Abstract
B lymphopoiesis arrests in rabbits by 4 months of age. To identify molecules that contribute to this arrest, cDNA–representational difference analysis on BM stromal cells from young and adult rabbits showed that expression of Postn that encodes for the extracellular matrix protein periostin dramatically reduced with age. Postn–small interfering RNA OP9 cells lost their capacity to support B-cell development from rabbit or murine BM cells, and reexpression of periostin restored this potential, indicating an in vitro requirement for periostin in B lymphopoiesis. In our system, we determined that periostin deficiency leads to increased cell death and decreased proliferation of B-lineage progenitors. Further, RGD peptide inhibition of periostin/αvβ3 interaction resulted in a marked decrease in B lymphopoiesis in vitro. Microarray analysis of the Postn–small interfering RNA OP9 cells showed decreased expression of key B-lymphopoietic factors, including IL-7 and CXCL12. In vivo, unidentified molecule(s) probably compensate periostin loss because Postn−/− mice had normal numbers of B-cell progenitors in BM. We conclude that the decline in periostin expression in adult rabbit BM does not solely explain the arrest of B lymphopoiesis. However, the interaction of periostin with αvβ3 on lymphoid progenitors probably provides both proliferative and survival signals for cells in the B-cell development pathway.
Introduction
The differentiation and proliferation of B-lineage cells in the BM is orchestrated internally by a set of nonredundant transcription factors that are crucial for B-cell development.1 B-cell progenitors also require fine-tuned interactions with the supportive BM stroma and extracellular matrix (ECM) molecules that create specialized microenvironments, or niches, within the BM. These niches provide both nurturing cell contacts and cytokines that guide all of the hematopoietic cell differentiation programs.2 The ECM molecules, including periostin, fibronectin, laminin, and proteoglycans, are ligands of integrins that not only control anchorage, spreading, and migration of hematopoietic cells but also induce signal transduction pathways in these cells.3-5
Unlike mice and humans, rabbits generate high levels of new B-cell precursors only until a few weeks of age, after which time B lymphopoiesis gradually wanes; by 4 months of age, essentially no new pro–B or pre–B-cell precursors are found in the BM.6 Lymphoid progenitors are present in the BM of adult rabbits as evidenced by the finding that total BM cells from adults differentiated into B-cell precursors after adoptive transfer into young recipients and also that rabbit lymphoid progenitors differentiated into B- and T-cell precursors when cocultured with OP9 or OP9-DL1 stromal cells.7 These data suggested to us that the suppression of B lymphopoiesis in the BM of adult rabbits might be mediated through age-related changes in the BM environment. To investigate this hypothesis, a cDNA representational difference analysis (cDNA-RDA) was performed to identify genes down-regulated in BM stromal cells from adults compared with BM stromal cells from young rabbits. We found several ECM molecules, including periostin, whose expression was down-regulated in adult rabbit stroma. Periostin, a secreted ECM protein of poorly defined function, was originally identified in cells of the mesenchymal lineage, leading to suggestions that it contributed to bone development. Periostin has also been implicated in heart development (reviewed in Hoersch and Andrade-Navarro8 ). Using the murine OP9 stromal cell system, we show in vitro that periostin is required for B lymphopoiesis in mouse and rabbit.
Methods
Animals
Periostin (perilacZ knockin) knockout mice were as described9 ; all animal experimentation was performed in accordance with guidelines from the National Institutes of Health, and protocols were approved by the respective Institutional Animal Care and Use Committees of all participating institutions.
Antibodies and reagents
Antibodies used in this study are listed in Table 1. Reagents used are LIVE/DEAD cell stain Kit (Invitrogen); 7-amino-actinomycin D (7-AAD; BD Bioscience); Annexin V (BD Bioscience); CFSE (Invitrogen); RGD-peptide cyclo (Arg-Gly-Asp-DPhe-Lys; PCI-3661-PI; Peptide International); RGD-peptide cyclo (Arg-Gly-Asp-DPhe-Lys; PCI-3661-PI; Peptide International).
Antibodies used in this study
| Anti-CD79a (BD clone HM47) . | Anti–ms-Ter119 (clone Ter-119) . |
|---|---|
| Biotin-labeled Anti–rb-IgG (clone C101-167; BD Bioscience) | Anti–ms-CD49b (clone DX5) |
| Anti–ms-B220 (clone RA3-6B2) | |
| Anti–ms-CD3 (clone RA3-6B2) | |
| Anti–ms-CD8 (H35-17.2) | |
| Anti–ms-Gr-1 (clone RB6-8C5) | |
| Anti–ms-CD11b (clone M1/70) | |
| Anti–ms-Sca1 (clone D7) | |
| Anti–ms-c-Kit (clone ACK2) | |
| Anti–ms-CD51/CD61 (clone LM609) | |
| Anti–ms-IgM (clone R6-8Cs) | |
| Anti–ms-CD43 (clone R2/60) | |
| Anti–ms-Cd19 (clone 1D3) | |
| Anti–ms-periostin (gift of Dr LanBo Chen, Harvard University) | |
| Anti-6xHis (clone G-18) | |
| Anti-actin (clone AC-15) | |
| Rabbit IgG anti–ms-Cxcl12 (polyclonal) | |
| Anti–ms-CD29 (clone KM16) | |
| Anti–ms-MHC-I (clone 24-1-4-8) |
| Anti-CD79a (BD clone HM47) . | Anti–ms-Ter119 (clone Ter-119) . |
|---|---|
| Biotin-labeled Anti–rb-IgG (clone C101-167; BD Bioscience) | Anti–ms-CD49b (clone DX5) |
| Anti–ms-B220 (clone RA3-6B2) | |
| Anti–ms-CD3 (clone RA3-6B2) | |
| Anti–ms-CD8 (H35-17.2) | |
| Anti–ms-Gr-1 (clone RB6-8C5) | |
| Anti–ms-CD11b (clone M1/70) | |
| Anti–ms-Sca1 (clone D7) | |
| Anti–ms-c-Kit (clone ACK2) | |
| Anti–ms-CD51/CD61 (clone LM609) | |
| Anti–ms-IgM (clone R6-8Cs) | |
| Anti–ms-CD43 (clone R2/60) | |
| Anti–ms-Cd19 (clone 1D3) | |
| Anti–ms-periostin (gift of Dr LanBo Chen, Harvard University) | |
| Anti-6xHis (clone G-18) | |
| Anti-actin (clone AC-15) | |
| Rabbit IgG anti–ms-Cxcl12 (polyclonal) | |
| Anti–ms-CD29 (clone KM16) | |
| Anti–ms-MHC-I (clone 24-1-4-8) |
All antibodies were purchased from eBioscience, Inc unless otherwise stated.
rb indicates rabbit; and ms = mouse.
cDNA-RDA
Tissue culture flasks were seeded with 25 × 106 nucleated BM cells from newborn and 1-year-old rabbits in 5 mL of α-MEM. Nonadherent cells were removed after 24-48 hours, and stromal cells were passaged after reaching ∼ 90%-100% confluence. On the third passage, RNA was isolated with the use of CsCl (Sigma-Aldrich) gradient centrifugation, and cDNA-RDA was performed.10 First difference products were cloned into pGEMT (Promega) and identified by National Center for Biotechnology Information BLASTn.
End point, semiquantitative, and quantitative RT-PCR
Primers used are listed in Table 2. Total cellular RNA was isolated with an RNeasy kit (QIAGEN) and reverse-transcribed with SuperScript III reverse transcriptase (Invitrogen). For quantitative (q) PCR a total volume of 20 μL with ∼ 6 ng of cDNA template and 0.5 μL of each primer and SYBR Green PCRMaster Mix (Applied Biosystems) was analyzed in triplicate. Gene expression was analyzed with an ABI Biosystems 7300 Real-Time PCR Sequence Detector and ABI Prism Sequence Detection Software (Applied Biosystems). Results were normalized to a housekeeping gene control as described in each experiment. For semiquantitative (sq) RT-PCR, the thermocycler was paused after 28, 30, 32, and 34 cycles, and at each time point a 15-μL aliquot of the PCR product was removed and resolved on a 5% polyacrylamide gel. Visualization was performed on a Typhoon imaging system (GE Healthcare).
PCR Primers used in this study
| . | Primers . |
|---|---|
| End point PCR | |
| Mouse Postn | 5′-ATTGGTACCTCCATCCAAGACT-3′ |
| 5′-TGTGTCAGGAGATCTTTCAAAT-3′ | |
| Gapdh control | 5′-TGAGTATGTCGTGGAGTCTACTGG-3′ |
| 5′-GTGGCAGTGATGGCATGGAC-3′. | |
| sqRT-PCR | |
| Rabbit Postn | 5′-CATGAACAGATCTCTTGACACAA-3′ |
| 5′-TCCAATGGGTATGTCTGCT-3′ | |
| Gapdh control | 5′-CATCACTGCCACCCAGAAGA-3′ |
| 5′-CCTGCTTCACCACCTTCTTGA-3′ | |
| qRT-PCR | |
| Mouse IL-7 | 5′-GGCAGGTTGTGCAAACCAGTAACA-3′ |
| 5′-AAAGGAAGCCTCTGTTCCCATGTGT-3′ | |
| Mouse CXCL12 | 5′-ACACTCCAAACTGTGCCCTTCAGA-3′ |
| 5′-AAGAGGCTCAAGATGTGAGAGGCT-3′ | |
| Mouse Postn | 5′-AGGCAAACAGCTCAGAGTCTTCGT-3′ |
| 5′-ATGGAGGGATTTCTCTGCTGGCTT-3′ | |
| Hgprt control | 5′-GTCAACGGGGGACATAAAAG-3′ |
| 5′-TGGGGCTGTACTGCTTAACC-3′ | |
| Postn mutagenesis primers* | 5′-GCTGCCCCGCAGTCATGCCTATA' |
| GATCATGTTTATGGCAC-3′ | |
| 5′-GTGCCATAAACATGATCTATAGG' | |
| CATGACTGCGGGGCAGC-3′ |
| . | Primers . |
|---|---|
| End point PCR | |
| Mouse Postn | 5′-ATTGGTACCTCCATCCAAGACT-3′ |
| 5′-TGTGTCAGGAGATCTTTCAAAT-3′ | |
| Gapdh control | 5′-TGAGTATGTCGTGGAGTCTACTGG-3′ |
| 5′-GTGGCAGTGATGGCATGGAC-3′. | |
| sqRT-PCR | |
| Rabbit Postn | 5′-CATGAACAGATCTCTTGACACAA-3′ |
| 5′-TCCAATGGGTATGTCTGCT-3′ | |
| Gapdh control | 5′-CATCACTGCCACCCAGAAGA-3′ |
| 5′-CCTGCTTCACCACCTTCTTGA-3′ | |
| qRT-PCR | |
| Mouse IL-7 | 5′-GGCAGGTTGTGCAAACCAGTAACA-3′ |
| 5′-AAAGGAAGCCTCTGTTCCCATGTGT-3′ | |
| Mouse CXCL12 | 5′-ACACTCCAAACTGTGCCCTTCAGA-3′ |
| 5′-AAGAGGCTCAAGATGTGAGAGGCT-3′ | |
| Mouse Postn | 5′-AGGCAAACAGCTCAGAGTCTTCGT-3′ |
| 5′-ATGGAGGGATTTCTCTGCTGGCTT-3′ | |
| Hgprt control | 5′-GTCAACGGGGGACATAAAAG-3′ |
| 5′-TGGGGCTGTACTGCTTAACC-3′ | |
| Postn mutagenesis primers* | 5′-GCTGCCCCGCAGTCATGCCTATA' |
| GATCATGTTTATGGCAC-3′ | |
| 5′-GTGCCATAAACATGATCTATAGG' | |
| CATGACTGCGGGGCAGC-3′ |
Inserted mutations are underlined.
CFSE labeling
Cells (106) were washed, resuspended in 1 mL of 1× PBS, and CFSE was added to a final concentration of 5μM. The cell suspension was then mixed and incubated for 10 minutes at 37°C. Cold cell culture medium (1 mL) was added and incubated for 1 minute at room temperature. Cells were washed twice with PBS and counted.
Stromal cell cultures
Murine OP9-DL1, OP9-GFP stromal cells11,12 and OP9-GFP variants transfected with small interfering RNA (siRNA) GFP or Postn constructs were seeded at ∼ 10 000 OP9 cells/well in 96-well plates. After 24 hours, the OP9 cells were overlaid with ∼ 10 000 total rabbit or mouse BM cells. Initially, most of the BM cells died; however, after 1 week, stromal-associated cell clusters became visible. Growth of nonadherent cells (B-lineage cells) in the cocultures was monitored by harvesting the cells, straining them (70-μm cell strainer; BD Biosciences) to remove loose feeder cells, and quantifying them using trypan blue exclusion. Cells arising from the cocultures were fixed and permeabilized (Cytofix/Perm; BD PharMingen); rabbit cells were stained with anti-CD79a to identify B-lineage cells, and murine cells were stained with anti-B220 and anti-IgM to identify B-lineage cells, and with anti-CD3 to identify T-lineage cells. Stained cells were examined by flow cytometry with the use of FACSCanto (BD Biosciences); only cells that fell within a lymphocyte forward scatter versus side scatter gate were analyzed by FlowJo software (Version 7.2.2; TreeStar Inc). Cultures were routinely supplemented with IL-7 (10 ng/mL).
RGD-peptide inhibition
BM cocultured on OP9 cells were supplemented with RGD peptides at the concentrations stated. After 7 days in culture, inhibition of lymphopoiesis was assayed by cell enumeration with the use of trypan blue and by flow cytometry for analysis of differentiation.
Murine hematopoietic stem cells (Lin−Sca1+c-Kit+) and B-cell progenitors
BM cells were stained with a lineage cocktail (anti-Ter119, anti-CD49b, anti-B220, anti-CD3, anti-CD8; anti–Gr-1, and anti-CD11b), anti-Sca1 and anti–c-Kit to identify Lin−Sca1+c-Kit+ (LSK) cells. B-cell progenitors were identified as follows: CLPs were LSK–IL-7R+, pre-pro-B cells were B220+c-Kit−CD19−, pro-B cells were B220+CD43+CD19+, and pre-B cells were B220+CD43−CD19+. To identify cells expressing the αvβ3 receptor, cells were stained as above and also with anti-CD51/CD61 Ab. This monoclonal Ab (mAb) binds to a conformational epitope, resulting from the posttranslational association of the αv and β3 subunits. For coculture experiments, LSK αvβ3+ cells (3000-4000 cells/well) underwent FACS directly onto OP9 or OP9-DL1 cells.
siRNA transfection of OP9 stromal cells
OP9-GFP cells were transfected with GFP or murine Postn siRNA constructs. Two Postn siRNAs were designed (siRNAwizard; InvivoGen) with sequences 309 (GATGCCTATTGACCATGTTTA) and 2292 (GGTCTCCAAGGTCACAAAGTT), corresponding to the starting nucleotides, 309 and 2292, respectively. The siRNAs were cloned into psiRNA-h7Skzeo (InvivoGen), and stable siRNA transfectants of OP9-GFP cells were selected in zeocin. The siRNA for GFP was psiRNA-h7SKzE (InvivoGen). OP9 transfectants derived from Postn and GFP siRNAs were cloned by limiting dilution. DNA microarray analysis from one OP9-GFP siRNA clone and one OP9-OSF2 siRNA clone was performed in duplicate with Affymetrix Mouse Genome 430 2.0 Array, which included 45 K gene elements. Data were analyzed by the Affymetrix Microarray Suite Software (Version 5.0). All microarray data are deposited in ArrayExpress under accession no. E-MTAB-519.
Site-directed mutagenesis of Postn and transfection of OP9-309 cells
Silent mutations were introduced into murine His-tagged-Postn by PCR amplification of the pcDNA-Postn construct with the use of PCR primers shown in Table 2 (mutations are underlined). The PCR product was digested with DpnI restriction enzyme, gel-purified, and chemically transformed into XL1 Blue cells (Stratagene). Insertion of the desired mutations was confirmed by sequence analysis, and the mutated pcDNA-Postn construct was used to transfect OP9-309 cells. Stable clones were selected on neomycin (2 mg/mL). Reexpression of periostin was confirmed by staining with anti–ms-periostin and also by both sqRT-PCR and qRT-PCR.
Western blot analysis
Total cell lysates from 106 OP9-309 and OP9-309 cells transfected with mutated Postn were resolved by 7% SDS-PAGE and then transferred to an Immuno-Blot polyvinylidene difluoride membrane (Millipore). The blot was probed with anti-6xHis Ab and anti–actin Ab as a control.
Results
Age-related changes in gene expression in rabbit BM stromal cells
Throughout the lives of mice and humans, B cells are continuously generated in the BM.13 In contrast, B cells are produced in rabbits only during gestation and until ∼ 4 months after birth.6 Investigating the mechanism by which the observed B lymphopoiesis arrests in adult rabbits, Kalis et al7 showed that BM stroma from adult rabbits loses its potential to support proliferation and differentiation of HSCs into B-lineage cells. As a result of this finding, we searched for age-related differences in young and adult rabbit BM stromal cells. Stromal cells were cultured from BM of young and adult rabbits in α-MEM, and RDA was performed with cDNA from adult-derived stromal cells as the driver. We recovered 13 different cDNA clones and identified 8 by nucleotide sequence analysis and National Center for Biotechnology Information BLASTn search (Table 3). By sqRT-PCR we found that one of these molecules, Postn, which encodes for the ECM protein periostin, was robustly expressed in the BM of 2- and 4-week-old rabbits (Figure 1). Its expression began to wane soon thereafter, and by 8 months of age Postn expression was undetectable. The decline in expression of Postn after ∼ 4 weeks of age correlated with the timing of decreased B-cell precursor production in the BM. This initial decline in B lymphopoiesis is followed by a gradual waning of B-cell precursors that continues until 4 months of age, after which time, B lymphopoiesis is arrested.6 We therefore investigated whether reduced Postn expression plays a role in the decline and ultimate arrest of B lymphopoiesis.
Genes identified by cDNA-RDA of BM stromal cells from young and adult rabbits
| Clone no. . | Encoding for . | Function . |
|---|---|---|
| 1 | Periostin | ECM protein |
| 2 | Fibronectin | ECM protein |
| 3 | Collagen type I | ECM protein |
| 4 | Thrombospondin | ECM protein |
| 5 | Ubiquitin Ct hydrolase | Protein ubiquitination |
| 6 | FAT | Tumor suppressor (cell-cell interaction) |
| 7 | Intersectin | Clathrin mediated endocytosis |
| 8 | Frizzled 4 | Wnt signaling |
| Clone no. . | Encoding for . | Function . |
|---|---|---|
| 1 | Periostin | ECM protein |
| 2 | Fibronectin | ECM protein |
| 3 | Collagen type I | ECM protein |
| 4 | Thrombospondin | ECM protein |
| 5 | Ubiquitin Ct hydrolase | Protein ubiquitination |
| 6 | FAT | Tumor suppressor (cell-cell interaction) |
| 7 | Intersectin | Clathrin mediated endocytosis |
| 8 | Frizzled 4 | Wnt signaling |
Driver was cDNA from adult rabbit (2 years old); tester was cDNA from newborn rabbit.
Semiquantitative RT-PCR of expressed Postn in BM from young and adult rabbits. Aliquots were removed 4 times at 2-cycle intervals during the PCR and analyzed by PAGE. Age of rabbits was from 2 weeks to 2.5 years. Postn indicates periostin; Gapdh was used as a control. Results are representative of 2 experiments.
Semiquantitative RT-PCR of expressed Postn in BM from young and adult rabbits. Aliquots were removed 4 times at 2-cycle intervals during the PCR and analyzed by PAGE. Age of rabbits was from 2 weeks to 2.5 years. Postn indicates periostin; Gapdh was used as a control. Results are representative of 2 experiments.
In vitro inhibition of B lymphopoiesis by Postn siRNA
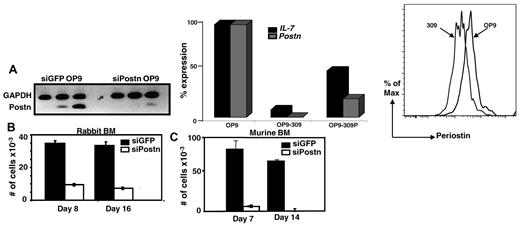
Previous in vitro studies have shown that the ECM protein collagen type I is required for hematopoiesis,14,15 and, similarly, fibronectin has been shown both in vitro and in vivo to promote proliferation of HSCs.16 Whether the ECM protein periostin is also important for hematopoiesis, specifically B lymphopoiesis, has not been investigated. We tested this possibility using the OP9 stromal cell coculture system that preferentially promotes B lymphopoiesis from BM cells.11 We stably transfected OP9-GFP cells with a Postn siRNA construct (no. 309) or a control GFP siRNA construct and found considerable reduction in the expression of Postn as determined by sqRT-PCR, qRT-PCR, and flow cytometry (Figure 2A left, middle, and right panels, respectively). To determine whether the Postn siRNA OP9 (OP9-309) cells retained the capacity to support B-cell development, we overlaid 10 000 total BM cells from adult rabbits on Postn siRNA OP9 and on control GFP siRNA OP9 cells and found that 1 and 2 weeks later the number of cells that grew out on the Postn siRNA OP9 cells was decreased 4- to 5-fold compared with those that grew out on the control GFP siRNA OP9 cells (Figure 2B). Similar results were obtained with a second OP9 variant transfected with the Postn siRNA construct no. 2292 (data not shown). As shown previously, the cells that grew out from rabbit BM were confirmed as B lineage on the basis of their expression of CD79a (Figure 5C in Kalis et al7 ). We repeated these experiments with the use of murine BM cells and found an 18-fold reduction in the number of cells arising from Postn siRNA OP9 cells compared with cells arising from the GFP siRNA OP9 cells (Figure 2C). The murine cells that developed on the GFP siRNA OP9 cells were confirmed by flow cytometry to be early B-lineage cells (B220+CD43+; data not shown). These data showed that enforced reduction of Postn expression in OP9 cells greatly inhibited their capacity to promote B-cell development from both rabbit and murine BM progenitors, suggesting that periostin is required for B lymphopoiesis in vitro.
Rabbit and murine BM cells cocultured with Postn siRNA OP9 or GFP siRNA OP9 cells. (A) SqRT-PCR for Postn in stable transfectants of GFP siRNA OP9 and Postn siRNA cells (left). QRT-PCR for Postn and IL-7 in OP9, OP9-309, and OP9-309P cells; expression levels were normalized to Hgprt housekeeping gene (similar results were obtained in 2 independent experiments; middle). Flow cytometric analysis of OP9 and OP9-309 cells stained with anti–periostin Ab (right). (B-C) Total number of nonadherent cells harvested 1 and 2 weeks after coculture of 10 000 total rabbit (B) and murine (C) BM cells with GFP siRNA OP9 and Postn siRNA OP9 (OP9-309) cells. Error bars are SEM from triplicate wells. Results are representative of 3 experiments.
Rabbit and murine BM cells cocultured with Postn siRNA OP9 or GFP siRNA OP9 cells. (A) SqRT-PCR for Postn in stable transfectants of GFP siRNA OP9 and Postn siRNA cells (left). QRT-PCR for Postn and IL-7 in OP9, OP9-309, and OP9-309P cells; expression levels were normalized to Hgprt housekeeping gene (similar results were obtained in 2 independent experiments; middle). Flow cytometric analysis of OP9 and OP9-309 cells stained with anti–periostin Ab (right). (B-C) Total number of nonadherent cells harvested 1 and 2 weeks after coculture of 10 000 total rabbit (B) and murine (C) BM cells with GFP siRNA OP9 and Postn siRNA OP9 (OP9-309) cells. Error bars are SEM from triplicate wells. Results are representative of 3 experiments.
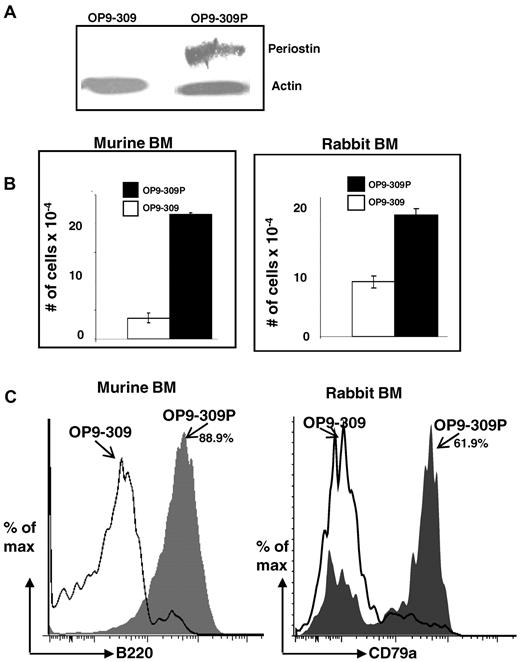
To confirm that the siRNA-mediated knockdown of Postn in the OP9-309 cells was responsible for the marked reduction of differentiation and proliferation of BM cells into B-lineage cells, we tested if the re-expression of periostin would restore their capacity to support differentiation and proliferation of BM lymphoid progenitors into cells of the B lineage. We generated a construct of Postn containing silent mutations in the hybridizing region of the Postn siRNA (see “Site-directed mutagenesis of Postn and transfection of OP9-309 cells”). We reasoned that transfection of OP9-309 cells with the mutated construct would reestablish expression of periostin because the mutated construct is impervious to the silencing effects of the Postn siRNA. After transfection and selection of cells on neomycin, a stable transfectant was obtained (OP9-309P), which by Western blot analysis (Figure 3A) and qRT-PCR (Figure 2A middle) had restored expression of periostin. To determine whether reexpression of periostin restored the capacity of Postn siRNA OP9 cells to support B lymphopoiesis, we cocultured rabbit and mouse BM cells on the OP9-309 and OP9-309P cells, and after 7 days, the cells arising from the cocultures were quantified (Figure 3B) and analyzed by flow cytometry (Figure 3C). We found that reexpression of periostin restored the capacity of OP9-309 cells to support differentiation of BM cells to B-lineage cells. Five-fold (mouse) or 2-fold (rabbit) more cell growth was found in cocultures with the OP9-309P cells than with the OP9-309 cells (Figure 3B), and nearly all cells (88.9%) arising in the mouse BM cocultures with OP9-309P were B220+ (Figure 3C left). Greater than 60% of the cocultures of rabbit BM cells with OP9-309P were CD79a+ B-lineage cells; in contrast < 5% of cells growing on OP9-309 were B-lineage cells (Figure 3C). These data indicate that with the use of the OP9 stromal cell system, periostin is required for B lymphopoiesis in vitro.
Restoration of capacity to support B lymphopoiesis by OP9-309 cells transfected with periostin. (A) Western blot analysis of OP9-309 and OP9-309P cells reexpressing his-tagged periostin. Blot probed with anti–his Ab and anti–actin Ab (control). (B) Cocultures of murine and rabbit BM with OP9-309 and OP9-309P cells; number of total cells in cocultures is indicated; error bars shown as SEM from a range of 2 samples. (C) Flow cytometric analysis of B220+ (murine) and CD79a+ (rabbit) cells arising from cocultures (gray histograms indicate cells from OP9-309P cultures; and open histograms, cells from OP9-309 cultures). Similar results were obtained in 3 independent experiments.
Restoration of capacity to support B lymphopoiesis by OP9-309 cells transfected with periostin. (A) Western blot analysis of OP9-309 and OP9-309P cells reexpressing his-tagged periostin. Blot probed with anti–his Ab and anti–actin Ab (control). (B) Cocultures of murine and rabbit BM with OP9-309 and OP9-309P cells; number of total cells in cocultures is indicated; error bars shown as SEM from a range of 2 samples. (C) Flow cytometric analysis of B220+ (murine) and CD79a+ (rabbit) cells arising from cocultures (gray histograms indicate cells from OP9-309P cultures; and open histograms, cells from OP9-309 cultures). Similar results were obtained in 3 independent experiments.
Proliferation and survival of BM cells in periostin-deficient OP9-309 cultures
Periostin can act as a ligand for αvβ3 and αvβ5 cellular integrins17 and αvβ3-ligand interactions have been shown to activate the Akt/protein kinase B signaling pathway, promoting cell survival, proliferation, and cell motility in vitro.17-20 We investigated if the reduction in B lymhpopoiesis observed in cocultures with the periostin-deficient OP9-309 cells was because of a decrease in proliferation or survival or both of B-lineage cells. Murine CFSE-labeled total BM cells were cultured on OP9 or OP9-309 cells, and cell proliferation, survival, and differentiation were assayed 2-7 days later. After 2 days in culture, the percentage of cells in the lymphocyte gate was similar in both cultures, whereas by day 4 a noticeable reduction in the population from the OP9-309 cultures was observed (Figure 4A) with 43% of the cells being dead and only 22% dead in the OP9 cultures (data not shown). By day 7 we found a dramatic reduction of lymphocyte-sized cells in 309-OP9 cultures (∼ 10-fold less) compared with the cells arising in the OP9 cultures (Figure 4A). Further, most of the lymphocytes from the OP9-309 cultures were dead (53%) compared with 6% from the OP9 cultures, showing that most lymphocyte-sized cells from the periostin-deficient OP9-309 cultures were either dead or dying.
Cell death, proliferation, and differentiation of BM cells with periostin-deficient OP9-309 cells. (A) Flow cytometric analysis of cells arising from cocultures of total BM and OP9 or OP9-309 cells after 2, 4, and 7 days. The lymphocyte gates are indicated in percentages; cell death was determined at day 7 (LIVE/DEAD staining). (B) After 7 days in cultures, apoptosis in the lymphocyte populations from OP9 and OP9-309 cultures was assayed by staining cells with 7-AAD and Annexin V and analyzing the cells by flow cytometry. (C) Flow cytometric analysis of CFSE distribution in BM cells after 4 and 7 days in culture (solid gray and black lines indicate cells from OP9 cultures; and dotted black and gray lines, cells from OP9-309 cultures). (D) Flow cytometry of LSK cultured 7 days on either OP9 or OP9-309 cells. The data are representative of 2 experiments.
Cell death, proliferation, and differentiation of BM cells with periostin-deficient OP9-309 cells. (A) Flow cytometric analysis of cells arising from cocultures of total BM and OP9 or OP9-309 cells after 2, 4, and 7 days. The lymphocyte gates are indicated in percentages; cell death was determined at day 7 (LIVE/DEAD staining). (B) After 7 days in cultures, apoptosis in the lymphocyte populations from OP9 and OP9-309 cultures was assayed by staining cells with 7-AAD and Annexin V and analyzing the cells by flow cytometry. (C) Flow cytometric analysis of CFSE distribution in BM cells after 4 and 7 days in culture (solid gray and black lines indicate cells from OP9 cultures; and dotted black and gray lines, cells from OP9-309 cultures). (D) Flow cytometry of LSK cultured 7 days on either OP9 or OP9-309 cells. The data are representative of 2 experiments.
Integrin signaling activates the Akt pathway, which promotes cell survival by inhibiting apoptosis.20,21 We predicted that the observed cell death in the periostin-deficient 309 cells was because of increased apoptosis. By staining with 7-AAD and Annexin V, we found > 60% of the lymphocytes from the OP9-309 cultures were apoptotic (7-AAD+/−Anenexin V+), compared with only <25% from the OP9 cultures (Figure 4B). These data indicate that lymphoid cells in the periostin-deficient OP9-309 cultures are dying primarily by apoptosis.
To determine the effect of periostin deficiency on cell proliferation, we analyzed the distribution of CFSE in B220+ cells arising from both OP9 and OP9-309 cultures by flow cytometry and found that at day 4 essentially all B220+ cells from both OP9 and OP9-309 cultures had proliferated (Figure 4C left). By day 7, consistent with the high level of apoptosis (Figure 4B), we found almost no B220+ cells that had proliferated in the OP9-309 cultures (Figure 4C right), indicating that the B220+ cells seen at day 4 had died. Interestingly, the few B220− cells from the OP9-309 cultures had proliferated albeit poorly, signifying that periostin is probably required for the development/expansion of B220− cells as well as B220+ cells. We repeated these experiments with the use of FACS of LSK cells (1000 cells/well) instead of total BM cells to investigate the requirement of periostin in B lymphopoiesis with the use of a homogenous progenitor population. After 7 days in culture, almost no B220+ cells could be identified in the OP9-309 cultures compared with 23% from the OP9 cultures (Figure 4D arrowheads). However, unlike cultures with total BM cells in which most cells are dead after 7 days, most of the lymphocyte-sized cells were alive in these cultures. We think that this is because of the self-renewal potential of LSK cells.22,23 This self-renewal effect is largely undetectable when total BM cells are cultured on OP9-309, because LSK cells make up only 0.05% of total BM cells.24,25 Most of the cells arising from the OP9-309 cultures were B220− and some of these were Gr-1+ monocytes, although the number of monocytes was 3-fold less (55% vs 18%) than from the OP9 cultures. This finding confirms our previous observation that periostin is probably required for the development/expansion of non–B-lineage cells (Figure 4C). Taken together, the data from the experiments that used LSK cells confirm the data obtained with total BM cells. We conclude that the periostin-deficient OP9-309 cells do not support the proliferation of B-lineage progenitors, indicating a requirement for periostin in B-lineage development in vitro.
Receptor for periostin on mouse BM precursors
Our results show that periostin is required for B lymphopoiesis in vitro most probably by promoting cell proliferation and survival. Umemoto et al26 reported that murine HSCs express the β3 chain (CD61) common to multiple integrin receptors,27 and we investigated if murine LSK and other early progenitors express this β3 complexed with αv (CD51), forming a functional periostin receptor αvβ3.17 With the use of a mAb against an αvβ3 conformational epitope resulting from the posttranslational association of the αv and β3 subunits,28 we found a population of LSK cells expressing the αvβ3 hetrodimer (Figure 5A). To confirm that this LSK αvβ3+ population contained HSCs, the cells underwent FACS and were cocultured on either OP9 or OP9-DL1 stromal cells. After 7 days, the cells arising from the cocultures were analyzed by flow cytometry, and we identified B- and T-lineage cells arising from the OP9 and OP9-DL1 cultures, respectively (Figure 5A), indicating that the LSK αvβ3+ cells include HSCs or early lymphoid progenitors or both. We also found that some common lymphoid progenitors (CLPs), pre-pro-B, pro-B, and pre-B cells express αvβ3 (Figure 5B). These results corroborate and extend the findings of Umemoto et al,26 demonstrating that on murine HSCs, the β3 chain (CD61) is expressed in a heterodimer with an αv chain (CD51) forming the receptor for periostin.
Identification and characterization of BM B-cell progenitors expressing the periostin receptor αvβ3. (A) Flow cytometry of Lin−Sca1+c-Kit+ αvβ3+ murine BM cells after 7 days of coculture with OP9 or OP9-DL cells. T-lineage cells identified with anti-CD3; B-lineage cells identified with anti-B220. (B) Flow cytometry of B-cell progenitors for αvβ3; gray histograms represent isotype controls. The data are representative of 2 experiments.
Identification and characterization of BM B-cell progenitors expressing the periostin receptor αvβ3. (A) Flow cytometry of Lin−Sca1+c-Kit+ αvβ3+ murine BM cells after 7 days of coculture with OP9 or OP9-DL cells. T-lineage cells identified with anti-CD3; B-lineage cells identified with anti-B220. (B) Flow cytometry of B-cell progenitors for αvβ3; gray histograms represent isotype controls. The data are representative of 2 experiments.
The presence of the periostin receptor αvβ3 on lymphoid progenitor cells in BM (Figure 5) suggests that an αvβ3-mediated interaction between BM progenitor cells and periostin molecules on the OP9 cells is probably required to facilitate proliferation, survival, and differentiation. Cell-surface integrins, including αvβ3, interact with ECM proteins by recognizing a specific RGD protein sequence shared by several ECM proteins, including periostin, collagen, and fibronectin.29-31 We reasoned that, if periostin interacts with αvβ3 on LSK and B-cell progenitors to drive B lymphopoiesis, then inhibiting this interaction would prevent B lymphopoiesis. Because it has been shown that millimolar concentrations of synthetic RGD peptides can inhibit this receptor-ligand interaction in a variety of cells in vitro,30-34 we cultured mouse BM cells on OP9 stromal cells in the presence of an RGD inhibitory peptide (RGD-3661) or a control peptide (RGD-3883) and determined whether B lymphopoiesis was inhibited in vitro. After 7 days, the cells arising from the cocultures were examined for evidence of proliferation and differentiation. The addition of inhibitory RGD-3661 exhibited a ∼ 2-fold reduction in differentiation of BM progenitors to B-lineage cells compared with cells cocultured with control peptide (Figure 6A). This reduction in differentiation was reflected by marked reduction in cell growth at 3 different concentrations of the RGD peptide (Figures 6B). However, supplementation of cocultures with the control RGD-3883 had no inhibitory effect, neither on differentiation (Figure 6A right) nor on the proliferation of B-lineage cells (Figure 6B). We conclude that early BM progenitors need stimulation through their αvβ3 receptor, probably through periostin and perhaps other stromal ligands, for optimal proliferation and differentiation into B-lineage cells.
In vitro inhibition of B lymphopoiesis by synthetic RGD peptides. (A) Flow cytometric analysis of cells arising from cocultures of murine BM and OP9 cells (left) supplemented with inhibitory RGD-3661 peptide (1 mg/mL; middle) or control RGD-3883 peptide (1 mg/mL; right). (B) Quantification of B-lineage cells arising from the cocultures. Error bars are SEM of 3 independent experiments. Left panel depicts cell numbers from experiments with 1 mg/mL RGD peptide; right panel depicts cell numbers from RGD dose-dependent experiments.
In vitro inhibition of B lymphopoiesis by synthetic RGD peptides. (A) Flow cytometric analysis of cells arising from cocultures of murine BM and OP9 cells (left) supplemented with inhibitory RGD-3661 peptide (1 mg/mL; middle) or control RGD-3883 peptide (1 mg/mL; right). (B) Quantification of B-lineage cells arising from the cocultures. Error bars are SEM of 3 independent experiments. Left panel depicts cell numbers from experiments with 1 mg/mL RGD peptide; right panel depicts cell numbers from RGD dose-dependent experiments.
B and T lymphopoiesis in Postn−/− mice
After determining that periostin is required for B lymphopoiesis in vitro, we tested if periostin is required for B or T lymphopoiesis in vivo by examining B- and T-cell populations in Postn−/− mice.9 BM, spleen, thymus, and peripheral blood cells from a total of 10 mice aged 3-16 weeks were examined by flow cytometric analysis to determine whether the number and distribution of lymphocytes and lymphoid progenitors were altered. Flow cytometric analysis of these cells from both 3- and 16-week-old mice showed that the percentages of BM B-cell precursor subsets (Figure 7) and the number of splenic B cells (data not shown) was similar to age-matched controls. Although Postn−/− mice had slightly lower levels of splenic CD4 T cells (73% wild type vs 57% Postn−/−) and slightly increased levels of splenic CD8 T cells (20% wild type vs. 37% Postn−/−), the development of thymocyte subsets was also not altered (data not shown). These results indicate that periostin is not required in vivo for murine B and T lymphopoiesis.
Flow cytometric analysis of BM cells from wild-type (WT) and Postn−/− mice. BM cells from 16-week-old mice were analyzed for the expression of B220 and for CD43 (left) and for surface IgM and CD43 within the B220+ population (right). Numbers indicate the percentages of various lymphocyte stages: pre indicates pre-B stage; and pro, pro-B stage. Similar results were obtained in ≥ 3 separate experiments and also with BM of 3-week-old mice; a total of 10 mice were examined.
Flow cytometric analysis of BM cells from wild-type (WT) and Postn−/− mice. BM cells from 16-week-old mice were analyzed for the expression of B220 and for CD43 (left) and for surface IgM and CD43 within the B220+ population (right). Numbers indicate the percentages of various lymphocyte stages: pre indicates pre-B stage; and pro, pro-B stage. Similar results were obtained in ≥ 3 separate experiments and also with BM of 3-week-old mice; a total of 10 mice were examined.
Microarray analysis of Postn siRNA and GFP siRNA OP9 cells
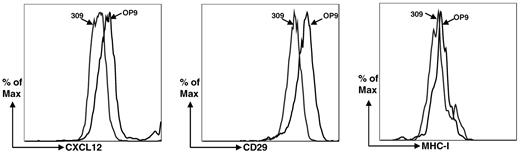
To begin elucidating changes in OP9 cells that occur in response to reduced periostin expression, we compared expression profiles of the Postn siRNA OP9 cell line derived from siRNA no.309 and the GFP siRNA OP9 cell line by microarray analysis. We found that several critical lymphoid-supporting factors were down-regulated in the Postn siRNA OP9 cells, including IL-7 and CXCL12 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Expression of IL-7 and CXCL12 were reduced by ∼ 8-fold. Down-regulation of IL-7 was confirmed by qRT-PCR (Figure 2 middle) and down-regulation of CXCL12 was confirmed by flow cytometry (Figure 8). By flow cytometry, we also found that CD29 (integrin β1) was down-regulated (Figure 8). This was expected from the microarray data because anti-CD29 mAb recognizes the β1 common chain associated with integrin α11 (ITga11),35 the expression of which is down-regulated in OP9-309 cells.
Flow cytometric analysis of OP9-309 cells for down-regulation of CXCL12 and integrin α11 (ITga11). Anti–MHC-1 serves as a control for a molecule not found by microarray analysis to have dysregulated expression.
Flow cytometric analysis of OP9-309 cells for down-regulation of CXCL12 and integrin α11 (ITga11). Anti–MHC-1 serves as a control for a molecule not found by microarray analysis to have dysregulated expression.
The down-regulation of CXCL12 in the periostin-deficient OP9-309 cells may in part account for the decreased capacity of the OP9-309 cells to support B lymphopoiesis. The reduction in CXCL12 expression would limit its interaction with CCR4 on B-cell progenitors. This interaction is important for adhesion, migration, and retention of the B-cell progenitors in the BM hematopoietic niche as well as for signaling via the Akt pathway. The addition of exogenous IL-7 and CXCL12 to BM:OP9-309 cocultures did not restore B lymphopoiesis (data not shown), indicating that still other molecules are needed to restore the OP9-309 cells. The microarray data provide the basis for continued studies to delineate the role of periostin in B lymphopoiesis.
Discussion
Throughout the life of mice and humans, HSCs in the BM continuously proliferate and differentiate into B-lineage cells.13 In other species, including chickens, sheep, and rabbits, B lymphopoiesis occurs primarily during gestation,36,37 and in rabbits it ceases a few months after birth.6 The proliferation and differentiation of HSCs to B-lineage cells requires vital signals from the BM microenviroment, including cytokines, growth factors, and adhesion molecules secreted by stromal cells and the ECM.38-40 Previous investigation of the mechanism responsible for the observed arrest of B lymphopoiesis in rabbits led Kalis et al7 to suggest that the BM stroma in adult rabbits loses its potential to support B lymphopoiesis. Surprisingly, IL-7, a cytokine critical for B lymphopoiesis, was found to be up-regulated in adult rabbits.7 A cDNA-RDA assay to compare cultured stromal cells from adult and young rabbits identified the ECM molecule periostin as a molecule that appeared to be down-regulated in adult BM stromal cells. Periostin is a secreted ECM protein of poorly defined function but has been associated with development of bone, teeth, and heart.41-45 Periostin is also deposited on osteoblasts,46 which have been shown to support B-lymphocyte commitment and differentiation from HSCs.47 This finding in combination with a recent report showing the importance of another ECM protein, fibronectin, in lymphopoiesis48,49 led us to test if periostin could similarly be important for B lymphopoiesis. Our results indicate that enforced reduction of Postn expression in OP9 cells (OP9-309) led to the near ablation of proliferation and differentiation of mouse and rabbit BM progenitors to B-lineage cells. We confirmed that the decline in B lymphopoiesis in the OP9-309 cells was the result of reduction of periostin by reexpressing periostin in the OP9-309 cells and showing that the capacity to support differentiation and proliferation of mouse and rabbit BM progenitors into B-lineage cells was regained. These results indicate a novel role for periostin in B lymphopoiesis.
Periostin is the ligand for intergrin αvβ3, and αvβ3-ligand interactions activate the Akt signaling pathway resulting in cell survival and proliferation.16,18,19,21 We postulate that in vitro periostin deficiency prevents signaling via the Akt pathway, leading to loss of proliferation and cell death, thereby inhibiting differentiation of BM progenitors into B-lineage cells. Consistent with this idea, we found pronounced apoptosis and almost no proliferation or differentiation of BM progenitors cultured on periostin-deficient OP9 cells. This requirement for periostin in B lymphopoiesis was confirmed with the use of purified LSK cells in coculture experiments. Although cell death was minimal in these cultures, probably because of the self-renewal potential of LSK,22,23 almost no differentiation of LSK into the B lineage occurred. Further, although cell viability was extremely low, we found almost no live cells in purified pre-pro-B cells from OP9-309 cultures (data not shown).
Additional evidence that BM progenitors interact with periostin was the finding that αvβ3 is expressed on LSK, CLP, pre-pro-B, pro-B, and pre-B cell subsets and also that inhibiting the periostin-αvβ3 interactions with RGD peptide inhibited the proliferation and differentiation of BM progenitors into B-lineage cells. On the basis of these data, we conclude that in vitro, periostin-αvβ3 interactions are required to drive proliferation and differentiation of BM progenitors into B-lineage cells, most probably by activating the Akt signaling pathway. Because early B-cell precursors reside in the subendosteal region of the BM cavity,50 we hypothesize that, during aging, disruption of the ECM in the subendosteal region could diminish attachments and survival signals between B-lineage progenitors and the stroma and lead to a decline in the levels of B lymphopoiesis.
We were surprised to find no decline in either B or T lymphopoiesis in young or adult Postn−/− mice, and, in addition, β3-deficient mice appear to have normal lymphocyte compartments.51 It is probable that in vivo other stimuli compensate for the loss of periostin in activating the Akt signaling pathway. Another possibility is that other ECM molecules present in vivo compensate for the loss of periostin expression, because, for example, a possible redundancy of integrin (α4β5 and α4β7) interactions with fibronectin has been reported (discussed in Bungartz et al52 ). However, we found that the exogenous addition of fibronectin to cocultures of BM cells and Postn siRNA OP9 cells was not sufficient to compensate for the loss of periostin (data not shown). Further studies are warranted to explore these possibilities as a means of delineating this novel role of periostin in B lymphopoiesis.
Even though we found a decline in expression of periostin in the BM of adult rabbits, we think this decline is isoform specific. Periostin isoforms have been identified in other species,8 and using PCR primers for domains 5′ of those used in the present study, we found periostin expressed in the BM throughout life (data not shown). Isoforms of rabbit periostin will need to be identified and examined before conclusions can be made about the potential role of specific periostin isoforms in rabbit B lymphopoiesis, in vivo. Taken together the data presented herein highlight the importance of the ECM in B lymphopoiesis, with the identification of a novel in vitro requirement for periostin. This interaction between periostin and its receptor αvβ3 on early BM progenitors is required for proliferation and differentiation of these progenitors into B-lineage cells in vitro.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Haiyan Wang for animal husbandry and Shi-Kang Zhai and Pi-Chen Yam for excellent technical assistance and Patricia Simms of the Loyola University Medical Center Flow Cytometry Facility.
This work was supported by from the National Institutes of Health (grant AI 054508 to K.L.K.), NRF of Korea (grant 2010-0016256 to S.C.), and grants from the Riley Foundation and IU Department of Pediatrics/Cardiology (S.J.C.).
National Institutes of Health
Authorship
Contribution: B.T.S. and S.L.K. designed research, performed research, analyzed data, and wrote the paper; P.T.L. and P.L.W. designed research and analyzed data; S.C. performed microarray research; S.J.C. provided vital mice and scientific expertise; L.D. performed research; and K.L.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katherine L. Knight, Department of Microbiology and Immunology, Stritch School of Medicine, Loyola University Chicago, 2160 S First Ave, Maywood, IL 60153; e-mail: kknight@lumc.edu.
References
Author notes
B.T.S. and S.L.K. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal