Abstract
Splenic marginal zone (MZ) B cells are a lineage distinct from follicular and peritoneal B1 B cells. They are located next to the marginal sinus where blood is released. Here they pick up antigens and shuttle the load onto follicular dendritic cells inside the follicle. On activation, MZ B cells rapidly differentiate into plasmablasts secreting antibodies, thereby mediating humoral immune responses against blood-borne type 2 T-independent antigens. As Krüppel-like factors are implicated in cell differentiation/function in various tissues, we studied the function of basic Krüppel-like factor (BKLF/KLF3) in B cells. Whereas B-cell development in the bone marrow of KLF3-transgenic mice was unaffected, MZ B-cell numbers in spleen were increased considerably. As revealed in chimeric mice, this occurred cell autonomously, increasing both MZ and peritoneal B1 B-cell subsets. Comparing KLF3-transgenic and nontransgenic follicular B cells by RNA-microarray revealed that KLF3 regulates a subset of genes that was similarly up-regulated/down-regulated on normal MZ B-cell differentiation. Indeed, KLF3 expression overcame the lack of MZ B cells caused by different genetic alterations, such as CD19-deficiency or blockade of B-cell activating factor-receptor signaling, indicating that KLF3 may complement alternative nuclear factor-κB signaling. Thus, KLF3 is a driving force toward MZ B-cell maturation.
Introduction
Sp/Krüppel-like factors (KLFs) encompass more than 20 transcription factors in mice or humans. All KLFs share a characteristic DNA-binding domain at their C-terminus consisting of 3 Krüppel-like zinc-finger domains. One subset of KLFs, containing the PXDLS/T motive, recruits the C-terminal binding protein 2 general transcriptional repressor (CtBP2).1 Apart from this motive, no consensus sequences are found in the N-terminal domain of KLFs. For KLF3 an interaction with four and a half LIM domains 3 protein (FHL3) has been demonstrated.2 KLFs are involved in differentiation, proliferation, or trafficking of cells in various tissues.3 They are differentially expressed in lymphocytes, including T cells. Here, KLF2 and few other KLFs have been studied extensively.4-8 To date, little is known about the role of KLFs in B-cell development.
Before colonizing secondary lymphoid organs, such as spleen or lymph nodes, B cells differentiate from hematopoietic precursors in the bone marrow. After the developmental processes there, most of the immature/transitional B cells that are IgMhi CD24hi IgDlo CD93hi (AA4.1, 493) CD21/35− CD23− migrate via the blood to the spleen where they complete their maturation. During splenic maturation, they down-modulate CD93, gain CD21/35 and CD23 expression, and, finally, appear as mature follicular or marginal zone (MZ) B cells, together referred to as B2 cells. Mature follicular and MZ B cells differentially express several cell surface markers: follicular B cells are CD21/35+ CD23+ CD1dlo, whereas MZ B cells are CD21/35hi CD23− CD1dhi; both express IgM and IgD, but to different levels (IgMlo IgDhi and IgMhi IgDlo for follicular and MZ B cells, respectively).9
Another lineage of B cells, the B1 subset, localizes predominantly to the peritoneum. These derive mostly from fetal hematopoietic stem cells, as opposed to adult bone marrow stem cells, and require the spleen for maturation. In the spleen, follicular and MZ B cells are localized differently: naive follicular B cells constitute the white pulp follicles that enwrap the T cell-rich periarteriolar zone, surrounding the central ateriole. Branching off the central ateriole, small vessels end openly into the marginal sinus at the white pulp and red pulp interface. This zone is termed “marginal zone” and contains resident macrophages, dendritic cells, and MZ B cells. Here, blood-borne pathogens and microbial products come by contact with the immune systems' tissue first. Thought of as a first line of defense, MZ B cells can initiate rapid T cell-independent antibody responses.10 Thus, the function of MZ B cells is dependent on their proper localization.
Whereas integrins mediate their adhesion,11,12 chemoattractant molecules define where MZ B cells home to. They are attracted by sphingosine-1-phospate (S1P), with the highest concentration found in blood, by the S1P-receptor 1 (S1P1, encoded by Edg1) and/or S1P3 (Edg3). Physiologically, the dominance of S1P1 (and/or S1P3) signaling is regularly interrupted, allowing the temporary shuttling of MZ B cells into the follicle to transport antigens captured via complement receptors onto follicular dendritic cells.13 Here, MZ B cells migrate toward the CXCL13 gradient sensed by CXCR5. On activation, they also rapidly relocate into the follicle, after S1P1 down-regulation,14 and appear to exit via the bridging channels into the red pulp as plasmablasts.10
The molecular basis of MZ B-cell differentiation is not fully understood. To date, signals received by the following receptors are known to be involved: B-cell activating factor (BAFF)-receptor (BAFF-R, BR3); the B-cell receptor (BCR) and its coreceptors, such as CD19 or CD22; and signaling via Notch-2/RBP-J by binding to delta-like 1, expressed by red pulp endothelial cells. Signaling via BAFF-R is essential for the survival of mature B cells and operates via the nuclear factor-κB (NF-κB) pathway to induce prosurvival genes. Indeed, B-cell cellularity can be restored in the absence of either BAFF or BAFF-R by forced expression of bcl-2. Interestingly, though, this led to the accumulation of physiologic numbers of follicular B cells but not of MZ B cells, indicating that BAFF/BAFF-R signaling also has a differentiation inducing component.15,16 The latter might be mediated via the alternative NF-κB pathway.17,18 However, complementing BAFF-R deficiency by classical NF-κB signaling using a constitutively active IκB kinase 2 restores the pool of B2 B cells, including MZ B cells.19 This result could be explained by the provision of p100 substrate, essential for alternative NF-κB signaling, downstream of the BCR/classical NF-κB pathway.20
Signaling via the BCR is essential for mature B-cell survival and subtle changes affecting BCR signaling strength alter B2 and B1 B-cell subsets: btk-deficient or xid (btk-mutant) mice have less B1 cells but increased MZ B cells,21,22 CD19-deficient mice lack MZ B cells and B1 cells,22-25 and CD22-deficient mice lack MZ B cells but have normal numbers of B1 cells.26
Finally, signaling via Notch-2 is essential for MZ B cells as in the absence of Notch-2, Notch-2 interacting protein RBP-J, or Notch-ligand delta-like 1; or when Notch processing is deficient (ADAM10 deficiency or pharmacologic inhibition of γ-secretases), MZ B cells are absent.9
To study the function of KLF3 in B-cell development and function, we generated transgenic mice expressing KLF3 in B cells. We show that KLF3 promotes MZ B-cell differentiation and complements BAFF-receptor signaling defects if further complemented by bcl-2. In KLF3-transgenic mice, MZ B cells are dramatically increased in number, reside in their usual niche, and show several other characteristics in line to normal MZ B cells, including being sessile and dependent on Notch2, a general property of MZ B cells.
Methods
Mice
KLF3 transgenic mice were generated at the Basel Institute for Immunology by microinjection into C57BL/6 (B6) fertilized eggs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Unless indicated, KLF3 transgenic mice were from founder line 2 or 23, which were phenotypically indistinguishable. KLF3-deficient mice were on FVB/NJ background.27 Further lines are: B6.TACI-Ig,16 floxed Notch-2 allele (Notch-2fl),28 B6;Eμ:bcl-2 line 3629 (T. Rolink), CD19cre25 (E. Hobeika), B6.IgHa (B6.Cg-IghaThy1a), and B6.Ly-5.1 (B6.SJL-ptprca; The Jackson Laboratory). Mice were crossed to obtain allele combinations as indicated. Parabiosis was carried out as previously described with female mice of 7 to 9 weeks of age.30 Chimeric mice were prepared by γ-irradiation from a 137Cs-source, followed by reconstitution with hematopoietic stem cells equivalent to 0.3 to 1 fetal liver (FL, day 14.5) per recipient given intravenously. Studies on animals were approved by local animal committees (Regierungspräsidium Freiburg/Service Vétérinaire cantonal Lausanne/CIPA-CHUM Montréal/Sydney University).
FACS and immunofluorescence
Cells were isolated from the indicated organs. For samples from spleen or bone marrow, erythrocytes were lysed in ammonium chloride/Tris-buffer. Sytox-blue (Invitrogen) or propidium-iodide was used to exclude dead cells. Antibodies were from BD Biosciences, eBioscience, or prepared by Protein G affinity chromatography followed by derivatization using standard techniques. Cells were analyzed on Calibur, Canto, or LSRII (all BD Biosciences) instruments with CellQuest Version 33 or Diva Versions 4.1 to 6.1 software. Data analysis was done using these or FlowJo Version 6.4.7 software. For histology, cryosections (5 μm) were cut, fixed in acetone, blocked, and stained with the indicated antibodies. Sections were mounted in Fluoromount-G (Southern Biotech Assoc) or a mixture of 1 part glycerol with 2 parts of 25% (w/v) Moviol 4-88 in 0.2M Tris pH 8.5. Pictures were acquired on a Zeiss Axioscope (equipped with a Hamamatsu C4880 CCD camera), Apotome (Axio ComMR camera), or LSM510 meta confocal microscopes using Zeiss Axiovision and LSM software. For graphical presentation, Photoshop 7.0 and/or Illustrator 10.0.3 software (Adobe) was used.
Gene array analysis
Viable follicular and MZ B cells from 10- to 11-week-old female KLF3 transgenic and nontransgenic littermate mice were purified by cell sorting using a FACSAria (Sytox− CD19+ CD23+ CD21/35+ and Sytox− CD19+ CD23− CD21/35hi, respectively, purity > 98%). RNA arrays were performed as previously described (supplemental Figure 2).31 Complete microarray data are in supplemental Table 1 (individual log2 probe matrices and population comparisons).
In vitro assays
For lipopolysaccharide (LPS) stimulation, spleen cells were stained directly as below and sorted to isolate follicular B cells. MZ B cells were pre-enriched by depleting T cells and CD23+ cells using bead cocktails of anti-Thy-1.2 admixed with anti-CD23-biotin precoated streptavidin beads (Dynal). Cells were stained for CD21/35, CD23, and CD19 and subjected to sorting. For assays on follicular B cells without MZ B cells, lymph node cells were used as such or depleted of T cells using anti-Thy-1.2 beads.
Cell culture was in Iscove modified Dulbecco medium (Biochrom, purchased as powder) supplemented with nonessential amino acids, 100 IU/mL/100 μg/mL penicillin-streptomycin (both from Invitrogen), β-mercaptoethanol (50μM), glutamine (4mM), 0.03% (weight/volume) Primatone RL (Quest, 5×59051), 5 μg/mL insulin (I5500, Sigma-Aldrich), and 2% to 4% fetal calf serum at 7.5% CO2, 37°C.
Transwell migration assays were performed as described.12 Briefly, 106 splenocytes resuspended in Dulbecco modified Eagle medium supplemented with 0.1% bovine serum albumin were added into the upper chamber (5 μm pore size, Corning), and dilutions of CXCL13 (R&D Systems) or S1P (Sigma-Aldrich) prepared in the same media were added into the dish. After 2.5 hours at 37°C 5% CO2, cells were recovered from both compartments and stained. Dead cells were excluded by Sytox-blue staining, and lymphocyte cell populations were quantified by fluorescence-activated cell sorter (FACS) relative to an internal bead standard (Flow-Count fluorospheres, Beckman Coulter).
Western blotting was performed using standard techniques with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium detection of alkaline phosphatase-labeled secondary antibodies. Briefly, follicular B cells were purified from lymph node cells by live cell enrichment over Ficoll-Paque followed by T-cell depletion (anti-Thy-1.2 magnetic beads, Dynal) resulting in more than 95% purity. The KLF2 peptide specific rabbit antiserum was a gift of W. Schuh.32
Results
Generation of KLF3 transgenic mice
We generated KLF3 transgenic mice in which the KLF3 cDNA is expressed under the control of the CD19 promoter (T. Winkler, Erlangen, Germany). Four independent transgenic lines with less than 10 and one line with more than 50 copies of the transgene were obtained and expressed transgenic KLF3 (supplemental Figure 1A-B). Nuclear extracts of splenocytes from control and transgenic mice contained material that bound to a β-globin promoter probe, as would be expected for KLF3. Indeed, one of the retarded bands was KLF3, as tested by adding an antiserum to KLF3. Adding an antibody against the c-myc tag confirmed that the activity corresponded to the transgene encoded tagged KLF3 (supplemental Figure 1C).
KLF3 favors B-cell maturation toward the MZ B-cell subset
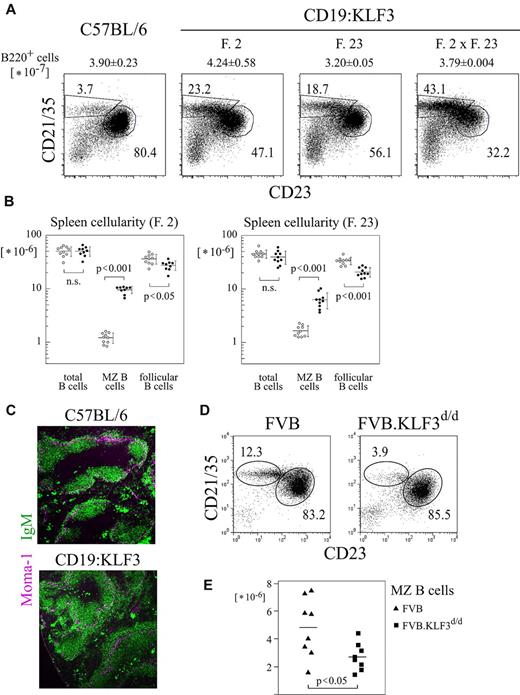
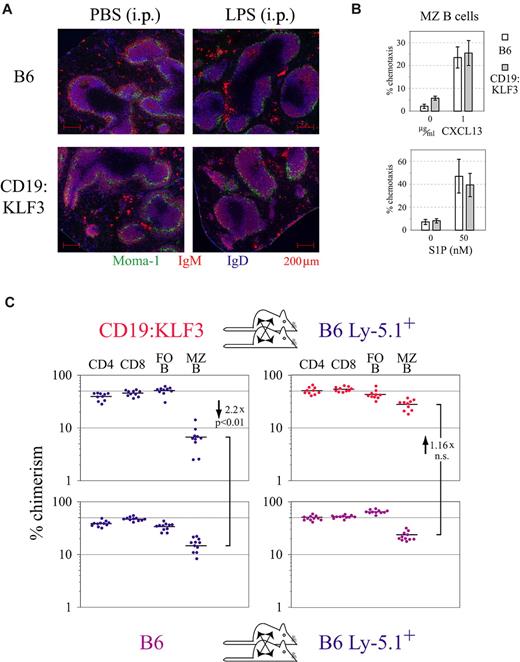
All low-copy number lines showed a similar peripheral B-cell phenotype, characterized by roughly normal B-cell numbers in lymph nodes and spleen but a dramatic 6- to 10-fold increase in the proportion and, notably, in the number of MZ B cells in the spleen (Figure 1A-B; supplemental Figure 1A). These cells were indeed located to the splenic MZ (Figure 1C). In contrast, MZ B cells were reduced in KLF3-deficient mice. As some of these cells remained, there is, however, redundancy in the function of KLF3 (Figure 1D-E). To test from which stage onwards the KLF3 transgene mediates its effect and whether the increase in MZ B-cell differentiation was cell autonomous, as would be expected because expression was controlled by the B cell-specific CD19 promoter, we prepared competitive FL chimeras. Congenic Rag-2-deficient recipients were reconstituted with allotype marked normal control or KLF3 transgenic FL cells and normal FL cells. Donor-derived T cells or immature/transitional B cells served as internal control to estimate the grafting efficiency of the given donor hematopoietic stem cells within the individual chimera. As shown in Figure 2A and B, in the chimeras KLF3 transgenic cells dominated the recipients' MZ B-cell compartment. In contrast, immature and follicular B cells showed a similar proportion of Ly-5.1+ cells as observed among T cells, irrespective of whether derived from normal or KLF3 transgenic FL cells. Interestingly, in the peritoneum, transgenic cells dominated the B1 compartment (Figure 2C). These data indicate that KLF3 acts to establish MZ B cells in the spleen and B1 cells in the peritoneum.
Changes in proportion and absolute number of MZ B cells in CD19:KLF3 and KLF3-deficient mice. (A) Spleen follicular (CD21/35− CD23+) and MZ B-cell (CD21/35hi CD23−) proportions in normal and CD19:KLF3 transgenic lines (founder line 2, 23, and double-transgenic mice). Cells were stained for B220, CD21/35, and CD23. Data shown are gated on B220+ cells. Total numbers of B220+ cells are indicated above the plots (± SD). (B) Numbers of the indicated subsets were determined among CD19:KLF3 and nontransgenic littermates of founder lines 2 and 23 at 6 to 7 or 9 to 12 weeks of age, respectively, as shown. (C) Immunofluorescent analysis of spleen cryosections of CD19:KLF3 and littermate control mice stained for Moma-1 and IgM (R33-24-12). (D) Splenic B cells from normal (FVB/NJ) and KLF3-deficient (FVB/NJ.KLF3d/d) mice at 12 weeks of age. Data shown are gated on B220+ cells. (E) MZ B-cell numbers in mice as shown in panel D (n = 8 each). Double-transgenic mice were identified by Southern blot (A, n = 2 each). (B-E) Each dot represents an individual animal. (C) Immunohistologic analysis has been performed on more than 5 mice each of CD19:KLF3 and normal genotype. Micrographs were obtained with Axioscope instrumentation, using a 5× objective.
Changes in proportion and absolute number of MZ B cells in CD19:KLF3 and KLF3-deficient mice. (A) Spleen follicular (CD21/35− CD23+) and MZ B-cell (CD21/35hi CD23−) proportions in normal and CD19:KLF3 transgenic lines (founder line 2, 23, and double-transgenic mice). Cells were stained for B220, CD21/35, and CD23. Data shown are gated on B220+ cells. Total numbers of B220+ cells are indicated above the plots (± SD). (B) Numbers of the indicated subsets were determined among CD19:KLF3 and nontransgenic littermates of founder lines 2 and 23 at 6 to 7 or 9 to 12 weeks of age, respectively, as shown. (C) Immunofluorescent analysis of spleen cryosections of CD19:KLF3 and littermate control mice stained for Moma-1 and IgM (R33-24-12). (D) Splenic B cells from normal (FVB/NJ) and KLF3-deficient (FVB/NJ.KLF3d/d) mice at 12 weeks of age. Data shown are gated on B220+ cells. (E) MZ B-cell numbers in mice as shown in panel D (n = 8 each). Double-transgenic mice were identified by Southern blot (A, n = 2 each). (B-E) Each dot represents an individual animal. (C) Immunohistologic analysis has been performed on more than 5 mice each of CD19:KLF3 and normal genotype. Micrographs were obtained with Axioscope instrumentation, using a 5× objective.
Cell autonomous induction of MZ B-cell development by KLF3. (A) FACS analysis of lethally irradiated B6.Rag-2−/− mice that had been reconstituted with FL cells from B6.IgHa (blue) and either normal B6.Ly-5.1 (purple) or CD19:KLF3 Ly-5.1 (red) embryos. Mice were analyzed 2 months after reconstitution by staining splenocytes for B220, CD21/35, CD23, and Ly-5.1. The proportion of Ly-5.1− T cells was 33.1% and 39.9% in chimeras reconstituted with KLF3 transgenic and nontransgenic FL cells, respectively. (B) The ratio of cells derived either from normal or from KLF3 transgenic Ly-5.1+ FL cells divided by the proportion derived from control IgHa FL cells as calculated for the indicated subset for chimeras as shown in panel A. (C) FACS analysis of cells from the peritoneal cavity of the chimeras as presented in panel A. Cells were stained for CD19, CD5, and Ly-5.1 as indicated. Data are representative of more than 5 mice of each group.
Cell autonomous induction of MZ B-cell development by KLF3. (A) FACS analysis of lethally irradiated B6.Rag-2−/− mice that had been reconstituted with FL cells from B6.IgHa (blue) and either normal B6.Ly-5.1 (purple) or CD19:KLF3 Ly-5.1 (red) embryos. Mice were analyzed 2 months after reconstitution by staining splenocytes for B220, CD21/35, CD23, and Ly-5.1. The proportion of Ly-5.1− T cells was 33.1% and 39.9% in chimeras reconstituted with KLF3 transgenic and nontransgenic FL cells, respectively. (B) The ratio of cells derived either from normal or from KLF3 transgenic Ly-5.1+ FL cells divided by the proportion derived from control IgHa FL cells as calculated for the indicated subset for chimeras as shown in panel A. (C) FACS analysis of cells from the peritoneal cavity of the chimeras as presented in panel A. Cells were stained for CD19, CD5, and Ly-5.1 as indicated. Data are representative of more than 5 mice of each group.
KLF3 induces a MZ B-cell signature within follicular B cells
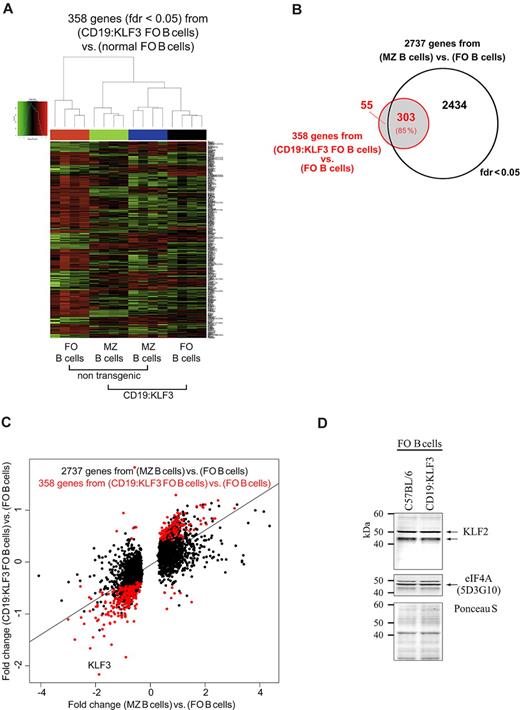
Using RNA arrays, we determined the expression of genes involved in normal MZ B-cell differentiation by comparing sorted follicular and MZ B cells (supplemental Table 1; available at www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE26948). The resulting set of 2737 genes associated with MZ B cells versus follicular B cells included most of those previously identified in a similar comparison.33 As shown in supplemental Figure 2B, using these 2737 genes to determine the relatedness of follicular and MZ B-cell subsets from normal and transgenic mice, we found that transgenic MZ B cells, and, surprisingly, transgenic follicular B cells, clustered together with normal MZ B cells. This was unlike in a purely computed (unsupervised) clustering where follicular and MZ B cells were grouped together irrespective of being transgenic or not (supplemental Figure 2A). Together, this indicates that KLF3 targets, directly or indirectly, many genes that are normally differentially regulated during MZ B-cell differentiation. Indeed, clustering based on 358 genes identified here that were differentially regulated between normal and KLF3 transgenic follicular B cells resulted in the separation of normal follicular cells from all other populations (normal MZ B cells, transgenic follicular, and MZ B cells), and this subset of genes overlapped considerably (85%) to the MZ B cell-specific set (Figure 3A-B). Furthermore, almost all of these 358 genes were similarly differentially regulated during normal MZ B-cell differentiation as visualized by the dot plot of expression intensities (Figure 3C). Thus, by focusing on genes that are regulated by KLF3 within follicular B cells, we have identified a reduced set of genes that are clearly differentially regulated during normal MZ B-cell maturation (supplemental Table 1). Among other KLF-family members, KLF2, KLF4, and KLF6 were differentially regulated in MZ B cells (all down); however, their expression in follicular B cells was not affected by the KLF3 transgene, excluding that they are down-regulated by KLF3 (Table 1). KLF2 protein levels were indeed not altered in follicular B cells of CD19:KLF3 mice (Figure 3D). A total of 704 genes (fdr < 0.05) were differentially expressed between normal and KLF3 transgenic MZ B cells. However, this KLF3-mediated change in expression did not correlate well to those associated with MZ B cells versus follicular B cells of normal mice (supplemental Table 1).
Gene expression analyses of follicular and MZ B cells from normal and CD19:KLF3 mice. (A) Cluster analysis based on 358 genes identified here as differentially expressed in CD19:KLF3 follicular B cells versus normal follicular B cells (fdr < 0.05). As shown by the genealogic tree above, CD19:KLF3 follicular and normal MZ B cells are related. Indeed, the subset of CD19:KLF3 follicular B cells is more related to MZ B cells of normal or CD19:KLF3 mice than to normal follicular B cells, which appear as a distinct entity. (B) Venn diagram of genes identified by the 2 comparisons based on normal MZ B cells versus normal follicular B cells (black circle) or CD19:KLF3 follicular B cells versus normal follicular B cells (red circle) (fdr < 0.05). (C) Dot plot analysis for expression differences of 2737 genes identified here as being differentially expressed in normal MZ versus follicular B cells (x-axis) and their corresponding expression difference in CD19:KLF3 follicular B cells versus normal follicular B cells (y-axis; all dots). The 358 genes that were differentially expressed in CD19:KLF3 follicular and normal follicular B cells are shown in red. Note that in the arrays KLF3 is detected by a probe outside the coding region of KLF3 that is not part of the transgene. Thus, KLF3 appears here not to be expressed in transgenic mice, whereas indeed transgenic KLF3 is expressed efficiently. Indeed, transgenic KLF3 expression appears to limit the expression from the endogenous KLF3 locus (supplemental Figure 1A). Array experiments were performed twice with follicular and MZ B cells of normal and CD19:KLF3 mice (n = 4 each). Representative data of one of 2 experiments, giving similar results, are shown. (D) Western blot analysis for KLF2 among normal and CD19:KLF3 follicular B cells. Two KLF2 specific bands are revealed.32 Equal loading is demonstrated by reprobing for the ribosomal elongation initiation factor 4A (eIF4A). Note that the eIF4A signal appears in between the KLF2 specific bands revealed earlier. Also shown is the Ponceau S stain of the blot before probing. A second experiment was performed giving similar results.
Gene expression analyses of follicular and MZ B cells from normal and CD19:KLF3 mice. (A) Cluster analysis based on 358 genes identified here as differentially expressed in CD19:KLF3 follicular B cells versus normal follicular B cells (fdr < 0.05). As shown by the genealogic tree above, CD19:KLF3 follicular and normal MZ B cells are related. Indeed, the subset of CD19:KLF3 follicular B cells is more related to MZ B cells of normal or CD19:KLF3 mice than to normal follicular B cells, which appear as a distinct entity. (B) Venn diagram of genes identified by the 2 comparisons based on normal MZ B cells versus normal follicular B cells (black circle) or CD19:KLF3 follicular B cells versus normal follicular B cells (red circle) (fdr < 0.05). (C) Dot plot analysis for expression differences of 2737 genes identified here as being differentially expressed in normal MZ versus follicular B cells (x-axis) and their corresponding expression difference in CD19:KLF3 follicular B cells versus normal follicular B cells (y-axis; all dots). The 358 genes that were differentially expressed in CD19:KLF3 follicular and normal follicular B cells are shown in red. Note that in the arrays KLF3 is detected by a probe outside the coding region of KLF3 that is not part of the transgene. Thus, KLF3 appears here not to be expressed in transgenic mice, whereas indeed transgenic KLF3 is expressed efficiently. Indeed, transgenic KLF3 expression appears to limit the expression from the endogenous KLF3 locus (supplemental Figure 1A). Array experiments were performed twice with follicular and MZ B cells of normal and CD19:KLF3 mice (n = 4 each). Representative data of one of 2 experiments, giving similar results, are shown. (D) Western blot analysis for KLF2 among normal and CD19:KLF3 follicular B cells. Two KLF2 specific bands are revealed.32 Equal loading is demonstrated by reprobing for the ribosomal elongation initiation factor 4A (eIF4A). Note that the eIF4A signal appears in between the KLF2 specific bands revealed earlier. Also shown is the Ponceau S stain of the blot before probing. A second experiment was performed giving similar results.
Deregulated KLF3 expression does not result in altered gene expression of KLFs but endogenous KLF3
| FC* . | CD19:KLF3 FO B cells vs FO B cells . | MZ B cells vs FO B cells . | CD19:KLF3 MZ B cells vs CD19:KLF3 FO B cells . | CD19:KLF3 MZ B cells vs MZ B cells . |
|---|---|---|---|---|
| KLF2 | 1.06 | -3.63† | -5.79† | -1.50 |
| KLF3 | -4.49† | -3.66† | -2.20† | -2.69† |
| KLF4 | -1.17 | -1.48† | -1.20 | 1.05 |
| KLF6 | 1.07 | -1.69‡ | -1.36 | 1.34 |
| KLF7 | 1.39 | -1.23 | -1.49 | 1.16 |
| KLF9 | 1.16 | 1.09 | 1.36 | 1.46 |
| FC* . | CD19:KLF3 FO B cells vs FO B cells . | MZ B cells vs FO B cells . | CD19:KLF3 MZ B cells vs CD19:KLF3 FO B cells . | CD19:KLF3 MZ B cells vs MZ B cells . |
|---|---|---|---|---|
| KLF2 | 1.06 | -3.63† | -5.79† | -1.50 |
| KLF3 | -4.49† | -3.66† | -2.20† | -2.69† |
| KLF4 | -1.17 | -1.48† | -1.20 | 1.05 |
| KLF6 | 1.07 | -1.69‡ | -1.36 | 1.34 |
| KLF7 | 1.39 | -1.23 | -1.49 | 1.16 |
| KLF9 | 1.16 | 1.09 | 1.36 | 1.46 |
Data are fold changes in the expression of the indicated genes.
fdr < 0.01; changes in fold change are significant only where fdr is reported.
fdr < 0.05; changes in fold change are significant only where fdr is reported.
We conclude that the transcription factor KLF3 is a major factor inducing/regulating MZ B-cell differentiation, directly or indirectly, as its expression in transgenic mice results in a partial MZ B-cell gene signature among follicular B cells. We therefore tested whether KLF3 could by itself restore the MZ B-cell compartment affected by various genetic alterations.
KLF3 overcomes MZ B-cell maturation arrest caused by various alterations
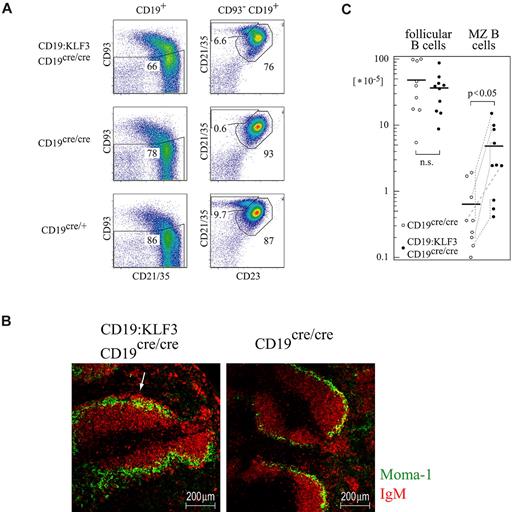
Deleting BCR accessory molecules such as CD19 or CD22 changes BCR signaling strength. MZ B cells are particularly sensitive to such alterations.22,24,26 Indeed, whereas CD19-deficient mice lacked MZ B cells, we found that CD19-deficient mice that were KLF3 transgenic still had MZ B cells, although there was some variability in their number (Figure 4A,C). Nevertheless, they properly located into the marginal zone (Figure 4B).
KLF3 expression overcomes the MZ B-cell developmental arrest caused by CD19 deficiency. (A) Splenocytes from mice of the indicated genotypes were stained for CD19, CD93, CD21/35, and CD23, and analyzed by FACS. (B) Spleen cryosections were stained for Moma-1 and IgM and analyzed by immunofluorescent microscopy. The white arrow indicates an area rich in MZ B cells. Such areas were lacking entirely in CD19-deficient mice. (C) Cell numbers of follicular and MZ B cells in mice of the indicated genotype. Dashed lines indicate sex-matched littermates analyzed in parallel. Three FACS experiments were performed. For histology, 3 CD19:KLF3 and 3 nontransgenic CD19cre/cre mice were analyzed. Micrographs were obtained with a LSM610 meta confocal microscope, using a 16×/0.5 NA objective.
KLF3 expression overcomes the MZ B-cell developmental arrest caused by CD19 deficiency. (A) Splenocytes from mice of the indicated genotypes were stained for CD19, CD93, CD21/35, and CD23, and analyzed by FACS. (B) Spleen cryosections were stained for Moma-1 and IgM and analyzed by immunofluorescent microscopy. The white arrow indicates an area rich in MZ B cells. Such areas were lacking entirely in CD19-deficient mice. (C) Cell numbers of follicular and MZ B cells in mice of the indicated genotype. Dashed lines indicate sex-matched littermates analyzed in parallel. Three FACS experiments were performed. For histology, 3 CD19:KLF3 and 3 nontransgenic CD19cre/cre mice were analyzed. Micrographs were obtained with a LSM610 meta confocal microscope, using a 16×/0.5 NA objective.
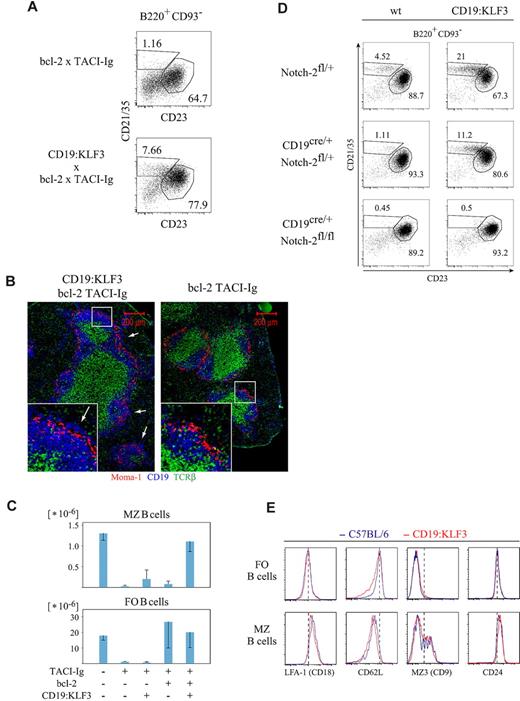
Recently, it was shown that canonical NF-κB signaling, downstream of the BCR, led to an increased availability of NF-κB p100 substrate to be processed to p52 in the (alternative) NF-κB2 signaling pathway downstream of the BAFF-R.17,20 Reduced BCR signaling in CD19-deficient mice might cause MZ B-cell paucity by limiting p100 availability, basically abrogating BAFF-R signaling via the alternative NF-κB pathway. This finding (ie, that KLF3 transgenic CD19-deficient mice have MZ B cells) indicates that KLF3 might itself act downstream of alternative NF-κB signaling. We therefore tested whether KLF3 expression would overrule defects in BAFF-R signaling. BAFF and BAFF-R deficiency or sequestering BAFF by expression of a soluble decoy receptor (soluble BAFF-R or TACI-Ig) dramatically reduced mature B-cell numbers. As demonstrated previously, on bcl-2 expression, follicular B-cell numbers were restored in the absence of BAFF signaling. Yet, in this situation, MZ B cells specifically remained absent.15,16 Adding the KLF3 transgene on top of the Eμ:bcl-2 transgene in TACI-Ig transgenic mice restored the proportion and number of MZ B cells back to levels of normal mice (Figure 5A,C). Indeed, as defined by histology, the KLF3 transgene led to the reappearance of B cells in the MZ of these mice (Figure 5B). Together, these data demonstrate that KLF3 expression does overcome the developmental block in MZ B-cell differentiation specifically caused by the absence of BAFF signaling.
KLF3 expression overcomes the MZ B-cell developmental arrest caused by a lack of BAFF, but not by Notch-2 deficiency, and does not alter expression of various surface markers. In the absence of BAFF, KLF3 complements bcl-2 to induce MZ B-cell development: (A) Splenocytes from mice of the indicated genotypes were stained for CD19, CD93, CD21/35, and CD23, and analyzed by FACS as shown. (B) Cryosections of similar mice were stained for Moma-1, CD19, and T-cell receptor-β and analyzed by immunofluorescent microscopy. (C) Cell numbers of follicular and MZ B cells in mice of the indicated genotypes (± SD). FACS data derive from 2 mice of each genotype; histologic analysis was done comparing 2 mice each of CD19:KLF3 or nontransgenic TACI-Ig, Eμ:bcl-2 mice. MZ B cells in CD19:KLF3 mice depend on Notch-2: (D) Spleen cells from mice of the indicated genotypes were stained for B220, CD93, CD21/35, and CD23. Data shown are gated on B220+ CD93− cells. Proportions of follicular and MZ B cells are indicated. Two mice of each genotype were analyzed; a similar analysis was performed by pharmacologic inhibition of Notch-2 signaling (DBZ, 0.4 μmol intraperitoneally for 5 consecutive days, analysis on day 6), resulting in more than 10-fold reduction of MZ B cells both in proportion and absolute number, relative to carrier-treated controls (n = 2 each, data not shown). (E) Histograms showing expression of CD18 (LFA-1), CD62L, CD9 (MZ3), or CD24 (HSA) by spleen follicular and MZ B cells of CD19:KLF3 and normal mice. Analysis was performed twice independently, using in total more than or equal to 3 mice of each genotype. Micrographs were obtained with an Apotome microscope, using a 10×/0.45 NA objective and the MosaiX Axiovision software module.
KLF3 expression overcomes the MZ B-cell developmental arrest caused by a lack of BAFF, but not by Notch-2 deficiency, and does not alter expression of various surface markers. In the absence of BAFF, KLF3 complements bcl-2 to induce MZ B-cell development: (A) Splenocytes from mice of the indicated genotypes were stained for CD19, CD93, CD21/35, and CD23, and analyzed by FACS as shown. (B) Cryosections of similar mice were stained for Moma-1, CD19, and T-cell receptor-β and analyzed by immunofluorescent microscopy. (C) Cell numbers of follicular and MZ B cells in mice of the indicated genotypes (± SD). FACS data derive from 2 mice of each genotype; histologic analysis was done comparing 2 mice each of CD19:KLF3 or nontransgenic TACI-Ig, Eμ:bcl-2 mice. MZ B cells in CD19:KLF3 mice depend on Notch-2: (D) Spleen cells from mice of the indicated genotypes were stained for B220, CD93, CD21/35, and CD23. Data shown are gated on B220+ CD93− cells. Proportions of follicular and MZ B cells are indicated. Two mice of each genotype were analyzed; a similar analysis was performed by pharmacologic inhibition of Notch-2 signaling (DBZ, 0.4 μmol intraperitoneally for 5 consecutive days, analysis on day 6), resulting in more than 10-fold reduction of MZ B cells both in proportion and absolute number, relative to carrier-treated controls (n = 2 each, data not shown). (E) Histograms showing expression of CD18 (LFA-1), CD62L, CD9 (MZ3), or CD24 (HSA) by spleen follicular and MZ B cells of CD19:KLF3 and normal mice. Analysis was performed twice independently, using in total more than or equal to 3 mice of each genotype. Micrographs were obtained with an Apotome microscope, using a 10×/0.45 NA objective and the MosaiX Axiovision software module.
Finally, we addressed the requirement for Notch-2 signaling for the formation of MZ B cells in KLF3 transgenic mice. Dependency on Notch signaling would be an affirmation that the increased MZ B-cell population observed in CD19:KLF3 mice by phenotype and location represents bona fide MZ B cells. We first used DBZ [(s,s)-2-[2-(3,5-difluorophenyl)-acetylamino]-N-(5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-propionamide], a pharmacologic inhibitor of γ-secretases, to abolish Notch signaling. Indeed, 5 days of treatment led to the complete disappearance of MZ B cells in the spleen of normal and KLF3 transgenic mice (not shown). To rule out that this was caused by side effects of the drug, we also bred the KLF3 transgene into mice harboring one Notch-2fl allele, such that they were otherwise normal or heterozygous for CD19cre. As observed in Notch-2 heterozygous KO mice before, Notch-2fl/wt CD19cre mice already showed a pronounced reduction in MZ B cells, indicating a form of Notch-2 haploinsufficiency.34 Interestingly, this effect was somewhat ameliorated when mice were KLF3 transgenic (Figure 5D, compare top and middle panels). This is consistent with the previously reported synergy between Notch-2 and NF-κB signaling for the formation of MZ B cells.35 However, once both Notch-2 alleles were deleted, no MZ B cells remained, irrespective of the presence of the KLF3 transgene (Figure 5D bottom panels). Taken together, these results indicate that Notch-2 signaling is essential for the formation and maintenance of MZ B cells in normal and CD19:KLF3 mice.
MZ B cells in CD19:KLF3 mice are similar to normal MZ B cells
Whereas MZ B cells in CD19:KLF3 mice appeared normal by both gene signature and the requirement for Notch-2 signaling, we further tested whether these cells are equivalent to normal MZ B cells. We found that the expression of CD18 (LFA-1), CD9 (MZ3), CD24 (HSA), CD1d, CD36, and CD62L was identical among transgenic and nontransgenic follicular and MZ B cells (Figure 5E; and data not shown). Among follicular B cells, there was an increased proportion of cells expressing lower levels of CD62L in transgenic animals, but the peak expression level was unchanged. The “tail” of CD62Llo cells in transgenic mice may be the result of an enrichment of cells intermediate between follicular and MZ B-cell phenotype.
A hallmark of MZ B cells is their prompt T-independent activation in vivo. Thus, after triggering by bacterial products, such as LPS, they down-regulate S1P1 and S1P3 and migrate into the follicle, following the CXCL13 chemokine gradient sensed by CXCR5.12,14 Indeed, similar to normal MZ B cells, KLF3 transgenic cells moved rapidly into the follicle upon LPS stimulation (Figure 6A), indicating that chemokine driven migration of transgenic MZ B cells toward CXCL13 does take place as for nontransgenic cells. In addition, in vitro the ability of transgenic MZ B cells to sense and migrate toward CXCL13 or S1P was similar to normal MZ B cells (Figure 6B).
Intact MZ B-cell migration in vivo and in vitro. (A) Intact B-cell chemotaxis toward CXCL13 in vivo: After LPS stimulation, CD19:KLF3 transgenic MZ B cells move into the B-cell follicle. Migration was induced by injecting 50 μg LPS (Fluka) in 200 μL of phosphate-buffered saline intraperitoneally.14 Mice were killed for analysis 3 hours later, and spleen cryosections were stained for Moma-1, IgM, and IgD. (A) Micrographs were obtained with an Apotome microscope, using a 10×/0.45 NA objective and the MosaiX Axiovision software module. (B) The proportion of MZ B cells migrating toward CXCL13 or S1P in vitro is the same for normal and KLF3 transgenic cells. Experiments were performed in duplicate or quadruplicate, respectively. Results were obtained from 2 independent experiments. Spontaneous migration was variable between experiments but always less than or equal to 10%. (C) Preestablished CD19:KLF3 MZ B cells are sessile and resistant to replacement by normal cells: B6 and B6.Ly-5.1 or CD19:KLF3 and B6.Ly-5.1 mice were parabiosed 3 weeks before analysis. The chimerism among the indicated lymphocyte subset was evaluated using FACS (CD4 T cells: CD3+ CD4+; CD8 T cells: CD3+ CD8+; follicular B cells: CD19+ CD21+ CD23+; MZ B cells: CD19+ CD21hi CD23−). Graphs depict the chimerism among lymphocyte subsets in B6.Ly-5.1 mice (right panels) that were parabiosed with B6 mice (bottom left panel) or CD19:KLF3 mice (top left panel). Results derived from 2 independent experiments are shown (using 11 and 10 parabiotic couples, respectively).
Intact MZ B-cell migration in vivo and in vitro. (A) Intact B-cell chemotaxis toward CXCL13 in vivo: After LPS stimulation, CD19:KLF3 transgenic MZ B cells move into the B-cell follicle. Migration was induced by injecting 50 μg LPS (Fluka) in 200 μL of phosphate-buffered saline intraperitoneally.14 Mice were killed for analysis 3 hours later, and spleen cryosections were stained for Moma-1, IgM, and IgD. (A) Micrographs were obtained with an Apotome microscope, using a 10×/0.45 NA objective and the MosaiX Axiovision software module. (B) The proportion of MZ B cells migrating toward CXCL13 or S1P in vitro is the same for normal and KLF3 transgenic cells. Experiments were performed in duplicate or quadruplicate, respectively. Results were obtained from 2 independent experiments. Spontaneous migration was variable between experiments but always less than or equal to 10%. (C) Preestablished CD19:KLF3 MZ B cells are sessile and resistant to replacement by normal cells: B6 and B6.Ly-5.1 or CD19:KLF3 and B6.Ly-5.1 mice were parabiosed 3 weeks before analysis. The chimerism among the indicated lymphocyte subset was evaluated using FACS (CD4 T cells: CD3+ CD4+; CD8 T cells: CD3+ CD8+; follicular B cells: CD19+ CD21+ CD23+; MZ B cells: CD19+ CD21hi CD23−). Graphs depict the chimerism among lymphocyte subsets in B6.Ly-5.1 mice (right panels) that were parabiosed with B6 mice (bottom left panel) or CD19:KLF3 mice (top left panel). Results derived from 2 independent experiments are shown (using 11 and 10 parabiotic couples, respectively).
In rats, MZ B cells are sessile and do not emigrate out of the spleen.36 To determine whether normal and CD19:KLF3 murine MZ B cells share this property, we performed parabiosis experiments and evaluated the chimerism in the various lymphocyte populations (ie, for a given cell population, the proportion of cells originating from the contralateral parabiont). As expected, 3 weeks after parabiosis of B6 B6.Ly-5.1 mice, the chimerism was close to 50% among CD4 T cells, CD8 T cells, and follicular B cells (Figure 6C bottom panels). This was also the case in the CD19:KLF3 B6.Ly-5.1 parabiosis pairs for the T-cell populations (Figure 6C top panels). The slightly lower chimerism observed for follicular B cells in B6.Ly-5.1 mice parabiosed with CD19:KLF3 mice compared with those parabiosed with nontransgenic B6 mice (Figure 6C right panels) can be explained by the somewhat decreased absolute number of follicular B cells in CD19:KLF3 mice (Figure 1B). For MZ B cells, these experiments confirmed that the exchange of normal MZ B cells from B6 mice to the contralateral B6.Ly-5.1 parabiont, and vice versa, is very low after 3 weeks of parabiosis with only 10% to 30% of MZ B cells originating from the contralateral parabiont (Figure 6C lower panels). This shows that MZ B cells are static and do not exchange well via the bloodstream. Analysis of the chimerism among MZ B cells in parabiosed KLF3 transgenic mice revealed that Ly-5.1 cells cannot establish as efficiently as they do in nontransgenic animals (Figure 6C left panels, 2.2-fold less). This suggests that CD19:KLF3 MZ B cells are well adapted to their niche, persist in situ, and do not emigrate. This is confirmed by the result obtained from the contralateral B6.Ly-5.1 parabiont: the chimerism among MZ B cells was identical whether parabiosis was with B6 or CD19:KLF3 mice; thus, it was independent of the genotype of the contralateral parabiont (Figure 6C right panels). As total MZ B-cell numbers are increased in CD19:KLF3 animals (Figure 1B), the rate of immigration of transgenic MZ B cells into the B6.Ly-5.1 spleen is far less than expected. Thus, on a per-cell basis, CD19:KLF3 MZ B cells appear as or even more sessile than normal MZ B cells. Because the chimerism observed in the various B-cell compartments in the spleen of B6.Ly-5.1 animals parabiosed with normal or KLF3 transgenic mice was relatively similar, we also conclude that the rate of production of B2 cells is not modified in KLF3 transgenic mice.
In vitro, KLF3 transgenic and nontransgenic B cells are equivalent
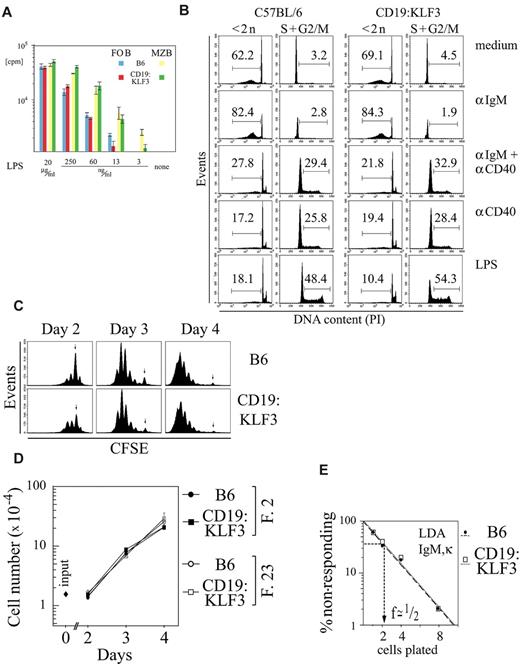
In normal mice, MZ B cells are hyperresponsive to LPS relative to follicular B cells. Indeed, MZ B cells from KLF3 transgenic mice were also more sensitive to LPS stimulation, while transgenic follicular B cells were similar in their LPS responsiveness to follicular B cells from normal mice (Figure 7A). This is unlike in c-fos transgenic and PTEN-deficient mice that both show increased numbers of MZ B cells, but in which all B cells responded equally to LPS or demonstrated a general hyperproliferative state, respectively.37-39
Similar proliferative responsiveness of FO and MZ B cells of normal and KLF3 transgenic mice in vitro. (A) FO and MZ B cells were isolated from the spleens of nontransgenic and KLF3 transgenic littermates using magnetic bead enrichment and cell sorting based on CD21/35, CD23, and CD19 staining. A total of 2 × 104 cells were cultured in 0.2 mL medium to which graded amounts of LPS were added as indicated. 3H-Thymidine (0.5 μCi/well) was added on day 2, and incorporated radioactivity was measured on day 3. Assays were performed in triplicate; mean values and error bars (representing SDs) are shown. (B) DNA G1/G2/S profiles at 42 hours after stimulation by anti-IgM, anti-CD40 + IgM, anti-CD40 alone, or LPS. (C) CFSE profiles on days 2 to 4 upon LPS stimulation of lymph node cells depleted of Thy-1.2+ cells. (D) Increase in cell numbers on days 2 to 4 on LPS stimulation of lymph node cells depleted of Thy-1.2+ cells. Cells were counted by FACS using beads of known concentration as reference. (E) Limiting dilution assay for the determination of the frequency of IgM,κ-secreting cells upon LPS stimulation on rat feeder cells. Graded amounts of Thy-1.2+ depleted lymph node cells were seeded onto 4 × 105 rat thymus feeder cells in 96-well plates. Mouse IgM,κ secretion was determined by enzyme-linked immunosorbent assay. Wells were counted positive if a given well gave an OD that differed by more than 3 SDs from the mean of negative wells. (A) One of 2 independent experiments is shown. (B-D) The experiment was performed in parallel with transgenic and nontransgenic littermate cells of 2 founder lines, giving similar results, and was repeated once. (E) The experiment was repeated once, giving similar results.
Similar proliferative responsiveness of FO and MZ B cells of normal and KLF3 transgenic mice in vitro. (A) FO and MZ B cells were isolated from the spleens of nontransgenic and KLF3 transgenic littermates using magnetic bead enrichment and cell sorting based on CD21/35, CD23, and CD19 staining. A total of 2 × 104 cells were cultured in 0.2 mL medium to which graded amounts of LPS were added as indicated. 3H-Thymidine (0.5 μCi/well) was added on day 2, and incorporated radioactivity was measured on day 3. Assays were performed in triplicate; mean values and error bars (representing SDs) are shown. (B) DNA G1/G2/S profiles at 42 hours after stimulation by anti-IgM, anti-CD40 + IgM, anti-CD40 alone, or LPS. (C) CFSE profiles on days 2 to 4 upon LPS stimulation of lymph node cells depleted of Thy-1.2+ cells. (D) Increase in cell numbers on days 2 to 4 on LPS stimulation of lymph node cells depleted of Thy-1.2+ cells. Cells were counted by FACS using beads of known concentration as reference. (E) Limiting dilution assay for the determination of the frequency of IgM,κ-secreting cells upon LPS stimulation on rat feeder cells. Graded amounts of Thy-1.2+ depleted lymph node cells were seeded onto 4 × 105 rat thymus feeder cells in 96-well plates. Mouse IgM,κ secretion was determined by enzyme-linked immunosorbent assay. Wells were counted positive if a given well gave an OD that differed by more than 3 SDs from the mean of negative wells. (A) One of 2 independent experiments is shown. (B-D) The experiment was performed in parallel with transgenic and nontransgenic littermate cells of 2 founder lines, giving similar results, and was repeated once. (E) The experiment was repeated once, giving similar results.
MZ B cells may be considered preactivated, as they show some features of activated cells (eg, being enlarged relative to naive follicular B cells). Thus, a general increase in the ability to proliferate may favor MZ B-cell development. KLF3 was isolated as proviral integration site in a tumor cell line, suggesting that it may promote growth or limit apoptosis.40 However, when we tested KLF3 transgenic follicular B cells from lymph nodes (to avoid variable contamination with MZ B cells) for growth abnormalities in vitro, we did not detect significant differences. As defined by proliferation/apoptosis induction after BCR stimulation, in the presence or absence of anti-CD40, by anti-CD40 alone, or by stimulation with LPS, no major differences were observed comparing normal and transgenic lymph node B cells (Figure 7B). When cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), a small difference in the fraction of nonproliferating cells was indeed visible at day 2 after LPS stimulation (Figure 7C), and this may correspond to the slight decrease in the proportion of cells undergoing apoptosis (18.1% vs 10.4% at 42 hours; Figure 7B). However, this difference could not be detected in the CFSE at later time points. In addition, absolute numbers of cells that accumulated over time in such cultures (Figure 7D) and the frequency of LPS-responsive cells as measured by limiting dilution (Figure 7E) were similar for normal and transgenic cells. Taken together, deregulated expression of KLF3 does not affect proliferation/apoptosis in B cells.
Discussion
Here we demonstrate that the transcription factor KLF3 drives the formation of MZ B cells. We identified a KLF3-regulated set of genes by determining the differential gene expression pattern in normal versus KLF3 transgenic follicular B cells. Most interestingly, this set overlaps extensively with those genes that are differentially regulated between normal MZ and follicular B cells (Figure 3C; supplemental Table 1). Apparently, the gene regulation exerted by KLF3 is sufficient to favor MZ B-cell differentiation, as its deregulated expression leads to considerably increased MZ B-cell numbers. Further, this driving force of KLF3 is sufficient to overcome several genetic defects normally associated with MZ B-cell paucity. Finally, we show that KLF3-induced MZ B cells represent bona fide MZ B cells by several in vivo and in vitro criteria.
BAFF, a member of the TNF family, is an essential cytokine ensuring B-cell survival. It is also essential for the generation of MZ B cells, as in its absence complementation with bcl-xL or bcl-2 only rescues follicular, but not MZ B cells. Here we show that, by expressing KLF3, MZ B cells were rescued in this situation. However, KLF3 itself could not sustain follicular or MZ B cells in the absence of BAFF. Thus, KLF3 has a clear maturation-inducing ability that complements genes controlling survival. Both need to act downstream of BAFF-R to allow for MZ B-cell maturation. BAFF-R-mediated cell growth and survival are a complex process that apparently requires the contribution of both the alternative NF-κB pathway and mTORC1 pathways downstream of BAFF-R.41,42 In contrast, BAFF-R-mediated differentiation to MZ B cells would only require the alternative NF-κB pathway. Indeed, in p100 knock-out mice that still express the p52 subunit, the MZ B-cell compartment is increased because of constitutive alternative NF-κB signaling.43 Therefore, it is possible that KLF3 acts as a downstream target of BAFF-mediated NF-κB signaling, below the bifurcation of survival and differentiation inducing branches. Indeed, KLF3 was among the specific targets isolated in a microarray screen for NF-κB target genes performed in pre-B cells.44 We have also shown that KLF3 expression restored MZ B cells in CD19-deficient mice. It is conceivable that here KLF3 acts in the same way as for complementation of BAFF-deficiency, namely, complementing alternative NF-κB signaling: BCR signaling in maturating peripheral B cells leads to sustained expression of c-Rel, a component of the canonical NF-κB pathway, which in turn increases expression of BAFF-R and p100.45 p100 then can be processed within the alternative NF-κB pathway triggered by BAFF.17,20 Thus, ablation of BCR “costimulatory” molecules such as CD19 could lead to an absence of MZ B cells because of insufficient activation of alternative NF-κB signaling within MZ B cell(s)/precursors. As a possible downstream target of alternative NF-κB signaling, constitutive KLF3 expression did overcome this defect.
Somewhat counterintuitively, KLF3 expression drives MZ B-cell differentiation, as shown here, whereas it is actually normally being down-regulated in MZ B cells33 (current results). We interpret this such that KLF3 is a relevant factor in the initiation of MZ B-cell differentiation but is superfluous thereafter, or may only be transiently expressed in a fraction of MZ B cells. This is supported by the fact that KLF3-deficient mice had less MZ B cells. The expression of other KLF-family members KLF2, KLF4, and KLF6, which were differentially regulated in MZ B cells, was not affected by the KLF3 transgene. This excludes that KLF3 acts by regulating those factors. Yet, regulation within the KLF family has been observed previously (eg, KLF1 activating KLF3, KLF3 regulating KLF8, or KLF2 autoregulation).46 Here we show that KLF3 expression is also regulated via a feedback loop, as in KLF3 transgenic animals endogenous KLF3 mRNA and protein expression was dramatically down-regulated in B cells (supplemental Figure 1B; and data not shown). Indeed, KLF3 protein did not accumulate much higher in B cells of CD19:KLF3 mice than endogenous KLF3 does in normal mice. However, as the temporal pattern of KLF3 expression will be dysregulated in transgenic B cells, this may constitute the most relevant difference.
For T cells, KLF2 has been shown to influence migration/homing by directly altering expression of S1P1 and CD62L.4,47-49 By FACS, expression of CD62L is down-regulated in MZ B cells. However, the KLF3 transgene did not change the expression level of CD62L within follicular or MZ B cells, ruling against KLF3 regulating CD62L expression. Regarding S1P1, there is apparently normal expression on transgenic MZ B cells as they can follow the S1P gradient to take residency in the MZ. Moreover, upon LPS injection, S1P1 expression appears to be down-regulated as in normal mice because both normal and KLF3 transgenic MZ B cells moved into the follicle. Thus, in B cells, these genes do not appear to be regulated by KLF3 only.
Recently, Hoek et al observed that KLF2-deficiency resulted in a similar increase in MZ B cells as seen here.50 Thus, KLF2 and KLF3 may constitute transcriptional antagonists, as the lack of KLF2 or increased KLF3 expression leads to similar outcomes. However, KLF3 transgenic follicular B cells showed normal KLF2 expression. In addition, the results on KLF2-deficient B cells differ from those in CD19:KLF3 mice in several aspects: KLF2 deficiency not only increased MZ B cells but also follicular B-cell numbers. In addition, all major functional differences reported for KLF2-deficient cells were among follicular B cells.50 For example, migration of KLF2-deficient follicular B cells toward CXCL13 was affected, whereas we observed no such difference for KLF3 transgenic cells (not shown). Thus, KLF2 may regulate target genes that are indeed more relevant to follicular B cells. Of note, B1 B cells in the peritoneum were lacking in KLF2-deficient mice, whereas the KLF3 transgene favored these cells, even when in competition with normal cells. Thus, KLF2 and KLF3 may act in a rather complex transcriptional regulatory network, as proposed for KLFs in general.46
Note added in proof:
While the revision of this paper was under review, 2 studies reported on the B-cell phenotype of KLF2-deficient mice: Winkelmann R, Sandrock L, Porstner M, et al. B cell homeostasis and plasma cell homing controlled by Kruppel-like factor 2. Proc Natl Acad Sci U S A. 2011;108(2):710-715. Hart GT, Wang X, Hogquist KA, Jameson SC. Kruppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proc Natl Acad Sci U S A. 2011;108(2):716-721.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J.-P. Dangy, Y. Joos, D. Schwarzkopf, and N. Sawyer for technical assistance; U. Müller for transgenesis; A. Würch, L. Lejeune, and A. Gosselin for cell sorting; the animal caretakers in Basel, Freiburg, Montréal (S. Vincent and L. Thibault), and Sydney for help; J. Kearney and W. Schuh for antibodies; R. Ceredig and T. Defrance for comments on the manuscript; S.C. Jameson and H.-M. Jäck for sharing unpublished results; H. Acha-Orbea for hosting J. Kirberg in Lausanne; and P. Ancuta, N.H. Shoukry, and R.P. Sekaly for supporting F.A. in Montreal.
This work is submitted in partial fulfillment of the requirement for the PhD thesis of G.T.; parts of this study were submitted to the Faculty of Biology, University of Freiburg, Freiburg, Germany.
Authorship
Contribution: G.T. designed and performed research, analyzed and interpreted data, and started a draft of the manuscript; T.T.V., F.F., J. Kranich, S.S., M.A., J.-B.L., J.-P.G., and R.P. designed and performed research and analyzed data; M.C., R.P., U.Z.-S., and P.S. provided essential tools; M.C., J.B., and J. Kirberg initiated the study; and F.A. and J. Kirberg designed and performed research, analyzed and interpreted data, supervised the research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jörg Kirberg, Paul-Ehrlich-Institut, Paul-Ehrlich-Strasse 51-59, D-63225 Langen, Germany; e-mail: kirjo@gmx.de or joerg.kirberg@pei.de.
References
Author notes
F.F., J.K., and S.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal