In this issue of Blood, Brunner and colleagues demonstrate that uPAR, the urokinase plasminogen activator (uPA) receptor that drives endothelial invasion and proliferation in the initial phases of angiogenesis, undergoes down-regulation by density-enhanced phosphatase-1 (DEP-1) in confluent endothelial cells.1 Together, uPAR and DEP-1 provide a mechanism that regulates the response of endothelial cells to angiogenic stimuli.
The investigators who first identified uPAR also found that uPAR expression was inversely related to cell density. This find lead to some dismay as it was possible that the uPA, the uPAR ligand used for binding studies, could stick to culture-dish plastic in the absence of a cell monolayer. However, mud sticking to the sole of a shoe does not mean that shoe soles have mud receptors. The bulk of evidence regarding uPAR functions led to acceptance of uPAR down-regulation as having significant biologic function.2
uPAR is becoming more and more important as a target molecule for cancer therapy due to its role in regulating cell proliferation and invasion. Recent studies have shown that besides the classic extracellular matrix-degrading protease cascade triggered by uPA binding, uPAR also triggers robust proliferative cell-signaling responses and accounts for gripping properties of the cells to extracellular matrix via its interaction with vitronectin. Such features make the uPAR/uPA system an inviting target for control of malignant cell growth and spread.3 As endothelial cells exploit this cell-associated growth/invasion machinery, it is also an intriguing target for antiangiogenesis molecules.4
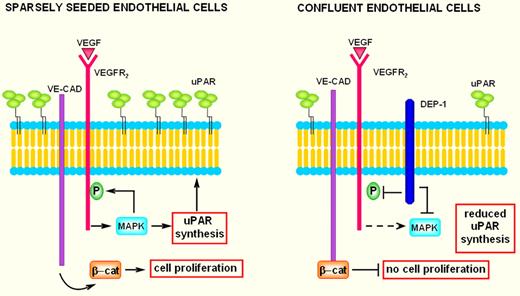
Twenty-five years after uPAR was first described, the scientific community finally has a satisfactory answer to the problem of the inverse relationship between uPAR and cell density. Using a battery of in vitro assays, Brunner and colleagues demonstrated that confluent endothelial cells not only show decreased responses toward VEGF stimulation, but also express less uPAR. The factor responsible for down-regulating ERK1/2 activation and subsequent decreased uPAR expression has been identified with the density-enhanced receptor-like tyrosine phosphatase DEP-1, a so-far undescribed regulator for uPAR. As suggested by its name, expression of DEP-1 is up-regulated with increasing cell density and inhibits the MAPK pathway in confluent seeded endothelial cells (see figure). The transcription factor Elk-1, downstream of ERK1/2, has been shown as a potential candidate to inhibit uPAR expression after DEP-1 up-regulation.
In subconfluent endothelial cells, interaction of VEGF with its type-2 receptor (VEGFR2) activates the MAPK pathway. MAPK (ERK1/2) phosphorylates VEGFR2 as well as transcription factors that stimulate uPAR synthesis. The phosphorylated VEGFR2 induces detachment of β-catenin from nearby VE-cadherin, thus promoting cell proliferation. Therefore, sparsely seeded endothelial cells show VEGF-dependent uPAR up-regulation with a strong increase of invasion and are provided with free β-catenin available for nuclear translocation, while the density-enhanced phosphatase-1 (DEP-1) is scarcely represented. Overall, these features foster endothelial-cell migration and growth. In confluent endothelial cells VEGFR2, VE-cadherin, and overexpressed DEP-1 undergo junction clustering. The presence of overexpressed DEP-1 impairs MAPK activation, thereby inhibiting uPAR synthesis and VEGFR-2 phosphorylation. The growth-inhibitory effects are believed to be mediated by the capacity of VE-cadherin to link β-catenin at the membrane, limiting in this way its nuclear translocation. Coupling growth inhibition with low uPAR levels results in exhaustion of the angiogenesis program.
In subconfluent endothelial cells, interaction of VEGF with its type-2 receptor (VEGFR2) activates the MAPK pathway. MAPK (ERK1/2) phosphorylates VEGFR2 as well as transcription factors that stimulate uPAR synthesis. The phosphorylated VEGFR2 induces detachment of β-catenin from nearby VE-cadherin, thus promoting cell proliferation. Therefore, sparsely seeded endothelial cells show VEGF-dependent uPAR up-regulation with a strong increase of invasion and are provided with free β-catenin available for nuclear translocation, while the density-enhanced phosphatase-1 (DEP-1) is scarcely represented. Overall, these features foster endothelial-cell migration and growth. In confluent endothelial cells VEGFR2, VE-cadherin, and overexpressed DEP-1 undergo junction clustering. The presence of overexpressed DEP-1 impairs MAPK activation, thereby inhibiting uPAR synthesis and VEGFR-2 phosphorylation. The growth-inhibitory effects are believed to be mediated by the capacity of VE-cadherin to link β-catenin at the membrane, limiting in this way its nuclear translocation. Coupling growth inhibition with low uPAR levels results in exhaustion of the angiogenesis program.
After its first description by Ostman et al,5 the receptor-like tyrosine phosphatase DEP-1 has been studied in both cancer cells and endothelial cells. In cancer cells DEP-1 is now referred to as a tumor suppressor, as it inhibits growth of cancer cells.6 In endothelial cells an increase of DEP-1 activity strongly inhibits angiogenesis and promotes contact inhibition of VEGF-induced proliferation.7 Evidence indicates that contact-inhibited cells, including endothelial cells, have a reduced response to specific growth factors after they reach confluence. It is likely that establishment of intercellular contacts transfers negative intracellular signals, which restrains the capacity of cells to respond to proliferative signals. The endothelial cell–specific cadherin named vascular endothelial cadherin (VE-cadherin) has been implicated in this effect. VE-cadherin expression and clustering at intercellular junctions block VEGFR-2 phosphorylation, thereby blunting the proliferative effect of VEGF. This mechanism requires several steps: (1) on stimulation with VEGF, the phosphorylated VEGFR-2 associates with the VE-cadherin and concentrates at cell-cell contacts; (2) DEP-1, which also is concentrated at cell-cell contacts, dephosphorylates VEGFR-2 by inhibiting the MAPK pathway; and (3) the growth-inhibitory effect is mediated by the intracellular partner of VE-cadherin, β-catenin. In fact, reduction of VEGFR-2 phosphorylation impairs translocation of β-catenin to the nucleus, where it normally up-regulates transcription of cyclinD1 and c-myc, which result into growth inhibition.8
The paper by Brunner et al1 establishes a new link in this scenario. DEP-1–dependent dephosphorylation not only impairs VEGFR-2 activation, thus inhibiting the activity of the most important among proangiogenesis factors, but also abolishes the activity of the uPAR system, the angiogenesis “workman” that obeys the orders of VEGF. As a consequence, endothelial cells do not receive angiogenesis command and do not express operational systems linked to cell migration, which results in inhibition of the angiogenesis signal once endothelial cells meet.
As uPAR is a molecule whose expression is controlled by binding of regulatory proteins to AU-rich regions of its mRNA, described to act as positive (mRNA stabilizing) or negative (mRNA destabilizing) elements, a natural extension of Brunner et al's work is to question whether the DEP-1 pathway might act by blunting activation of the relevant proteins that regulate uPAR mRNA stability, thus reducing its expression.9
Another question that arises from this work by Brunner and colleagues regards how DEP-1 is regulated in pathologies characterized by a decreased angiogenesis, such as diabetes and atherosclerosis characterized by impaired collateral vessel development; restenosis, which involves altered re-endothelialization; and systemic sclerosis where, independent of proangiogenesis factor overexpression, endothelial cells are unable to perform proper angiogenesis.10
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal