Abstract
Multiple observations support the existence of developmental differences in megakaryocytopoiesis. We have previously shown that neonatal megakaryocyte (MK) progenitors are hyperproliferative and give rise to MKs smaller and of lower ploidy than adult MKs. Based on these characteristics, neonatal MKs have been considered immature. The molecular mechanisms underlying these differences are unclear, but contribute to the pathogenesis of disorders of neonatal megakaryocytopoiesis. In the present study, we demonstrate that low-ploidy neonatal MKs, contrary to traditional belief, are more mature than adult low-ploidy MKs. These mature MKs are generated at a 10-fold higher rate than adult MKs, and result from a developmental uncoupling of proliferation, polyploidization, and terminal differentiation. This pattern is associated with up-regulated thrombopoietin (TPO) signaling through mammalian target of rapamycin (mTOR) and elevated levels of full-length GATA-1 and its targets. Blocking of mTOR with rapamycin suppressed the maturation of neonatal MKs without affecting ploidy, in contrast to the synchronous inhibition of polyploidization and cytoplasmic maturation in adult MKs. We propose that these mechanisms allow fetuses/neonates to populate their rapidly expanding bone marrow and intravascular spaces while maintaining normal platelet counts, but also set the stage for disorders restricted to fetal/neonatal MK progenitors, including the Down syndrome–transient myeloproliferative disorder and the thrombocytopenia absent radius syndrome.
Introduction
Megakaryocytopoiesis is the process by which hematopoietic stem cells undergo lineage commitment to become megakaryocyte (MK) progenitors, which proliferate and generate immature MKs. These immature MKs then undergo successive rounds of endomitosis that give rise to unique highly polyploid cells. The process of polyploidization is associated with the increasing production of proteins necessary for platelet formation and function,1 including membrane receptors such as CD41/61 and CD42, and platelet granule components such as VWF, platelet factor 4, and P-selectin. Polyploidization is also accompanied by progressive ultrastructural changes, particularly the formation of a complex demarcation membrane system (DMS), which, together with an accumulation of α granules, characterizes fully mature MKs. These events set the stage for the production of proplatelets and the release of platelets by mature MKs.2
Over the last decades, a mounting body of evidence has supported the existence of substantial biologic differences between fetal/neonatal and adult MKs. Several in vitro studies have shown that MK progenitors from fetuses and neonates proliferate at a much higher rate than adult progenitors.3-5 Neonatal MKs, however, are significantly smaller and of lower ploidy (and produce fewer platelets) than MKs from adults.6-8 Based on these characteristics, MKs from fetuses and neonates have been considered to be immature compared with adult MKs.9
Whereas the cellular and molecular mechanisms underlying these differences have remained elusive, it is clear that they play a critical role in the pathogenesis of several MK disorders with developmental stage–specific features. These include a transient myeloproliferative disorder with megakaryoblastic features seen exclusively in fetuses and neonates with Down syndrome and GATA-1 mutations,10 and 2 congenital disorders of the thrombopoietin (TPO)/TPO receptor (C-MPL) axis: thrombocytopenia absent radius (TAR) syndrome and congenital amegakaryocytic thrombocytopenia (CAMT).11 TAR is characterized by bilateral radial aplasia and severe thrombocytopenia beginning at birth. Affected individuals have no mutations in C-MPL or JAK2, but exhibit blocked MK maturation and abnormal TPO signaling.12 The platelet counts in patients with TAR improve to near-normal levels after the first year of life, indicating that the defect impairs TPO signaling in fetal/neonatal MKs, but not in adult MKs. CAMT is caused by mutations in C-MPL. Two variants of CAMT have been described. Type I CAMT, caused by mutations that completely block TPO signaling, presents with severe thrombocytopenia beginning at birth. Type II CAMT is caused by missense mutations in the C-MPL receptor, which allow residual function and variable amounts of TPO signaling. Patients with type II CAMT can have near normal platelet counts early in life, after which they develop severe thrombocytopenia and ultimately pancytopenia.13
The developmental differences in megakaryocytopoiesis are also thought to contribute to 2 relatively frequent hematologic problems. First, the small size and low ploidy of neonatal MKs might contribute to the delayed platelet engraftment after transplantation with cord blood (CB) stem cells. This is supported by the finding that CB-derived MKs remain significantly smaller than those derived from adult sources for at least 3 months after transplantation.14 The small size of neonatal MKs is also thought to contribute to the high incidence of thrombocytopenia among sick premature neonates.8,15
Although megakaryocytopoiesis is regulated by multiple cytokines, TPO is the main stimulatory factor for MK proliferation and maturation.16 Upon binding to its receptor, TPO induces conformational changes in the C-MPL molecule that lead to the activation of at least 3 intracellular signaling pathways: JAK-STAT,17,18 Shc-Ras-MAPK,19,20 and PI3K-AKT-mammalian target of rapamycin (mTOR).21,22 The biologic relevance of these pathways has been dissected through the use of chemical inhibitors and/or the introduction of mutant kinases. However, results from these studies have been inconsistent and at times contradictory, likely reflecting some differences in experimental conditions, but mostly in the source of cells used: murine or human cell lines, primary murine MKs, or primary human MKs.23 Based on our prior findings indicating that neonatal and adult MKs respond differently to TPO,24 we hypothesized that the developmental stage of the progenitor cell might also be an important variable. This study was undertaken to better characterize the maturational status of neonatal MKs and the molecular mechanisms underlying the developmental differences in megakaryocytopoiesis, particularly as they pertain to the TPO/C-MPL axis.
Methods
Cell cultures
Human umbilical CB-CD34+ cells were purchased from AllCells. G-CSF–mobilized adult peripheral blood (PB) CD34+ cells were purchased from the Fred Hutchinson Cancer Institute (Seattle, WA). CD34+ cells from either source were cultured in serum-free medium containing 50 ng/mL TPO with twice weekly medium changes, as described previously.24 Live cell number was quantified twice weekly after staining with 0.4% Trypan blue. For some experiments, rapamycin (Calbiochem), a specific inhibitor of mTOR, was added to the cultures. Rapamycin (50nM)25 was dissolved in DMSO, and an equivalent amount of DMSO (< 0.1%) was added to control cultures for these experiments.
Flow cytometry
After blocking with 5% mouse serum and 1% BSA, cells were stained with FITC-conjugated anti-human CD41a and allophycocyanin-conjugated anti-human CD42b (Becton Dickinson) or isotype-matched IgGs (negative controls). Cells were fixed with 1% paraformaldehyde and permeated with 70% ice-cold methanol. After incubation with 50 μL of 100 μg/mL RNAse, cells were stained with propidium iodide and evaluated by flow cytometry on an LSRII flow cytometer (Becton Dickinson). Data were analyzed using FlowJo Version 7.2.5 (TreeStar).
Immunofluorescence
Cultured cells were allowed to attach to poly-L-lysine–coated glass slides and fixed with 4% paraformaldehyde. Fixed cells were permeated by treatment with 0.5% Triton X-100. After blocking with 5% normal goat serum, cells were incubated with anti-VWF (Dako) and anti–P-selectin (Santa Cruz Biotechnology), followed by the appropriate Alexa Fluor 488 (green)– and Alexa Fluor 593 (red)–conjugated secondary antibodies. After 4′,6-diamidino-2-phenylindole, dihydrochloride counterstaining, cell images were analyzed in an Eclipse fluorescent microscope equipped with a DXM1200F camera (both from Nikon).
Ultrastructure of flow-sorted MKs
Cultured cells were incubated with 5 μg/mL Hoechst 33342 stain for 2 hours, followed by incubation with an FITC-conjugated anti-human CD41a antibody or an isotype-matched IgG control. CD41+ cells with ploidy levels of 2N and 4N were then flow-sorted using the MoFlo cell sorter (Becton Dickinson). For thin-section electron microscopy, flow-sorted cells were fixed with 1.5% glutaraldehyde in 0.1M cacodylate buffer. Cells were postfixed in osmium tetroxide, washed, and dehydrated through a series of alcohols, infiltrated with propylene oxide, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a G2 Spirit BioTWIN transmission electron microscope (Tecnai) at an accelerating voltage of 80 kV. Images were recorded with a 2k CCD camera (Advanced Microscopy Techniques).
Quantitative real-time PCR
Total RNA was isolated from CB-MKs and PB-MKs using the RNeasy Mini Kit (QIAGEN). Before reverse transcription, total RNA was treated with DNase I; 1 μg of RNA was then reverse transcribed using a M-MLV reverse transcriptase (Promega) containing a mixture of oligo(dT)20. The first-strand cDNA was used as a template in real-time PCR, using the SYBR Green Master Mix and an Applied Biosystem detection system. The cycling program was set as follows: denaturing at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Specific primers used for real-time RT-PCR of MK transcription factors are listed in the supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). β-Actin was used as the internal control.
G1ME cell studies
G1ME cells and viral supernatants containing GATA-1/MIGR1 or empty MIGR1 retroviruses were a generous gift from Dr Mitchell Weiss (University of Pennsylvania, Philadelphia, PA). G1ME cells were cultured and transfected with GATA-1–containing retrovirus or empty control viruses, as described previously.26 Briefly, G1ME cells were cultured in α-MEM supplemented with 20% FBS, 1% penicillin/streptomycin, 1% l-glutamine, and 20 ng/mL murine TPO (G1ME medium). After retroviral transduction, cells were placed in fresh G1ME medium and incubated for an additional 3 days. At the end of this period, cells were harvested and lysed, and protein was extracted for Western blot evaluation of GATA-1, mTOR, S6K, and p-4E-BP-1 (all from Cell Signaling Technology).
Western blots
Total protein was extracted from cultured MKs by cell lysis in radioimmunoprecipitation assay buffer with brief sonication. Proteins (50 μg) were separated on 4%-20% SDS-PAGE and transferred to a nitrocellulose filter. Blots were blocked using 5% BSA and incubated overnight with primary antibodies. Antibodies used for these studies were directed against human JAK-2, ERK1/2, p70S6K, p-4E-BP-1, cyclin D1, Cdk 4, and N-terminal GATA-1 (all from Cell Signaling Technology); C-terminal GATA-1 and p-21 (from Santa Cruz Biotechnology); C-MPL (from Millipore); and β-actin (from Sigma-Aldrich). Proteins were quantified by densitometric analysis of protein blots with ImageJ program Version 1.42g (National Institutes of Health).
For TPO-signaling analysis, at day 10 or 13 of cell culture, cells (2 × 106 cells/condition) were incubated overnight in serum-free medium without growth factors. Time- and dose-dependent TPO responses were evaluated by stimulating the starved cells with different concentrations of TPO for different periods of time, as indicated. To determine the effects of blocking the mTOR pathway, some cells were pretreated with 50nM rapamycin for 40 minutes, followed by 20 minutes of stimulation with 50 ng/mL TPO. Control cells for these experiments were pretreated with DMSO at an equivalent concentration. Phosphorylated proteins were evaluated using mAbs against phospho-JAK2 (Tyr 1007/1008), phospho-p70S6K (Thr421/Ser424), phospho–4E-BP-1 (Thr37/46), and phospho-ERK1/2 (Thr202/Tyr204; all from Cell Signaling Technology).
Statistical analysis
The statistical significance of the observed differences was determined using 2-way ANOVA for multiple comparisons and the Student t test for pairwise comparisons. All analyses were performed using GraphPad Prism Version 4 software. Statistical significance was set at P < .05. Data are presented as means ± SEM unless indicated otherwise.
Results
TPO-induced maturation of neonatal MKs
To determine the degree of cytoplasmic maturation of low-ploidy neonatal MKs, we first cultured CD34+ hematopoietic progenitor cells obtained from CB and from adult PB in the presence of TPO only. Cells from different donors (n = 5 per group) were cultured in independent experiments. After 14 days, 91.3% ± 4.01% of cells in CB cultures and 89.6% ± 3.61% in PB cultures were MKs (CD41+), but the number of MKs generated was highly dependent on the progenitor source: CB-CD34+ cells expanded approximately 70-fold, whereas PB-CD34+ cells only achieved a 5-fold expansion (supplemental Figure 1 shows proliferation, polyploidization, and proplatelet formation in CB-MKs and PB-MKs). The resulting PB-MKs, however, were much larger and reached significantly higher ploidy levels than the CB-MKs (31.7% ± 2.5% vs 8.0% ± 1.3% MKs with ploidy ≥ 8N, P < .001; supplemental Figure 1B-C). Proplatelets produced from PB-MKs were also large, with complicated long branches, whereas proplatelets formed by CB-MKs were smaller, with fewer branches and bulbs (supplemental Figure 1D).
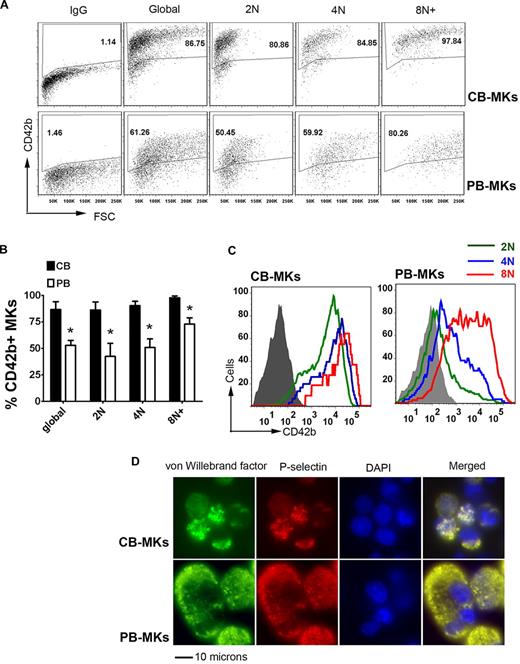
We next analyzed the cytoplasmic maturation of neonatal and adult MKs at different ploidy levels using CD42b (GpIbα) expression as a marker of mature MKs (Figure 1A).27,28 Surprisingly, we observed that after 14 days, CB cultures had significantly higher percentages of CD42b+ MKs than PB cultures (86.7% ± 7.3% vs 52.7% ± 4.8%; P = .003). When MKs at different ploidy levels were evaluated, the differences were more evident among low-ploidy MKs (Figure 1B). Specifically, 86.2% ± 7.6% of 2N CB-MKs were CD42b+, compared with 42.5% ± 12.2% of 2N PB-MKs (P = .006). Similarly, 90.3% ± 4.2% of 4N CB-MKs expressed CD42 compared with only 50.7% ± 8.2% of PB-MKs (P = .002). The difference between the 2 groups was less pronounced, but still significant, among MKs with a ploidy ≥ 8N (97.8% ± 1.8% vs 72.9% ± 6.0% in CB-MKs vs PB-MKs, respectively; P = .002). Furthermore, both the percentage of CD42b+ MKs and the mean fluorescent intensity (MFI) of CD42b staining increased in parallel to the ploidy level among PB-MKs, whereas there was no correlation among CB-MKs (Figure 1B-C).
Cytoplasmic maturation of CB-MKs. At day 14 of culture, MK cytoplasmic maturation and polyploidization were analyzed by flow cytometry and immunofluorescence. (A) Representative flow cytometric measurements of the percentage of CD42b+ MKs at each ploidy level in CB-MK and PB-MK cultures. (B) Compared with PB-MKs, CB-MKs exhibited significantly higher percentages of CD42b+ (mature) cells at each ploidy level. The bars represent the means ± SEM of 3 independent experiments. *P < .05. (C) When the intensity of CD42b staining was measured by MFI, CB-MKs exhibited almost equally intense expression at all ploidy levels. In contrast, among PB-MKs, the intensity of the staining increased gradually in parallel to the ploidy level. The figure shows a representative result of 3 independent experiments. (D) Like mature PB-MKs, low-ploidy CB-MKs formed α granules coexpressing P-selectin and VWF, as indicated by immunofluorescence staining. Images were taken on an Eclipse fluorescent microscope equipped with a DXM1200F camera (both from Nikon) at a magnification of 600×.
Cytoplasmic maturation of CB-MKs. At day 14 of culture, MK cytoplasmic maturation and polyploidization were analyzed by flow cytometry and immunofluorescence. (A) Representative flow cytometric measurements of the percentage of CD42b+ MKs at each ploidy level in CB-MK and PB-MK cultures. (B) Compared with PB-MKs, CB-MKs exhibited significantly higher percentages of CD42b+ (mature) cells at each ploidy level. The bars represent the means ± SEM of 3 independent experiments. *P < .05. (C) When the intensity of CD42b staining was measured by MFI, CB-MKs exhibited almost equally intense expression at all ploidy levels. In contrast, among PB-MKs, the intensity of the staining increased gradually in parallel to the ploidy level. The figure shows a representative result of 3 independent experiments. (D) Like mature PB-MKs, low-ploidy CB-MKs formed α granules coexpressing P-selectin and VWF, as indicated by immunofluorescence staining. Images were taken on an Eclipse fluorescent microscope equipped with a DXM1200F camera (both from Nikon) at a magnification of 600×.
To confirm the cytoplasmic maturation of CB-MKs, we also evaluated them for the presence of platelet granules using immunofluorescent staining for VWF and P-selectin, 2 components of α granules that accumulate in mature MKs. As shown in Figure 1D, VWF and P-selectin double-positive granules were observed in most CB-MKs and PB-MKs at day 14, indicating that these cells were mature MKs.
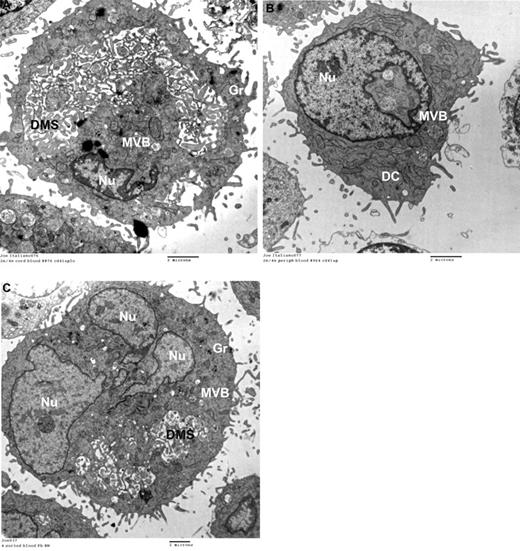
Based on the CD42b-expression patterns, we further hypothesized that neonatal 2N and 4N MKs would be more cytoplasmically mature than adult MKs of comparable ploidy. To investigate this, we flow-sorted 2N and 4N MKs generated from CB compared with PB cultures, and evaluated them by thin-section electron microscopy (n = 300 2N/4N MKs per group). Mature MKs were defined as those having an enlarged cytoplasm, abundant granules, and a well-developed DMS. Based on this definition, 58% of CB-derived 2N/4N MKs were mature (Figure 2A), compared with only 23% of 2N/4N PB-MKs (most low-ploidy PB-MKs were immature, as shown in Figure 2B). Interestingly, 54% of polyploid (≥ 8N) PB-MKs were mature, a percentage comparable with that of low-ploidy CB-MKs (Figure 2C).
Ultrastructure of MKs by transmission electron microscopy. Human MKs of different ploidy levels were flow sorted after anti-human CD41-FITC and Hoechst 33342 staining and examined by transmission electron microscopy. (A) Representative flow-sorted 2N/4N CB-MKs. The majority of these MKs were mature and exhibited abundant platelet granules and a well-developed DMS. (B) In contrast, 77% of flow-sorted 2N/4N PB-MKs were immature, as evidenced by the absence of a DMS and the paucity of granules (Gr) seen in this representative electron photomicrograph. These immature forms also exhibited multivesicular bodies (MVBs) and dense compartments (DCs), representing the precursors of platelet granules and of the DMS, respectively. (C) Fifty-four percent of all flow-sorted 8N PB-MKs were ultrastructurally similar to the mature neonatal 2N/4N MKs, although they were larger. All images were taken on a G2 Spirit BioTWIN transmission electron microscope (Tecnai) at an accelerating voltage of 80 kV, and recorded with a 2k CCD camera (Advanced Microscopy Techniques).
Ultrastructure of MKs by transmission electron microscopy. Human MKs of different ploidy levels were flow sorted after anti-human CD41-FITC and Hoechst 33342 staining and examined by transmission electron microscopy. (A) Representative flow-sorted 2N/4N CB-MKs. The majority of these MKs were mature and exhibited abundant platelet granules and a well-developed DMS. (B) In contrast, 77% of flow-sorted 2N/4N PB-MKs were immature, as evidenced by the absence of a DMS and the paucity of granules (Gr) seen in this representative electron photomicrograph. These immature forms also exhibited multivesicular bodies (MVBs) and dense compartments (DCs), representing the precursors of platelet granules and of the DMS, respectively. (C) Fifty-four percent of all flow-sorted 8N PB-MKs were ultrastructurally similar to the mature neonatal 2N/4N MKs, although they were larger. All images were taken on a G2 Spirit BioTWIN transmission electron microscope (Tecnai) at an accelerating voltage of 80 kV, and recorded with a 2k CCD camera (Advanced Microscopy Techniques).
To evaluate the maturational status of neonatal MKs in vivo, we then examined the ultrastructure of MKs in the livers of newborn mice on days 1 and 3 of life. As we have previously described, the liver is the main site of murine megakaryocytopoiesis at birth, and murine neonatal MKs (like human MKs) are significantly smaller than adult MKs.29,30 Using well-established parameters, neonatal MKs (n = 32) were classified as immature (stage I), DMS-forming (stage II), or platelet-forming (stage III).31 Based on these criteria, 50% of murine neonatal MKs were in stage II and 50% were in stage III (supplemental Figure 2 shows the ultrastructure of a representative MK in the liver of a newborn mouse). Using the same staging criteria, these percentages are similar to those found in adult murine marrow.31
Our data demonstrate that normal neonatal MKs are cytoplasmically mature despite their small size and low ploidy, and that fetuses and neonates generate large numbers of low-ploidy, fully mature MKs through a unique developmental uncoupling of proliferation, polyploidization, and cytoplasmic maturation.
Developmental differences in MK transcription factors
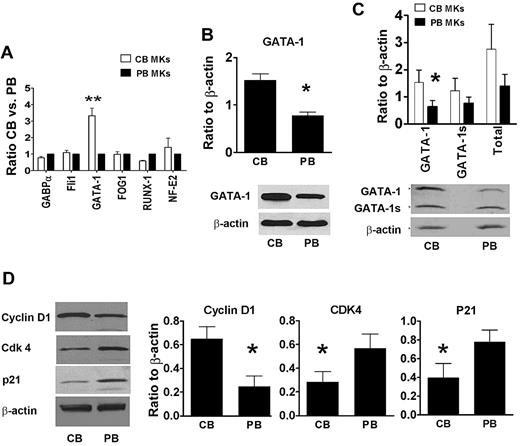
Given these findings, we then explored potential developmental differences in the mRNA expression levels of transcription factors involved in the regulation of MK proliferation and maturation, including GATA-1, FOG-1, RUNX-1, NF-E2, Fli-1, and GABP-α.32,33 As shown in Figure 3A, these studies revealed that GATA-1 mRNA levels were 3-fold higher in neonatal compared with adult MKs (P = .003). Western blot studies confirmed that full-length GATA-1 protein levels were also significantly higher in neonatal compared with adult MKs (Figure 3B).
GATA-1 levels in CB-MKs and PB-MKs. (A) The expression levels of multiple transcription factors related to MK development were analyzed by quantitative real-time PCR. Among the transcription factors studied, only GATA-1 was up-regulated in CB-MKs compared with PB-MKs. CB values were expressed as ratios to PB values. Bars represent the means ± SEM of 4 independent experiments. **P < .01. (B) Up-regulation of the GATA-1 protein was confirmed by Western blot analysis using an antibody targeting the N-terminal motif (no. 3535; Cell Signaling Technology), which only recognizes full-length GATA-1. *P < .05 (C) An antibody recognizing the C-terminal motif (SC-1233; Santa Cruz Biotechnology) was then used to differentiate between the full-length GATA-1 and GATA-1s. Only the levels of full-length GATA-1 were significantly higher in CB-MKs compared with PB-MKs. *P < .05. (D) The protein levels of cyclin D1, Cdk4, and p21 were also measured by Western blot analysis in human CB- and PB-derived mature MKs. Cyclin D1 protein levels were 2.6-fold higher in CB-MKs compared with PB-MKs, whereas Cdk4 and p21 protein levels were approximately 50%. In all cases, proteins were quantified by densitometric analysis of protein blots using the ImageJ program, and were expressed as a ratio to β-actin as an internal control. The bars represent the means ± SEM of 4 independent experiments, and the Western blots show representative results of each set of experiments. *P < .05.
GATA-1 levels in CB-MKs and PB-MKs. (A) The expression levels of multiple transcription factors related to MK development were analyzed by quantitative real-time PCR. Among the transcription factors studied, only GATA-1 was up-regulated in CB-MKs compared with PB-MKs. CB values were expressed as ratios to PB values. Bars represent the means ± SEM of 4 independent experiments. **P < .01. (B) Up-regulation of the GATA-1 protein was confirmed by Western blot analysis using an antibody targeting the N-terminal motif (no. 3535; Cell Signaling Technology), which only recognizes full-length GATA-1. *P < .05 (C) An antibody recognizing the C-terminal motif (SC-1233; Santa Cruz Biotechnology) was then used to differentiate between the full-length GATA-1 and GATA-1s. Only the levels of full-length GATA-1 were significantly higher in CB-MKs compared with PB-MKs. *P < .05. (D) The protein levels of cyclin D1, Cdk4, and p21 were also measured by Western blot analysis in human CB- and PB-derived mature MKs. Cyclin D1 protein levels were 2.6-fold higher in CB-MKs compared with PB-MKs, whereas Cdk4 and p21 protein levels were approximately 50%. In all cases, proteins were quantified by densitometric analysis of protein blots using the ImageJ program, and were expressed as a ratio to β-actin as an internal control. The bars represent the means ± SEM of 4 independent experiments, and the Western blots show representative results of each set of experiments. *P < .05.
Based on the prior finding that murine fetal liver MKs expressing exclusively a short form of GATA-1 (GATA-1s) exhibited a phenotype of increased proliferation and normal maturation similar to that observed in normal neonatal MKs,34 we then evaluated the expression levels of GATA-1s. GATA-1s is a 40-kDa protein lacking the N-terminal domain of GATA-1, and is normally produced from the same mRNA as the 47-kDa full-length form by alternative initiation of protein translation at amino acid 84 of the GATA-1 transcript.35 To test our hypothesis, we used an antibody that binds to the C-terminal domain of GATA-1 (Santa Cruz Biotechnology) and recognizes both GATA-1 forms. As seen in Figure 3C, there was a trend toward higher GATA-1s protein concentrations in CB-MKs compared with PB-MKs (1.22 ± 0.8 vs 0.76 ± 0.4, respectively; P = .2), but only full-length GATA-1 levels were significantly higher in CB-MKs compared with PB-MKs (1.53 ± 0.8 vs 0.63 ± 0.4, respectively, expressed as ratio to beta-Actin; P = .04).
Overexpression of GATA-1 in murine adult MKs accelerates maturation and substantially increases both ploidy and CD42b expression.36 However, it has been shown that GATA-1 regulates MK polyploidization and terminal maturation by controlling 2 different sets of target genes: one that regulates growth and polyploidization and a second one that drives terminal maturation.37 Prior studies in GATA-1–deficient MKs (from ΔneoΔHS GATA-1 mice) and in a GATA-1–null erythromegakaryocytic cell line (G1ME) identified cyclin D1/Cdk4 and STAT1 as downstream targets of GATA-1 that promote MK polyploidization independently of cytoplasmic maturation.36,37 To determine whether a down-regulation of these effectors contributed to the low ploidy levels of neonatal MKs, we quantified cyclin D1, Cdk4, and STAT-1 protein in neonatal and adult human MKs. These studies revealed no significant developmental differences in STAT-1 (data not shown). Cyclin D1 was present at 2.6-fold higher levels in CB-MKs compared with PB-MKs (P = .03), whereas Cdk4 was 50% lower in CB-MKs compared with PB-MKs (P = .04; Figure 3D).
TPO signaling in neonatal versus adult MKs
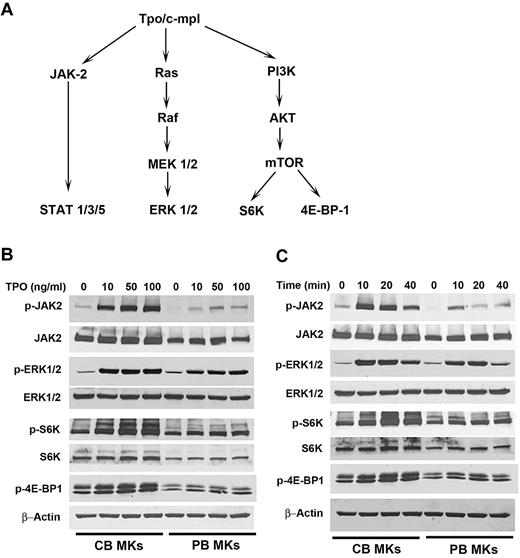
We hypothesized that the developmental differences in the response of MK progenitors to TPO could be associated with differences in TPO signaling. TPO activates at least 3 intracellular signaling pathways, JAK/STAT, MEK/MAPK, and PI3K/Akt/mTOR (Figure 4A). As shown in Figure 4B, the different concentrations of TPO examined (10-100 ng/mL) induced a similar degree of ERK1/2 phosphorylation in CB-MKs and PB-MKs. However, CB-MKs exhibited substantially stronger phosphorylation of JAK2, S6K, and 4E-BP-1 at all tested concentrations of TPO. To quantify these differences, we measured the degree of phosphorylation of these signaling molecules after stimulation with 10 ng/mL TPO for 20 minutes, and expressed the results as the -fold increase compared with the phosphorylation levels before TPO stimulation. In these experiments (n = 6), phosphorylation of JAK2, S6K, and 4E-BP-1 increased significantly more in CB-MKs compared with PB-MKs (4.7- ± 1.2-fold vs 2.3- ± 0.6-fold for JAK-2; 5.5- ± 3.5-fold vs 2.9- ± 1.7-fold for S6K; and 1.5- ± 0.2-fold vs 1.2- ± 0.2-fold for 4E-BP-1; P ≤ .01 for all).
TPO signaling in CB-MKs and PB-MKs. (A) Schematic representation of the major TPO-signaling pathways in MKs.25,38 (B) After 14-16 hours of starvation, MKs were stimulated with different concentrations of TPO (10-100 ng/mL) for 20 minutes, and TPO-induced protein phosphorylation was determined. At all TPO concentrations, JAK2, S6K, and 4E-BP-1 phosphorylation was significantly stronger in CB-MKs compared with PB-MKs. (C) After the same overnight starvation, MKs were stimulated with 50 ng/mL TPO for different time periods (10, 20, and 40 minutes). At all time points, JAK2, S6K, and 4E-BP-1 phosphorylation was stronger in CB-MKs compared with PB-MKs. The images shown are representative of 3 separate time courses and 2 dose-response experiments.
TPO signaling in CB-MKs and PB-MKs. (A) Schematic representation of the major TPO-signaling pathways in MKs.25,38 (B) After 14-16 hours of starvation, MKs were stimulated with different concentrations of TPO (10-100 ng/mL) for 20 minutes, and TPO-induced protein phosphorylation was determined. At all TPO concentrations, JAK2, S6K, and 4E-BP-1 phosphorylation was significantly stronger in CB-MKs compared with PB-MKs. (C) After the same overnight starvation, MKs were stimulated with 50 ng/mL TPO for different time periods (10, 20, and 40 minutes). At all time points, JAK2, S6K, and 4E-BP-1 phosphorylation was stronger in CB-MKs compared with PB-MKs. The images shown are representative of 3 separate time courses and 2 dose-response experiments.
In separate experiments, we tested the effects of TPO concentrations as low as 2 ng/mL (data not shown). Stimulation with this concentration for 20 minutes induced detectable phosphorylation signals of JAK2 and S6K in CB-MKs, but not in PB-MKs. ERK1/2 phosphorylation was detected in both CB-MKs and PB-MKs, although signals were stronger in CB-MKs. We further analyzed the time course of TPO-induced phosphorylation signals. As shown in Figure 4C, 40 minutes after stimulation, the signals were significantly stronger in CB-MKs compared with PB-MKs. In a separate experiment, the same was true at 60 minutes (data not shown). These studies revealed developmental differences in the kinetics of TPO-induced signaling, including up-regulated phosphorylation of JAK2 and mTOR pathway signals and increased sensitivity to low TPO concentrations in CB-MKs compared with PB-MKs.
Because of our findings of stronger and more sustained JAK2 phosphorylation in neonatal versus adult MKs, we also evaluated the protein levels of LNK, an adaptor protein that negatively regulates TPO signaling.39 As shown in Figure 5, the protein levels of LNK were similar in CB-MKs and PB-MKs, indicating that the longer and stronger phosphorylation of JAK2 and mTOR targets in neonatal MK is not related to lower LNK levels.
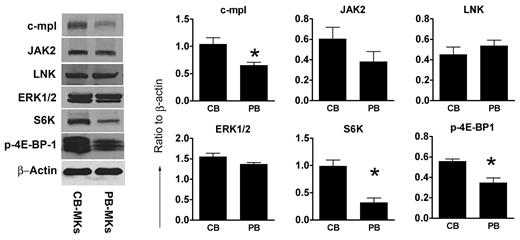
TPO-signaling proteins in CB-MKs and PB-MKs. Total levels of proteins related to TPO signaling were measured by Western blot in unstimulated mature MKs derived from CB and PB progenitor cells (after 14 days of culture with TPO only). C-MPL, S6K, and p-4E-BP-1 were present at significantly higher levels in CB-MKs compared with PB-MKs. There were no significant differences in JAK2, LNK, and ERK1/2 levels. The bars represent the means ± SEM of protein levels, expressed as a ratio to β-actin, in 4 independent experiments. *P < .05.
TPO-signaling proteins in CB-MKs and PB-MKs. Total levels of proteins related to TPO signaling were measured by Western blot in unstimulated mature MKs derived from CB and PB progenitor cells (after 14 days of culture with TPO only). C-MPL, S6K, and p-4E-BP-1 were present at significantly higher levels in CB-MKs compared with PB-MKs. There were no significant differences in JAK2, LNK, and ERK1/2 levels. The bars represent the means ± SEM of protein levels, expressed as a ratio to β-actin, in 4 independent experiments. *P < .05.
Developmental differences in TPO receptor and TPO-signaling protein levels
Based on findings from our signaling studies and on the high levels of GATA-1 in CB-MKs (which regulates C-MPL and JAK-2 expression), we then hypothesized that the concentrations of C-MPL and TPO-signaling proteins would be developmentally regulated. To investigate this, we quantified C-MPL, JAK2, ERK1/2, S6K, and p-4E-BP-1 protein levels in nonstimulated mature CB-MKs (n = 4) and PB-MKs (n = 4). As shown in Figure 5, CB-MKs expressed higher levels of C-MPL, S6K, and p-4E-BP-1 than PB-MKs. CB-MKs also showed a trend toward elevated JAK2 levels, although this was not statistically significant. Protein levels of ERK1/2 were similar in CB-MKs and PB-MKs.
Given these findings, we then investigated whether mTOR and its targets were regulated by GATA-1. To determine this, we evaluated the protein levels of mTOR, S6K, and p-4E-BP-1 in GATA-1–deficient (G1ME) cells 72 hours after transfection with either GATA-1/MIGR1 or an empty vector. These studies showed no correlation between GATA-1 expression and the protein levels of mTOR, S6K, or p-4E-BP-1 (supplemental Figure 3 shows GATA-1 and mTOR-signaling molecules in G1ME cells), indicating that the elevated levels of mTOR-signaling molecules in neonatal MKs do not result from their high GATA-1 levels.
MK maturation is mediated through the mTOR pathway
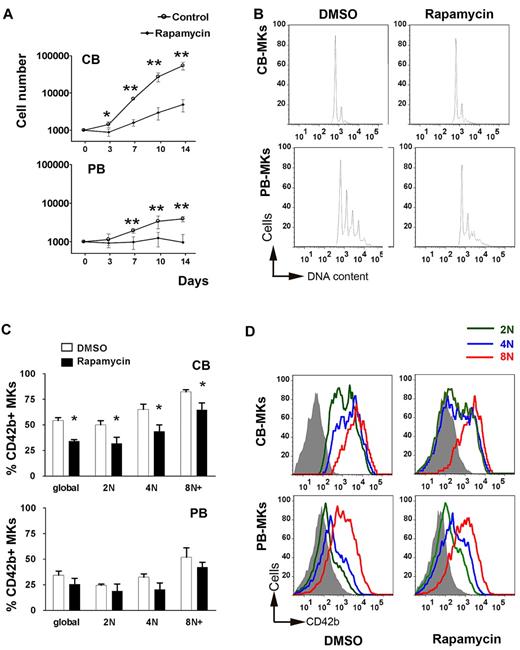
We next investigated the functional significance of up-regulated mTOR signaling in CB-MKs by adding the mTOR inhibitor rapamycin to MK cultures starting on day 0. Regardless of progenitor source (PB or CB), rapamycin substantially reduced the number of cells generated (Figure 6A). Differentiation into the MK lineage was also reduced, as evidenced by the decrease in the percentage of CD41+ cells in rapamycin-treated versus control cultures (77.2% ± 4.7% vs 94.6% ± 1.66% for CB cultures and 64.0% ± 7.2% vs 86.6% ± 2.7% for PB cultures). As a consequence of the combined reduction in proliferation and MK differentiation, the number of MKs generated in the presence of rapamycin was reduced by 90% in CB cultures and by 76% in PB cultures.
Effects of rapamycin on the proliferation, differentiation, and maturation of MKs. (A) Cell counts (± SD) in rapamycin (50nM) vs DMSO-treated (control) cultures of CB-MKs and PB-MKs. Rapamycin, added to the cultures starting on day 0, significantly reduced the cell counts in both CB-MK and PB-MK cultures, although the effect was somewhat more pronounced on CB-MKs. *P < .05; **P < .01. (B) Ploidy levels of rapamycin-treated versus control CB-MKs and PB-MKs as measured by flow cytometry. (C) Percentage of CD42b+ cells in rapamycin vs DMSO-treated CB-MKs and PB-MKs shown globally and classified by ploidy level. Bars represent the means ± SEM of 3 independent experiments. *P < .05. (D) Flow cytometric measurements of the MFI of CD42b staining among CB-MKs and PB-MKs cultured in the presence of rapamycin or DMSO classified by ploidy level. A representative result of 3 independent experiments is shown.
Effects of rapamycin on the proliferation, differentiation, and maturation of MKs. (A) Cell counts (± SD) in rapamycin (50nM) vs DMSO-treated (control) cultures of CB-MKs and PB-MKs. Rapamycin, added to the cultures starting on day 0, significantly reduced the cell counts in both CB-MK and PB-MK cultures, although the effect was somewhat more pronounced on CB-MKs. *P < .05; **P < .01. (B) Ploidy levels of rapamycin-treated versus control CB-MKs and PB-MKs as measured by flow cytometry. (C) Percentage of CD42b+ cells in rapamycin vs DMSO-treated CB-MKs and PB-MKs shown globally and classified by ploidy level. Bars represent the means ± SEM of 3 independent experiments. *P < .05. (D) Flow cytometric measurements of the MFI of CD42b staining among CB-MKs and PB-MKs cultured in the presence of rapamycin or DMSO classified by ploidy level. A representative result of 3 independent experiments is shown.
We then evaluated the effects of rapamycin on polyploidization. As shown in Figure 6B, in PB-MK cultures, the percentage of MKs ≥ 8N decreased from 31.7% ± 2.5% to 23.5% ± 2.6% in the presence of rapamycin (a 26% reduction; P = .002). In contrast, rapamycin did not significantly affect the percentage of ≥ 8N MKs in CB cultures (7.7% ± 1.3% in control vs 11.7% ± 3.6% in rapamycin-treated cultures; P = .2). Given these findings, we compared the CB-MK and PB-MK protein levels of p21, a cell-cycle inhibitor previously shown to be important for the regulation of MK ploidy via the mTOR pathway.38 As shown in Figure 3D, p21 levels in CB-MKs were 50% of those in PB-MKs (P = .02). Because the effects of rapamycin on ploidy are less pronounced in p21−/− mice compared with WT mice,38 the reduced levels of p21 in CB-MKs provide a potential mechanism for the lack of rapamycin effects on CB-MK ploidy.
PB-MKs exposed to rapamycin also exhibited a 26% reduction in global CD42b expression (from 34.4% to 25.5% CD42+ MKs), which is consistent with the overall decrease in polyploid MKs in the culture (Figure 6C). When the level of CD42b expression was analyzed at each ploidy level, we found no significant differences between the rapamycin-treated and the PB-MK control cultures in either the percentage of MKs expressing CD42b or the MFI of the staining within each ploidy class (Figure 6C-D). This indicates that rapamycin induced a synchronous decrease in the nuclear and cytoplasmic maturation of PB-MKs. In contrast, CB-MKs exhibited a substantial (37%) decrease in the percentage of CD42b+ MKs in response to rapamycin (34.1% ± 2.6% vs 54.4% ± 4.7% in controls; P = .02), without any effects on ploidy. Further analysis by ploidy level demonstrated that the reduction in CD42b expression was more pronounced in 2N and 4N MKs compared with 8N MKs (Figure 6C-D).
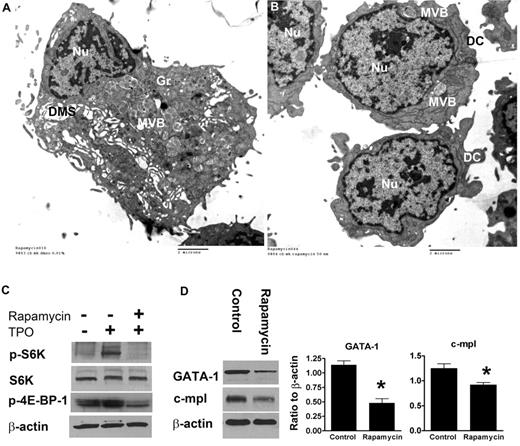
To confirm that the lower CD42b levels indeed reflected less-mature MKs, we also evaluated the ultrastructure of rapamycin-treated CB-MKs by electron microscopy. In this study, 81% of control MKs (DMSO-treated; n = 90) exhibited a DMS (Figure 7A), compared with 41% of rapamycin-treated MKs (n = 85). Most rapamycin-treated MKs exhibited scant cytoplasm, few granules, and an absent DMS (Figure 7B).
Ultrastructure and protein-expression levels of GATA-1 and C-MPL in rapamycin-treated versus control CB-MKs. (A) Representative electron photomicrograph of a CB-MK cultured in 0.01% DMSO (controls) exhibiting a large cytoplasm, a large cytoplasm/nucleus (Nu) ratio, abundant granules (Gr), and an open DMS. (B) Ultrastructural characteristics of MKs treated with rapamycin showing a striking reduction in cytoplasm, nearly absent granules, and a lack of DMS. Features of very immature MKs were frequently observed, including multivesicular bodies (MVB) and dense compartments (DC). (C) Under the same experimental setting described in Figure 4, MKs were starved overnight and then stimulated with TPO in the presence or absence of rapamycin (50nM). Pretreatment with rapamycin significantly suppressed phosphorylation of S6K and 4E-BP-1 upon TPO stimulation in CB-MKs. (D) In separate experiments, MKs treated with rapamycin from day 11 until day 14 of culture had significantly lower protein levels of GATA-1 and C-MPL compared with control MKs. Bars reflect the means ± SEM of 3 independent experiments. *P < .05.
Ultrastructure and protein-expression levels of GATA-1 and C-MPL in rapamycin-treated versus control CB-MKs. (A) Representative electron photomicrograph of a CB-MK cultured in 0.01% DMSO (controls) exhibiting a large cytoplasm, a large cytoplasm/nucleus (Nu) ratio, abundant granules (Gr), and an open DMS. (B) Ultrastructural characteristics of MKs treated with rapamycin showing a striking reduction in cytoplasm, nearly absent granules, and a lack of DMS. Features of very immature MKs were frequently observed, including multivesicular bodies (MVB) and dense compartments (DC). (C) Under the same experimental setting described in Figure 4, MKs were starved overnight and then stimulated with TPO in the presence or absence of rapamycin (50nM). Pretreatment with rapamycin significantly suppressed phosphorylation of S6K and 4E-BP-1 upon TPO stimulation in CB-MKs. (D) In separate experiments, MKs treated with rapamycin from day 11 until day 14 of culture had significantly lower protein levels of GATA-1 and C-MPL compared with control MKs. Bars reflect the means ± SEM of 3 independent experiments. *P < .05.
Finally, we evaluated the effects of rapamycin at the molecular level. After confirming in signaling studies that pretreatment with rapamycin suppressed the phosphorylation of mTOR targets after TPO stimulation (Figure 7C), we added rapamycin or DMSO to our CB cultures starting on day 11, when the majority of MKs are undergoing terminal maturation. After 3 days of culture, GATA-1 and C-MPL protein levels were substantially lower in rapamycin-treated compared with control MKs (Figure 7D). However, rapamycin did not affect GATA-1 mRNA levels measured 24, 48, and 72 hours after the addition of rapamycin (data not shown), suggesting that mTOR might regulate GATA-1 expression in human MKs at the posttranscriptional level.
Discussion
Several observations support the existence of substantial developmental differences in megakaryocytopoiesis. Some of them are directly related to the TPO/C-MPL axis, such as the clinical presentations of TAR and type II CAMT40 and our observation that TPO stimulates polyploidization in adult MKs but inhibits this process in neonatal MKs.24 Adding to this body of evidence and challenging the paradigm that neonatal MKs are relatively immature, our current study conclusively demonstrates that proliferation and cytoplasmic maturation are uncoupled during normal neonatal development, thus allowing for highly proliferative progenitors to give rise to fully mature MKs. The process of terminal differentiation is also developmentally unique in that polyploidization and cytoplasmic maturation are dissociated. As a net result of these different maturational patterns, 2N and 4N MKs derived from CB are more cytoplasmically mature than adult MKs of similar ploidy. These findings are consistent with those of Mattia et al, who observed that on days 9 and 12 of culture, CB-MKs had higher levels of CD42b surface expression than PB-MKs, despite their lower ploidy levels.7
Our study also provides novel insights into the molecular mechanisms underlying the profound developmental differences between neonatal and adult MKs. First, we observed developmental differences in TPO signaling, mostly characterized by an up-regulation of signaling through JAK-2 and the mTOR pathway. JAK-2 has been shown to mediate MK proliferation,41 and activating mutations of JAK-2 cause myeloproliferative disorders.42,43 mTOR is a Ser/Thr kinase that regulates cell size and cell-cycle progression through the G1/S transition. These effects are mediated via activation of 2 effector proteins critical for translational initiation, S6K and 4E-BP-1. mTOR is up-regulated in acute myeloid leukemias, where it stimulates cell proliferation, and mTOR inhibitors are currently being evaluated as therapeutic agents.44 In MKs, mTOR is activated by TPO through the PI3K-AKT pathway. Two prior studies have evaluated the functional relevance of mTOR in primary human adult MKs.25,38 In both, the addition of rapamycin during MK culture induced a significant reduction in MK proliferation, size, and polyploidization without inducing apoptosis. Raslova et al also observed a decrease in the CD42b expression levels, which paralleled the reduction in CD41 expression and ploidy. Consistent with their findings, we also observed a symmetric (∼ 25%) decrease in MK ploidy, size, and cytoplasmic maturation in adult MKs treated with rapamycin. CB-MK cultures exhibited a more pronounced suppression of MK proliferation in response to rapamycin than PB-MK cultures, which is not a surprising finding given the higher baseline proliferative rate of neonatal MK progenitors likely associated in part with the up-regulation of mTOR-induced cell-cycle progression. However, our studies also revealed a novel critical role of this pathway in mediating the cytoplasmic maturation of neonatal MKs independently of polyploidization.
An up-regulation of mTOR downstream of the insulin-like growth factor 1 receptor (IGF1R) pathway was also recently described in murine fetal megakaryocytic progenitors and in human DS-AMKL cells.45 These investigators observed that the IGF-IGF1R-mTOR pathway promoted proliferation in fetal MK progenitors through activation of the E2F transcription network and that full-length GATA-1 counteracted this E2F activation, thus serving as a developmental regulator of MK proliferation. Consistent with these observations, our findings support a critical role for mTOR (downstream of TPO) and GATA-1 in the regulation of normal human fetal/neonatal MK proliferation and terminal maturation.
Among the transcription factors important for MK proliferation and maturation, GATA-1 was the only one found to be substantially up-regulated in CB-MKs compared with PB-MKs. Our findings also suggest that mTOR regulates GATA-1 protein expression at the posttranscriptional level. Although this does not explain the elevated GATA-1 mRNA levels found in CB-MKs, it provides a potential mechanism through which mTOR might mediate the terminal maturation of MKs. GATA-1 directly regulates the expression of multiple genes critical for TPO signaling and MK maturation, including C-MPL, JAK-2, GPIIb, and GPIbα,46 most of which were found to be expressed at higher levels in neonatal compared with adult MKs. The role of GATA-1 in MK maturation has been shown in MK-specific, GATA-1–knockout mice (ΔneoΔHS GATA-1 mice), which exhibit increased MK proliferation and an arrest in maturation. Morphologically, GATA-1–deficient MKs are smaller than wild-type MKs and have scant cytoplasm, a paucity of granules, and an underdeveloped DMS.46,47 GATA-1 is also absolutely required for TPO to induce MK maturation, as evidenced by the failure of enhanced TPO signaling to induce polyploidization and CD42 expression in G1ME cells.36 In neonatal MKs, elevated GATA-1 levels are associated with high levels of cytoplasmic maturation, but a lack of polyploidization. Among the genes known to regulate polyploidization downstream of GATA-1, CB-MKs have high cyclin D1 but low Cdk4 levels. Interestingly, Muntean et al observed that the overexpression of cyclin D1 alone in GATA-1–deficient MKs resulted in a decrease in ploidy and size, and that the simultaneous overexpression of Cdk4 was absolutely required to induce MK polyploidization.37 Thus, it is possible that the low Cdk4 levels in neonatal MKs are one of the mechanisms contributing to their low ploidy despite elevated GATA-1.
We also investigated the possibility that the phenotype of neonatal MKs could be due to developmental differences in the relative expression levels of GATA-1s. In support of this hypothesis, murine fetal liver MKs expressing solely GATA-1s exhibit an uncoupling of proliferation and maturation.34 In addition, developmental differences in the relative expression of these 2 GATA-1 isoforms have been reported, with murine embryos at E8.5 expressing only GATA-1s.35 However, our data demonstrated that the elevated levels of GATA-1 in neonatal MKs were mostly accounted for by full-length GATA-1.
Consistent with the elevated GATA-1 levels, we found C-MPL protein levels to be significantly higher in CB-MKs compared with PB-MKs, as shown by Western blot analysis. This was in contrast to findings from a previous study, which reported a lower number of TPO receptors in CB-MKs by flow cytometry,48 a discordance likely due to methodologic differences. Nevertheless, our finding of higher C-MPL concentrations could explain the higher sensitivity of neonatal MKs to lower concentrations of TPO. First, according to the “receptor occupancy theory,” a high receptor density is favorable at low concentrations of ligands. Second, Millot et al observed that the degree of activation of all TPO-signaling pathways was directly correlated with the level of C-MPL expression in BaF-3 cells.49 Third, the activation of C-MPL depends on the dimerization of 2 receptors, a process favored by higher receptor density.
In summary, our data indicate that neonatal megakaryocytopoiesis is characterized by an uncoupling of proliferation, polyploidization, and cytoplasmic maturation, which results in the production of large numbers of highly cytoplasmically mature, low-ploidy MKs. This unique developmental pattern is associated with an up-regulation of TPO signaling through JAK2 and particularly mTOR, and high levels of GATA-1 and its target, cyclin D1, coupled with decreased Cdk4 and p21. We hypothesize that these developmental differences are all part of a carefully orchestrated molecular arrangement of pathways favoring increased proliferation and rapid terminal MK maturation, without the requirement for endomitosis. From an ontogenetic perspective, we propose that these mechanisms allow fetuses/neonates to populate their rapidly expanding bone marrow space and blood volume while maintaining normal platelet counts. However, characterizing these pathways and differences in TPO signaling during fetal/neonatal life is also critical to understanding those disorders of megakaryocytopoiesis with developmental stage-specific features and to guiding the use of novel TPO mimetics (which exhibit differences in the signaling pathways that they activate)50 in thrombocytopenic neonates and patients with delayed platelet engraftment after CB transplantation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Mitchell Weiss for generously providing the G1ME cells for these studies, Dr Maxim Pimkin for technical assistance, and Dr Alan Cantor for his critical reading and discussion of the manuscript.
This work was supported by National Institutes of Health Grant RO1 HL069990 (to M.S.-V.).
National Institutes of Health
Authorship
Contribution: Z.-J.L. designed and performed experiments, collected and analyzed data, and wrote the manuscript; J.I. performed and interpreted the electron microscopy studies; F.F.-M., R.G., M.B., and B.P. performed experiments; L.R. assisted with experimental design, data interpretation, and preparation of the manuscript; and M.S.-V. supervised and designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of R.G. is Department of Biochemistry, School of Life Sciences, University of Hyderabad, Hyderabad, India. The current affiliation of M.B. is Life Technologies, Eugene, OR.
Correspondence: Martha Sola-Visner, MD, Children's Hospital Boston, Division of Newborn Medicine, Enders Research Building, 300 Longwood Ave, Rm 961, Boston, MA 02115; e-mail: Martha.Sola-Visner@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal