B). T-cell proliferation was dependent on the expression of CD70 on target cells, and CD70dim tumor cells (Daudi) induced less T-cell proliferation than CD70bright tumor cells. In addition, we observed proliferation of nontransduced T cells after stimulation with SNT16 cells, which we attributed to low levels of IL-2 secretion by the SNT16 cells (10-50 pg/mL) and to their robust ability to costimulate, as judged by their ability to induce IL-2 production of CD70-specific T cells (Figure 1B). The expression of CD70 was low to absent on peripheral blood B and T cells from healthy donors (Figure 2). Accordingly, we could not detect IFN-γ or IL-2 production of CD70-specific T cells after coculture with primary B or T cells (data not shown). To confirm that CD70-specific T cells are not stimulated by B or T cells, we used an IFN-γ ELISPOT assay, which showed no activation of CD70-specific T cells after coculture with primary B or T cells (supplemental Figure 1A).
CD70-specific T cells kill CD70-positive tumor cells but not CD70-negative cells
We next measured the killing of CD70-positive targets by CD70-specific T cells in both a standard 4-hour 51Cr-release assay and a 5- to 7-day coculture assay. In the 4-hour 51Cr-release assay, CD70-specific T cells killed CD70-positive target cells (K562.70, Daudi, U266, SNK6, and SNK16) but not CD70-negative cells (K562). Nontransduced T cells showed no killing, thus confirming CD70 specificity (Figures 4A-B). For the coculture assays, CD70-specific or nontransduced T cells were labeled with CFSE and added to unlabeled tumor cells at a ratio of 2:1. After 5-7 days, tumor cells were enumerated by FACS analysis of the CD3-/CFSE-negative fraction; (Figure 4C). CD70-specific T cells eliminated all 4 CD70-positive lines tested (Daudi, U266, SNK6, and SNK16), whereas control T cells did not (Figure 4D). Whereas T cells stimulated with CD3/CD28 were not killed by CD70-specific T cells, B-cell blasts activated “super-physiologically” with the CD40 ligand on MRC5 cells were susceptible to CD70-specific T-cell killing (supplemental Figure 1B).
CD70-specific T cells kill CD70-positive tumor cell lines. (A) CD70-CAR T cells (solid lines) killed K562.70 cells but not parental K562 cells. Nontransduced control T cells (dashed lines) did not kill either target. (B) CD70-CAR T cells (solid lines) killed CD70-positive Daudi, U266, SNK6, and SNT16 tumor cell lines; control T cells (dashed lines) did not. (C) CD70-specific T cells or nontransduced T cells were labeled with CFSE and cocultured with SNT16 cells at a ratio of 2:1. CD70-specific T cells proliferated and killed SNT16 cells, as shown by CFSE dilution of CD3+ cells and by the lack of CD3/CFSE-negative cells in the culture compared with nontransduced T cells. (D) In all coculture experiments, only CD70-specific T cells eliminated the CD3/CFSE–negative CD70+ tumor cells Daudi, U266, SNK6, and SNT16.
CD70-specific T cells kill CD70-positive tumor cell lines. (A) CD70-CAR T cells (solid lines) killed K562.70 cells but not parental K562 cells. Nontransduced control T cells (dashed lines) did not kill either target. (B) CD70-CAR T cells (solid lines) killed CD70-positive Daudi, U266, SNK6, and SNT16 tumor cell lines; control T cells (dashed lines) did not. (C) CD70-specific T cells or nontransduced T cells were labeled with CFSE and cocultured with SNT16 cells at a ratio of 2:1. CD70-specific T cells proliferated and killed SNT16 cells, as shown by CFSE dilution of CD3+ cells and by the lack of CD3/CFSE-negative cells in the culture compared with nontransduced T cells. (D) In all coculture experiments, only CD70-specific T cells eliminated the CD3/CFSE–negative CD70+ tumor cells Daudi, U266, SNK6, and SNT16.
CD27 costimulation is important for T-cell survival after CD70-specific stimulation
To determine the role of the 23–amino acid costimulatory domain of CD27 located within the endodomain of the CD70-CAR (Figure 1A), we generated a CD70-CAR with a deleted CD27-costimulatory domain (ΔCD70-CAR). Functional absence of the costimulatory domain was confirmed by the inability of ΔCD70-CAR to bind to TRAF2, the key adaptor protein mediating CD27 signaling (Figure 5A). T cells were transduced with retroviral vectors encoding CD70-CAR-I-dsRed or ΔCD70-CAR-I-dsRed (supplemental Figure 2A). Transduction efficiencies of both constructs were similar, as judged by dsRed expression (65%-90%; supplemental Figure 2B), and in cytotoxicity assays CD70-CAR– and ΔCD70-CAR–expressing T cells killed CD70-positive targets with the same efficiency (Figure 5B). To assess the contribution of CD27 costimulation to T-cell activation, we took advantage of autologous fibroblasts, which are devoid of costimulatory molecules and genetically modified to express CD70 (Fib.CD70). Starting 3 days after T-cell stimulation with Fib.CD70, we observed significantly larger “clumps” of activated CD70-CAR T cells compared with ΔCD70-CAR T cells (Figure 5C). Whereas there was no difference in T-cell proliferation (Figure 5D) or in the production of IFN-γ or IL-2 (data not shown), ΔCD70-CAR T-cell viability was significantly reduced compared with CD70-CAR T cells (Figure 5D; P < .05). As reported previously, Bcl-xl, an important antiapoptotic protein, is induced by CD27 signaling.34 In agreement with this finding, CD70-CAR T cells consistently expressed higher levels of Bcl-xl compared with ΔCD70-CAR T cells (Figure 5E). These results indicate that the CD27-costimulatory domain located within CD70-CAR provides a costimulatory signal, resulting in enhanced T-cell survival. Therefore, for all subsequent experiments, we used CD70-CAR (CD70-specific) T cells.
CD27 costimulation enhances T-cell viability. (A) In coimmunoprecipitation experiments, only full-length CD27-ζ associated with TRAF2. (B) T cells expressing CD70-CAR or ΔCD70-CAR showed equivalent killing of CD70+ LCL and U266 cells, but did not kill CD70− K562 cells in 51Cr-release assays. (C) Microscopic evaluation (10×) of T cells expressing CD70-CAR or ΔCD70-CAR activated with autologous fibroblasts genetically modified to express CD70 revealed larger “T-cell clumps” of T cells expressing CD70-CAR; however, CFSE dilution analysis showed no significant differences in proliferation between groups. (D) The viability of ΔCD70-CAR T cells was 35% ± 16% that of T cells expressing CD70-CAR (n = 5). (E) Intracellular staining for Bcl-xl was performed on T cells 3 days after stimulation with CD70-transgenic autologous fibroblasts. Bcl-xl expression was consistently increased in CD70-CAR T cells compared with ΔCD70-CAR T cells (n = 3). One representative FACS analysis is shown.
CD27 costimulation enhances T-cell viability. (A) In coimmunoprecipitation experiments, only full-length CD27-ζ associated with TRAF2. (B) T cells expressing CD70-CAR or ΔCD70-CAR showed equivalent killing of CD70+ LCL and U266 cells, but did not kill CD70− K562 cells in 51Cr-release assays. (C) Microscopic evaluation (10×) of T cells expressing CD70-CAR or ΔCD70-CAR activated with autologous fibroblasts genetically modified to express CD70 revealed larger “T-cell clumps” of T cells expressing CD70-CAR; however, CFSE dilution analysis showed no significant differences in proliferation between groups. (D) The viability of ΔCD70-CAR T cells was 35% ± 16% that of T cells expressing CD70-CAR (n = 5). (E) Intracellular staining for Bcl-xl was performed on T cells 3 days after stimulation with CD70-transgenic autologous fibroblasts. Bcl-xl expression was consistently increased in CD70-CAR T cells compared with ΔCD70-CAR T cells (n = 3). One representative FACS analysis is shown.
CD70-specific T cells recognize and kill primary B- and T-cell lymphomas
Having shown that CD70-specific T cells recognize and kill CD70-positive lymphoma cell lines, we next validated the CD70 antigen as a target on primary B- and T-cell lymphomas. We cocultured primary CD70-positive B-cell non-Hodgkin lymphoma (MF1792, MF1731, and MF888) and T-cell acute lymphoblastic leukemia (T007) cells with CD70-specific T cells from a healthy donor for 24 hours, and measured IFN-γ in the supernatants. CD70-specific T cells but not control T cells produced IFN-γ secretion on exposure to CD70+ malignancies (Figure 6A). In 5-day coculture assays, CD70-specific T cells but not control T cells eliminated primary CD70-positive cells (Figure 6B-C). Therefore, CD70-specific T cells recognize and kill primary CD70-positive malignant cells in a CD70-specific manner.
CD70-specific T cells recognize and kill primary CD70-positive lymphomas. (A) CD70-overexpressing tumor cells from 3 patients with B-cell lymphoma and 1 patient with T-cell acute lymphoblastic leukemia were cocultured with CD70-specific or nontransduced T cells from healthy donors for 48 hours before performing IFN-γ ELISA. In all cases, CD70-specific T cells released IFN-γ in the presence of patient tumor cells, whereas nontransduced cells released little or no IFN-γ. (B-C) Coculture assays were performed with primary tumor cells and CFSE-labeled T cells to distinguish effector and target cells by FACS analysis. Only CD70-specific (CD3/CFSE–positive) T cells were able to eradicate patient tumor cells (P = .036).
CD70-specific T cells recognize and kill primary CD70-positive lymphomas. (A) CD70-overexpressing tumor cells from 3 patients with B-cell lymphoma and 1 patient with T-cell acute lymphoblastic leukemia were cocultured with CD70-specific or nontransduced T cells from healthy donors for 48 hours before performing IFN-γ ELISA. In all cases, CD70-specific T cells released IFN-γ in the presence of patient tumor cells, whereas nontransduced cells released little or no IFN-γ. (B-C) Coculture assays were performed with primary tumor cells and CFSE-labeled T cells to distinguish effector and target cells by FACS analysis. Only CD70-specific (CD3/CFSE–positive) T cells were able to eradicate patient tumor cells (P = .036).
In vivo regression of established lymphoma after administration of CD70-specific T cells
We measured the antitumor activity of CD70-specific T cells in a xenogenic SCID mouse model. We injected 5 × 105 Daudi.FFluc cells IP into sublethally irradiated SCID mice and followed tumor growth by serial bioluminescence imaging. After 10 days, mice received 3 injections of 1 × 107 CD70-specific T cells given 1 day and then 1 week apart (injection days 0, 1, and 7; n = 10). A second group of tumor-bearing mice was injected with nontransduced T cells, and in these mice the tumors grew exponentially, as judged by bioluminescence imaging (Figure 7A). In contrast, there was a significant difference in tumor burden between CD70-specific and nontransduced T-cell groups at day 7 after T-cell injection (P = .002) (Figure 7B). In 8 of 9 mice with growing tumors, photon emission returned to baseline after CD70-specific T-cell injection, indicating tumor regression that was sustained in 7 mice for > 2 weeks after T-cell transfer.
CD70-specific T cells exhibit in vivo antitumor activity in an IP and systemic xenograft model of lymphoma. (A-B) Daudi cells (5 × 105) expressing the eGFP-FFLuc gene were injected IP into SCID mice, and tumor growth was measured as increasing light signal (p/s/cm2/sr). On days 10, 11, and 17, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. Tumors treated with CD70-specific T cells regressed, whereas tumors treated with nontransduced T cells did not (P = .002) 7 days after treatment. Panel A shows images of representative animals. Panel B shows quantitative bioluminescence imaging. In panels C and D, Raji cells (2 × 105) were injected intravenously into SCID mice. On days 4, 5, and 11, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. (C) Systemic tumors were enumerated using bioluminescence imaging. At weeks 3 and 4 after tumor cell injection, there was a significantly higher tumor burden in mice receiving nontransduced T cells than CD70-specific T cells (week 3, P = .012; week 4 [n = 12], P = .010). (D) Mice treated with CD70-specific T cells displayed a significant survival advantage over those receiving nontransduced T cells (P < .05).
CD70-specific T cells exhibit in vivo antitumor activity in an IP and systemic xenograft model of lymphoma. (A-B) Daudi cells (5 × 105) expressing the eGFP-FFLuc gene were injected IP into SCID mice, and tumor growth was measured as increasing light signal (p/s/cm2/sr). On days 10, 11, and 17, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. Tumors treated with CD70-specific T cells regressed, whereas tumors treated with nontransduced T cells did not (P = .002) 7 days after treatment. Panel A shows images of representative animals. Panel B shows quantitative bioluminescence imaging. In panels C and D, Raji cells (2 × 105) were injected intravenously into SCID mice. On days 4, 5, and 11, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. (C) Systemic tumors were enumerated using bioluminescence imaging. At weeks 3 and 4 after tumor cell injection, there was a significantly higher tumor burden in mice receiving nontransduced T cells than CD70-specific T cells (week 3, P = .012; week 4 [n = 12], P = .010). (D) Mice treated with CD70-specific T cells displayed a significant survival advantage over those receiving nontransduced T cells (P < .05).
In a second in vivo study, we measured the antitumor activity of CD70-specific T cells using a systemic lymphoma model. We injected 2 × 105 Raji.FFluc cells IV into sublethally irradiated SCID mice. After 4 days, we gave the mice 3 IV injections of 1 × 107 CD70-specific or nontransduced T cells using the same treatment schema described in the previous paragraph. Systemic tumors were enumerated using bioluminescence imaging. At weeks 3 and 4 after tumor cell injection, there was a significantly higher (P = .012 and P = .10, respectively) tumor burden in mice receiving nontransduced T cells than CD70-specific T cells (Figure 7C). This translated into a significant increase (P < .05) in overall survival in mice treated with CD70-specific T cells (Figure 7D).
Discussion
We show that CD70, which is aberrantly expressed on several hematologic malignancies and carcinomas, can be targeted by T cells engineered to express CD27 as part of a CAR. T cells expressing a CD70-specific CAR recognized and killed CD70-positive tumor cell lines and primary tumor samples in vitro and eliminated human CD70 tumors in a mouse xenograft.
Although present on many leukemias and lymphomas, CD70 is not a lineage-specific marker, and physiologically it is only expressed transiently in subsets of highly activated T, B, and dendritic cells. The CD70 promoter contains transcription factor–binding sites for AP-1, AP-2, Sp1, and NF-κB, and is sensitive to methylation; however, the precise signaling pathways that regulate CD70 expression are poorly understood.35 CD70 is up-regulated in human T-lymphotropic virus type 1– and EBV-associated malignancies and Hodgkin lymphomas, likely in association with constitutive NF-κB activation, a pathway that might contribute to regulating CD70 expression.18,36 The role of aberrant CD70 expression on malignant cells is less well understood than its physiologic contributions, but it may contribute to immune evasion by non-Hodgkin lymphoma.37 Others have shown that the CD70/CD27 costimulatory pathway is critical for inducing leukemia-specific T-cell responses.38
The exodomains of most CARs consist of modified monoclonal antibody–binding sites that can be used to prepare antigen-specific T cells that recognize and kill tumor cells in a MHC-nonrestricted fashion. Unless these monoclonal antibody fragments are humanized, they may induce human anti–mouse antibody and/or endogenous T-cell responses that abbreviate the effector function of the infused cells.21,22,39 Thus, taking advantage of physiologically occurring receptor-ligand interactions23,24 bypasses this obstacle and should ensure that in vivo effector function in human subjects is not interrupted by an unwanted immune response to the transgene. We therefore constructed a CD70-specific CAR by fusing the CD3-ζ chain to the naturally occurring CD70 receptor CD27.
Stimulation of CD70-specific T cells with CD70-positive tumor cells resulted in the secretion of both IFN-γ and IL-2. Whereas triggering of CARs containing only a ζ-signaling domain results in IFN-γ production, IL-2 is generally only secreted in an antigen-dependent manner.26 Coculture of CD70-specific T cells with CD70-positive tumor cells resulted in the production of 4000-14 000 pg/mL of IFN-γ by CD70-specific T cells,40 which is within the range reported for other CAR-expressing T cells. Because CAR T-cell activation is dependent on the antigen density on target cells,41 as well as on the presence of costimulatory molecules,42 it is not surprising that IFN-γ production varied between individual CD70-positive tumor cell lines. Daudi cells, which induced the lowest level of IFN-γ secretion, had the lowest expression of CD70 as judged by FACS analysis. In addition to IFN-γ production, we observed significant—though variable—secretion of IL-2 after exposure to tumor cells. These differences were independent of tumor CD70 expression levels and did not appear to be dependent on the expression of conventional costimulation molecules, because we observed IL-2 secretion after T-cell stimulation with K562.70 cells, which do not express classic costimulatory molecules such as CD80 and CD86. These cells do, however, express NKG2D ligands, which can provide costimulatory signals by interacting with NKG2D expressed on human CD8-positive T cells.43 Moreover, SNT16 and SNK6 non-Hodgkin lymphoma cells induced high levels of IL-2 production from CD70-specific T cells, an effect consistent with the known high expression of adhesion molecules on EBV-positive, NK/T-cell non-Hodgkin lymphoma cells.44
CD27 costimulation prevents activation-induced cell death in T cells, in part by up-regulation of Bcl-xl, an antiapoptotic protein.34 In agreement with this finding, we observed that T cells expressing ΔCD70-CARs with a deleted CD27 costimulatory domain had decreased viability and lower levels of Bcl-xl expression than T cells expressing CD70-CARs with full-length CD27. These data suggest CD70-CAR T cells may also exhibit prolonged persistence in vivo. Interestingly, in vivo efficacy data of ex vivo–expanded tumor-infiltrating lymphocytes suggest that the expression of CD27 is correlated with antitumor activity.45 Studies are in progress to determine whether CD27 costimulation enhances the persistence of CAR-expressing T cells.
Whereas we observed complete killing of CD70-positive tumor cells in a 5- to 7-day coculture assay (Figure 4C-D), we observed more variable levels of tumor cell killing in a standard 4-hour 51Cr-release assay (Figure 4B). These differences were most likely T-cell independent, because the kinetics of tumor cell disintegration (chromium release) depends on their intrinsic sensitivity to T cell–derived cytotoxic molecules such as perforin or granzyme B rather than to differences in the effector function of the T cell itself.46
In our study, CD70-specific T cells expressing CD27-ζ CARs displayed significant in vivo antitumor activity in both an IP Daudi and IV Raji model of lymphoma. The observed antitumor activity of CD70-specific T cells in the IP Daudi model was similar to T cells expressing CD19-CARs, as reported previously.33,47,48 Interestingly, sustained antitumor responses, as observed with CD70-specific T cells, were only observed with CD19-specific T cells expressing CARs that contained costimulatory domains. This indicates that CD27-ζ CARs might also provide costimulatory signals in vivo, as we have shown in our in vitro experiments (Figure 5). The requirement for costimulatory domains for CD19-CARs to kill tumor cells in the IV Raji model is controversial and contradictory.31-33 These conflicting results might be explained by differences in the ex vivo preparation of genetically modified T cells, the strain of immunodeficient mice, and/or the particular Raji cell line derivative used for the in vivo experiments.
Because CD70 is physiologically expressed by a subset of immune cells during activation, the targeting of this receptor with CAR T cells might potentially impair cellular immune responses. However, we consider this unlikely because CD70 is only expressed transiently on a small proportion of activated lymphocytes and dendritic cells. In addition, CD27-knockout mice (lacking any CD27/CD70 costimulation) have only subtle changes in their immune systems, with protective primary antigen-specific T-cell responses but a smaller memory T-cell compartment compared with normal mice after pathogen exposure.17,18 These subtle changes are unlikely to be of major relevance in adult human subjects, in whom reactivation of preexisting memory populations is the dominant response to infection. In our studies, CD70-specific T cells showed no reactivity against peripheral blood B and T cells. We also showed that activated T cells are not killed by CD70-specific T cells in cytotoxicity assays (supplemental Figure 1B). In contrast, B-cell blasts were susceptible to CD70-specific T-cell killing, but only after activation with the CD40 ligand and after allogeneic feeder cells had induced CD70 expression (supplemental Figure 1B). Whereas these results indicate that CD70-specific T cells have the potential to kill activated B cells, the physiologic relevance of this finding remains uncertain because this type of “super-physiologic” B-cell activation, resulting in prolonged CD70 expression, does not occur in vivo. Indeed, it has been demonstrated that CD70 is readily expressed on the surface of murine B cells stimulated in vitro with CD40 monoclonal antibodies and lipopolysaccharide; however, mice challenged with influenza virus show virtually no surface expression of CD70 on B cells infiltrating the lungs and draining lymph nodes.49 Likewise, CD70-expressing B cells are rarely observed in humans, being found on a limited number of germinal center B cells in less than 10% of tonsils examined and on scattered lymphocytes in secondary lymphoid organs and peripheral blood.50 No side effects have been reported so far in 2 phase 1 clinical studies evaluating the safety and tolerability of CD70 monoclonal antibodies (MDX-1203, NCT00944905; SGN-75, NCT01015911).
In summary, CD70-specific T cells can be readily generated by gene transfer with CARs encoding CD27-ζ, and these cells can kill human tumors in vitro and in vivo. Adoptive transfer of CD70-redirected T cells may be an attractive immunotherapeutic approach for B or T cell–derived hematologic malignancies and other CD70-positive solid tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cliona M. Rooney and Malcolm K. Brenner for helpful discussions and advice.
This work was supported by National Institutes of Health grant PO1 CA94237. D.R.S. was supported by National Institutes of Health grant 5T32HL092332-07.
National Institutes of Health
Authorship
Contribution: D.R.S. designed and performed research, analyzed and interpreted data, and wrote the manuscript; Z.Y., K.K.H.C., and S.K. performed research and analyzed data; B.S., D.M.S., and G.D. designed research and analyzed and interpreted data; M.-F.W. and H.L. provided statistical support; S.K. designed research; and S.G. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Gottschalk, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates St, Ste 1770, Houston, TX 77030; e-mail: smg@bcm.edu.




![Figure 7. CD70-specific T cells exhibit in vivo antitumor activity in an IP and systemic xenograft model of lymphoma. (A-B) Daudi cells (5 × 105) expressing the eGFP-FFLuc gene were injected IP into SCID mice, and tumor growth was measured as increasing light signal (p/s/cm2/sr). On days 10, 11, and 17, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. Tumors treated with CD70-specific T cells regressed, whereas tumors treated with nontransduced T cells did not (P = .002) 7 days after treatment. Panel A shows images of representative animals. Panel B shows quantitative bioluminescence imaging. In panels C and D, Raji cells (2 × 105) were injected intravenously into SCID mice. On days 4, 5, and 11, mice were injected with 1 × 107 CD70-specific or nontransduced T cells. (C) Systemic tumors were enumerated using bioluminescence imaging. At weeks 3 and 4 after tumor cell injection, there was a significantly higher tumor burden in mice receiving nontransduced T cells than CD70-specific T cells (week 3, P = .012; week 4 [n = 12], P = .010). (D) Mice treated with CD70-specific T cells displayed a significant survival advantage over those receiving nontransduced T cells (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/16/10.1182_blood-2010-04-278218/4/m_zh89991169000007.jpeg?Expires=1765066932&Signature=4bk0tqfqn84SG4el-wpdtwn6Zo8z2pbQKNbFnq8WdDJE12H12eSdWQs8o11cGyG2IUx27wlkQkNl4GfbLlhuTigk9Au6aDMGqcpP6ul9qjCJ6vab20Z~osAg2mufzzxB0Vh3HnRitXSVv02PUrm9I5UtJZ9LzqFR~~aTSQupnGnmLhdJp3uX9NPOJYIG8Vb-0m1CO9aZPAuPbhu5MBDZ~uAPU33HKlYrF16DKO6it5xZa-WRqvuGZbmgl5Fy0qgRWEf~fu-gp7h~RiE6xOSD4kH70X2OXmegIxBgRSZA5fWRoOwU9PY2PIAF~vjpMhB5VimfUo62JU090Ncql-Z6Dw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal