Abstract
Richter syndrome (RS) represents the development of diffuse large B-cell lymphoma in the context of chronic lymphocytic leukemia. The scarcity of biologic information about RS has hampered the identification of molecular predictors of RS outcome. We addressed this issue by performing a comprehensive molecular characterization of 86 pathologically proven RS. TP53 disruption (47.1%) and c-MYC abnormalities (26.2%) were the most frequent alterations, whereas common genetic lesions of de novo diffuse large B-cell lymphoma were rare or absent. By multivariate analysis, lack of TP53 disruption (hazard ratio, 0.43; P = .003) translated into significant survival advantage with 57% reduction in risk of death. An algorithm based on TP53 disruption, response to RS treatment, and Eastern Cooperative Oncology Group performance status had 80.9% probability of correctly discriminating RS survival (c-index = .809). RS that were clonally unrelated to the paired chronic lymphocytic leukemia phase were clinically and biologically different from clonally related RS because of significantly longer survival (median, 62.5 months vs 14.2 months; P = .017) and lower prevalence of TP53 disruption (23.1% vs 60.0%; P = .018) and B-cell receptor stereotypy (7.6% vs 50.0%; P = .009). The molecular dissection of RS into biologically distinct categories highlights the genetic heterogeneity of this disorder and provides clinically relevant information for refining the prognostic stratification of patients.
Introduction
Richter syndrome (RS) represents the development of an aggressive lymphoma, most commonly a diffuse large B-cell lymphoma (DLBCL), in the context of chronic lymphocytic leukemia (CLL).1 The diagnosis of RS requires a histologic proof, in the absence of which RS can only be clinically suspected but not proven.2-4 RS differs from prolymphocytic transformation and from CLL “acceleration,” that are instead characterized by the expansion of proliferation centers in the absence of a histologic shift to DLBCL.1,5
Recent studies have indicated that, at the time of CLL diagnosis, the molecular features of the leukemic clone are important for the subsequent risk of RS development.6-9 Conversely, the pattern of genetic lesions acquired by RS on transformation is scarcely known, and available evidence is limited to small case series.10-18 Cytogenetic and molecular studies have shown that RS is associated with a higher degree of genomic complexity than is CLL, suggesting that transformation to RS might result from accumulation of novel genetic lesions that drive clinicopathologic shift and change the course of the disease.10-18
The conventional definition of RS is currently based on clinicopathologic grounds and does not take into account the immunogenetic heterogeneity of the disease. In fact, the clonal relationship between CLL and the developing DLBCL suggests that 2 types of RS may exist: (1) transformation of CLL to a clonally related DLBCL that accounts for the majority of cases and (2) development of a DLBCL unrelated to the CLL clone.9,19-22
The clinical outcome of patients with RS is generally considered poor.3,4 However, a large study of histologically proven RS has reported that survival of patients with RS can vary from a few weeks to 15 years.23 The recognition that RS outcome is not uniformly poor has prompted the development of the RS score that is currently the only available tool to predict RS prognosis.23 Given the limited understanding of the disease at the molecular level, the RS score was conceived on clinical grounds and does not take into account the biology of the disease.23 Despite the availability of the RS score, the identification of patients with RS projected to experience a long survival remains an unresolved issue that may benefit from the development of novel RS prognosticators.
This study aimed at a comprehensive molecular characterization of RS. By investigating the biology and the immunogenetic heterogeneity of the disease, we identified novel biologic predictors of RS survival with potentially important implications for the clinical management of patients with RS. First, this study shows that TP53 disruption critically affects RS survival, thus providing the rationale for exploring therapeutic agents that overcome TP53 disruption in this disease. Second, we report that clonally unrelated RS is clinically and biologically distinct from clonally related cases and is characterized by an outcome similar to that of de novo DLBCL. Accordingly, we propose that the diagnosis of RS should be restricted to clonally related cases.
Methods
Patients
The study was based on a multi-institutional series of 86 patients with RS developing from a previous surface immunoglobulin low/CD5+/CD23+ CLL defined according to guidelines from the International Workshop on Chronic Lymphocytic Leukemia–National Cancer Institute (IWCLL-NCI).2 The diagnosis of RS was based on the histology of lymph node or extranodal tissue excisional biopsies. After institutional pathologic review, all RS cases were classified as DLBCL according to the World Health Organization Classification of Tumors of the Hematopoietic and Lymphoid Tissues.24 Cases presenting with a large cell lymphoma during or immediately after alemtuzumab treatment were excluded from this analysis because they represent a clinicopathologic setting that is distinct from true RS.25 In all cases, molecular studies were performed on the biopsy used for RS diagnosis (frozen samples, 31 cases; formalin-fixed paraffin-embedded samples, 55 cases). Biologic samples from the paired CLL phase were available in 46 cases (53.4%). Patients provided informed consent in accordance with institutional review board requirements from all participating institutions and the Declaration of Helsinki.
Analysis of IGHV-D-J rearrangements
Rearrangements of IGHV-D-J genes were amplified with subgroup-specific primers as previously reported.9 Sequences were aligned to ImMunoGeneTics sequence directory.26 The following information was extracted: IGHV-D-J gene usage, percentage of identity to germline, and VH CDR3 length. The occurrence of stereotyped VH CDR3 was analyzed as reported.9 The clonal relationship between the CLL phase and the RS phase was established in 63 cases (73.2%) by comparing IGHV-D-J and VH CDR3 sequences.
Mutation analysis
Mutation analysis of TP53 (exons 4-8; GenBank accession no. NM_000546.4), CARD11 (exons 5-9; GenBank accession no. NM_032415.3), TNFAIP3/A20 (exons 2-9; GenBank accession no. NM_006290.2), PRDM1/BLIMP1 (exons 1-7; GenBank accession no. NM_001198), EZH2 (exon 15; GenBank accession no. NM_004456.3), CD79A (immunoreceptor tyrosine-based activating motif domain on exons 4-5; GenBank accession no. NM_001783.3), CD79B (immunoreceptor tyrosine-based activating motif domain on exons 5-6; GenBank accession no. NM_000626.2), BCL6 intron 1 (genomic region 413-1141; GenBank accession no. AY189709), BCL6 exon 1 (genomic region 1-381; GenBank accession no. AY189709), PIM1 (genomic region 1011-1971; GenBank accession no. AF386792), PAX5 (genomic region 629-1460; GenBank accession no. AF386791), RhoH/TTF (genomic region 21-1100; GenBank accession no. AF386789), and c-MYC (genomic regions 2291-3500 and 4283-5449; GenBank accession no. X00364) was performed by DNA direct sequencing on an ABI Prism 3100 automated DNA sequence analyzer (Applied Biosystems). Nucleotide variations were considered mutations after filtering out single nucleotide polymorphisms mapped on public databases.27-30
Interphase FISH
Fluorescence in situ hybridization (FISH) analysis was performed on the bioptic tissue used for RS diagnosis by staining as follows: (1) nuclei extracted from fresh or frozen cell suspensions or (2) formalin-fixed, paraffin-embedded, unstained 3-μm-thick tissue sections. The probes used for FISH analysis were (1) LSI13, LSID13S319, CEP12, LSIp53, LSIATM, LSI IGH/BCL2, LSI IGH/CCND1, LSI BCL6, LSI IGH/c-MYC/CEP8, c-MYC break-apart, LSI N-MYC, CEP2, CEP3, CEP18, CEP19 (Abbott); (2) BCL3 split signal (Dako); (3) 6q21/α-satellite (Kreatech Biotechnology); and (4) bacterial artificial chromosome clones 373L24-rel and 440P05-BCL11A. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole and antifade, and signals were visualized with the use of an Olympus BX51 microscope (Olympus Italia). For each probe, ≥ 400 interphase cells with well-delineated fluorescent spots were examined.
EBV analysis
Analysis of Epstein-Barr virus (EBV) infection was performed by polymerase chain reaction or in situ hybridization of EBV-encoded RNA transcripts, as previously reported.9
Immunohistochemistry
Cell of origin of DLBCL developing in the context of RS transformation was assigned by integrating the Hans algorithm31 with the Choi algorithm32 after staining for GCET1 (clone RAM341; Abcam), CD10 (clone 56C6; Ventana Medical Systems), BCL6 (polyclonal; Santa Cruz Biotechnology), IRF4/MUM1 (clone MRQ-43; Ventana Medical Systems), and FOXP1 (Clone JC12; Abcam).
Statistical analysis
The date of RS diagnosis was defined as the date of the diagnostic biopsy. RS survival was measured from date of RS diagnosis to date of death or last follow-up (censoring). Survival analysis was performed by the Kaplan-Meier method.33 Univariate and multivariate analyses were performed by the Cox regression model.34 False discovery rate was used to control for multiple statistical testing.35 Accordingly, along with the P value, also a q value was provided for each association analysis. The q value indicates the probability that a positive association identified by a P value < .05 might be a false-positive association because of chance. Only variables with a P value < .05 and a q value < .1 by univariate Cox analysis were retained for multivariate Cox analysis. Model minimization was performed by stepwise backward elimination (probability for entry, .05; probability for removal, .10). The following variables were analyzed in the multivariate analysis for risk of death: TP53 disruption, clonal relationship, age (≤ 60 years vs > 60 years), Eastern Cooperative Oncology Group Performance Status (ECOG PS; 0-1 vs 2-4), tumor size (≤ 5 cm vs > 5 cm), lactate dehydrogenase (LDH; ≤ 1.5 upper limit of normal [ULN] vs > 1.5 ULN), platelet count (≥ 100 × 109/L vs < 100 × 109/L), response after RS induction treatment (complete remission [CR] vs partial remission/no response), and allogeneic stem cell transplantation (SCT) as consolidation after induction. Response after RS induction treatment was treated as a time-dependent variable. To account for possible monotone likelihood in the multivariate analysis, the Firth bias correction method was applied to Cox regression when appropriate.36 The bias-corrected c-index for the final model was calculated with the validation function of the R Design library (http://www.r-project.org; accessed December 9, 2010) with 1000 bootstrap samples. This approach provides a bias-corrected estimate of prediction accuracy of the model to protect against overfitting during the stepwise regression of Cox multivariate analysis. Subsequently, the stability and predictive performance of the final model was internally validated with the use of a 2-step bootstrap resampling procedure.37,38 In the first step, 1000 bootstrap samples were generated randomly with replacement from the original population (n = 82). The stepwise Cox regression procedure was applied to each bootstrap sample with the same selection criteria as the original modeling. The percentage of bootstrap samples for which each variable was selected as significant in the model was then calculated. Percentage of inclusion reflects the prognostic importance of a variable, because it is expected that an important variable will be included for a majority of the bootstrap samples. In the second step, 1000 bootstrap samples were generated randomly with replacement from the original population. The stepwise Cox regression procedure was applied to each bootstrap sample with the same selection criteria as the original modeling. For each covariate, the mean standard deviation and confidence intervals (CIs) were computed for the 1000 bootstrap replications. The contribution of clinical covariates in determining the difference in risk of death between RS with TP53 disruption and RS with wild-type TP53 was estimated by the following formula: 100 × [1 − (adjusted HR for TP53 disruption ÷ unadjusted HR for TP53 disruption)].39 Harrell C statistics was used to compare the discriminatory value of the prognostic models (c-index = 1 indicates perfect discrimination, whereas c-index = 0.5 is equivalent to chance).40 U test statistics were calculated with the use of the rcorrp.cens function of the R Design library (http://www.r-project.org; accessed December 9, 2010) to test whether the c-index of one predictive model was significantly higher than the c-index of another predictive model. Recursive-partitioning analysis for censored survival data were performed to identify the factors that were most influential for RS survival and to permit the classification of patients into risk categories.41,42 Categorical variables were compared by chi-square test and Fisher exact test when appropriate. All statistical tests were 2-sided. Statistical significance was defined as P value < .05. The analysis was performed with the Statistical Package for the Social Sciences (SPSS) software Version 18.0 (and with R statistical package 2.8.1, http://www.r-project.org; accessed December 9, 2010).
Results
Clinicopathologic features of RS cases
The clinical features of RS (n = 86) were representative of this disease in terms of patient frailty, tumor burden, and history of previous CLL treatment (Table 1). Median time from CLL diagnosis to RS development was 3.7 years (range, 0.2-6.7 years). Eighty-three of 86 patients were evaluable for survival. After a median follow-up for surviving patients of 57 months, 52 of 83 patients (62.6%) died, accounting for a median survival from the time of RS development of 19 months.
Clinical characteristics of the RS series
| Characteristic . | Whole series . | Clonally related . | Clonally unrelated . | Undetermined clonal relationship . | ||||
|---|---|---|---|---|---|---|---|---|
| n/N . | % . | n/N . | % . | n/N . | % . | n/N . | % . | |
| Clinical features at RS diagnosis | ||||||||
| Age > 60 y | 56/86 | 65.1 | 31/50 | 62.0 | 11/13 | 84.6 | 14/23 | 60.9 |
| Male | 54/86 | 62.8 | 31/50 | 62.0 | 8/13 | 61.5 | 15/23 | 65.2 |
| Date of RS diagnosis | ||||||||
| 1990-1994 | 5/86 | 5.8 | 3/50 | 6.0 | 0/13 | 0 | 2/23 | 8.7 |
| 1995-1999 | 7/86 | 8.1 | 4/50 | 8.0 | 2/13 | 15.4 | 1/23 | 4.3 |
| 2000-2004 | 30/86 | 34.9 | 17/50 | 34.0 | 3/13 | 23.1 | 10/23 | 43.5 |
| 2005-2010 | 44/86 | 51.2 | 26/50 | 52.0 | 8/13 | 61.5 | 10/23 | 43.5 |
| ECOG PS > 1 | 30/82 | 36.6 | 21/49 | 42.9 | 2/11 | 18.2 | 7/22 | 31.8 |
| Ann Arbor stage III-IV | 75/82 | 91.5 | 45/49 | 91.8 | 9/11 | 81.8 | 21/22 | 95.5 |
| Binet stage | ||||||||
| A | 20/82 | 24.4 | 10/49 | 20.4 | 3/11 | 27.3 | 7/22 | 31.8 |
| B | 31/82 | 37.8 | 21/49 | 42.9 | 4/11 | 36.4 | 6/22 | 27.3 |
| C | 31/82 | 37.8 | 18/49 | 36.7 | 4/11 | 36.4 | 9/22 | 40.9 |
| B symptoms | 36/82 | 43.9 | 23/49 | 46.9 | 3/11 | 27.3 | 10/22 | 45.5 |
| Extranodal sites > 1 | 26/83 | 31.3 | 17/49 | 34.7 | 2/11 | 18.2 | 7/23 | 30.4 |
| Tumor size > 5 cm | 37/83 | 44.6 | 24/50 | 48.0 | 4/11 | 36.4 | 9/22 | 40.9 |
| Nodal areas ≥ 5 | 24/83 | 28.9 | 17/50 | 34.0 | 1/11 | 9.1 | 6/22 | 27.3 |
| ALC ≥ 5.0 × 109/L | 42/82 | 51.2 | 26/49 | 53.1 | 4/11 | 36.4 | 12/22 | 54.5 |
| Hb level < 10 g/dL | 18/82 | 22.0 | 8/49 | 16.3 | 4/11 | 36.4 | 6/22 | 27.3 |
| Platelet count < 100 × 109/L | 25/82 | 30.5 | 16/49 | 32.7 | 1/11 | 9.1 | 8/22 | 36.4 |
| LDH > 1.5 ULN | 37/82 | 45.1 | 22/49 | 44.9 | 6/11 | 54.5 | 9/22 | 40.9 |
| CD38 expression | 30/52 | 57.7 | 21/37 | 56.8 | 3/9 | 33.3 | 6/6 | 100 |
| ZAP70 expression | 20/31 | 64.5 | 17/26 | 65.4 | 3/4 | 75.0 | 0/1 | 0 |
| Prior CLL therapies > 1 | 32/83 | 38.6 | 18/49 | 36.7 | 5/12 | 41.7 | 9/22 | 40.9 |
| IPI | ||||||||
| Low | 11/82 | 13.4 | 4/49 | 8.2 | 2/11 | 18.2 | 5/22 | 22.7 |
| Low-intermediate | 26/82 | 31.7 | 17/49 | 34.7 | 4/11 | 36.4 | 5/22 | 22.7 |
| High-intermediate | 24/82 | 29.3 | 15/49 | 30.6 | 3/11 | 27.3 | 6/22 | 27.3 |
| High | 21/82 | 25.6 | 13/49 | 26.5 | 2/11 | 18.2 | 6/22 | 27.3 |
| RS score | ||||||||
| 0-1 point | 34/82 | 41.5 | 17/49 | 34.7 | 7/11 | 63.6 | 10/22 | 45.5 |
| 2 points | 15/82 | 18.3 | 12/49 | 24.5 | 0/11 | 0 | 3/22 | 13.6 |
| 3 points | 24/82 | 29.3 | 13/49 | 26.5 | 4/11 | 36.4 | 7/22 | 31.8 |
| 4-5 points | 9/82 | 11.0 | 7/49 | 14.3 | 0/11 | 0 | 2/22 | 9.1 |
| Pathologic features at RS diagnosis | ||||||||
| Site of RS development | ||||||||
| Nodal | 57/86 | 66.2 | 37/50 | 74.0 | 9/13 | 69.2 | 11/23 | 47.8 |
| Extranodal | 29/86 | 33.8 | 13/50 | 26.0 | 4/13 | 30.8 | 12/23 | 52.2 |
| CD5 expression | 40/83 | 48.2 | 23/48 | 47.9 | 7/12 | 53.8 | 10/23 | 43.5 |
| Non-GC phenotype | 76/82 | 92.7 | 43/46 | 93.5 | 11/13 | 84.6 | 22/23 | 95.7 |
| EBV infection | 5/85 | 5.9 | 3/50 | 6.0 | 2/13 | 15.4 | 0/22 | 0 |
| RS treatment | ||||||||
| Regimen | ||||||||
| R-CHOP/CHOP-like | 35/82 | 42.7 | 26/49 | 53.1 | 4/11 | 36.4 | 5/22 | 22.7 |
| CHOP/CHOP-like | 18/82 | 22.0 | 8/49 | 16.3 | 4/11 | 36.4 | 6/22 | 27.3 |
| Second-line regimens for NHL | 6/82 | 7.3 | 4/49 | 8.2 | 1/11 | 9.1 | 1/22 | 4.5 |
| Fludarabine-based regimens | 7/82 | 8.5 | 5/49 | 10.2 | 0/11 | 0 | 2/22 | 9.1 |
| Other | 16/82 | 19.5 | 6/49 | 12.2 | 2/11 | 18.2 | 8/22 | 36.4 |
| Rituximab-based | 47/82 | 57.3 | 33/49 | 67.3 | 4/11 | 36.4 | 10/22 | 45.5 |
| Allogeneic stem cell transplantation | 7/82 | 8.5 | 6/49 | 12.1 | 0/11 | 0 | 1/22 | 4.5 |
| CR after RS induction treatment | 23/83 | 27.7 | 10/49 | 20.4 | 6/12 | 50.0 | 7/22 | 31.8 |
| Characteristic . | Whole series . | Clonally related . | Clonally unrelated . | Undetermined clonal relationship . | ||||
|---|---|---|---|---|---|---|---|---|
| n/N . | % . | n/N . | % . | n/N . | % . | n/N . | % . | |
| Clinical features at RS diagnosis | ||||||||
| Age > 60 y | 56/86 | 65.1 | 31/50 | 62.0 | 11/13 | 84.6 | 14/23 | 60.9 |
| Male | 54/86 | 62.8 | 31/50 | 62.0 | 8/13 | 61.5 | 15/23 | 65.2 |
| Date of RS diagnosis | ||||||||
| 1990-1994 | 5/86 | 5.8 | 3/50 | 6.0 | 0/13 | 0 | 2/23 | 8.7 |
| 1995-1999 | 7/86 | 8.1 | 4/50 | 8.0 | 2/13 | 15.4 | 1/23 | 4.3 |
| 2000-2004 | 30/86 | 34.9 | 17/50 | 34.0 | 3/13 | 23.1 | 10/23 | 43.5 |
| 2005-2010 | 44/86 | 51.2 | 26/50 | 52.0 | 8/13 | 61.5 | 10/23 | 43.5 |
| ECOG PS > 1 | 30/82 | 36.6 | 21/49 | 42.9 | 2/11 | 18.2 | 7/22 | 31.8 |
| Ann Arbor stage III-IV | 75/82 | 91.5 | 45/49 | 91.8 | 9/11 | 81.8 | 21/22 | 95.5 |
| Binet stage | ||||||||
| A | 20/82 | 24.4 | 10/49 | 20.4 | 3/11 | 27.3 | 7/22 | 31.8 |
| B | 31/82 | 37.8 | 21/49 | 42.9 | 4/11 | 36.4 | 6/22 | 27.3 |
| C | 31/82 | 37.8 | 18/49 | 36.7 | 4/11 | 36.4 | 9/22 | 40.9 |
| B symptoms | 36/82 | 43.9 | 23/49 | 46.9 | 3/11 | 27.3 | 10/22 | 45.5 |
| Extranodal sites > 1 | 26/83 | 31.3 | 17/49 | 34.7 | 2/11 | 18.2 | 7/23 | 30.4 |
| Tumor size > 5 cm | 37/83 | 44.6 | 24/50 | 48.0 | 4/11 | 36.4 | 9/22 | 40.9 |
| Nodal areas ≥ 5 | 24/83 | 28.9 | 17/50 | 34.0 | 1/11 | 9.1 | 6/22 | 27.3 |
| ALC ≥ 5.0 × 109/L | 42/82 | 51.2 | 26/49 | 53.1 | 4/11 | 36.4 | 12/22 | 54.5 |
| Hb level < 10 g/dL | 18/82 | 22.0 | 8/49 | 16.3 | 4/11 | 36.4 | 6/22 | 27.3 |
| Platelet count < 100 × 109/L | 25/82 | 30.5 | 16/49 | 32.7 | 1/11 | 9.1 | 8/22 | 36.4 |
| LDH > 1.5 ULN | 37/82 | 45.1 | 22/49 | 44.9 | 6/11 | 54.5 | 9/22 | 40.9 |
| CD38 expression | 30/52 | 57.7 | 21/37 | 56.8 | 3/9 | 33.3 | 6/6 | 100 |
| ZAP70 expression | 20/31 | 64.5 | 17/26 | 65.4 | 3/4 | 75.0 | 0/1 | 0 |
| Prior CLL therapies > 1 | 32/83 | 38.6 | 18/49 | 36.7 | 5/12 | 41.7 | 9/22 | 40.9 |
| IPI | ||||||||
| Low | 11/82 | 13.4 | 4/49 | 8.2 | 2/11 | 18.2 | 5/22 | 22.7 |
| Low-intermediate | 26/82 | 31.7 | 17/49 | 34.7 | 4/11 | 36.4 | 5/22 | 22.7 |
| High-intermediate | 24/82 | 29.3 | 15/49 | 30.6 | 3/11 | 27.3 | 6/22 | 27.3 |
| High | 21/82 | 25.6 | 13/49 | 26.5 | 2/11 | 18.2 | 6/22 | 27.3 |
| RS score | ||||||||
| 0-1 point | 34/82 | 41.5 | 17/49 | 34.7 | 7/11 | 63.6 | 10/22 | 45.5 |
| 2 points | 15/82 | 18.3 | 12/49 | 24.5 | 0/11 | 0 | 3/22 | 13.6 |
| 3 points | 24/82 | 29.3 | 13/49 | 26.5 | 4/11 | 36.4 | 7/22 | 31.8 |
| 4-5 points | 9/82 | 11.0 | 7/49 | 14.3 | 0/11 | 0 | 2/22 | 9.1 |
| Pathologic features at RS diagnosis | ||||||||
| Site of RS development | ||||||||
| Nodal | 57/86 | 66.2 | 37/50 | 74.0 | 9/13 | 69.2 | 11/23 | 47.8 |
| Extranodal | 29/86 | 33.8 | 13/50 | 26.0 | 4/13 | 30.8 | 12/23 | 52.2 |
| CD5 expression | 40/83 | 48.2 | 23/48 | 47.9 | 7/12 | 53.8 | 10/23 | 43.5 |
| Non-GC phenotype | 76/82 | 92.7 | 43/46 | 93.5 | 11/13 | 84.6 | 22/23 | 95.7 |
| EBV infection | 5/85 | 5.9 | 3/50 | 6.0 | 2/13 | 15.4 | 0/22 | 0 |
| RS treatment | ||||||||
| Regimen | ||||||||
| R-CHOP/CHOP-like | 35/82 | 42.7 | 26/49 | 53.1 | 4/11 | 36.4 | 5/22 | 22.7 |
| CHOP/CHOP-like | 18/82 | 22.0 | 8/49 | 16.3 | 4/11 | 36.4 | 6/22 | 27.3 |
| Second-line regimens for NHL | 6/82 | 7.3 | 4/49 | 8.2 | 1/11 | 9.1 | 1/22 | 4.5 |
| Fludarabine-based regimens | 7/82 | 8.5 | 5/49 | 10.2 | 0/11 | 0 | 2/22 | 9.1 |
| Other | 16/82 | 19.5 | 6/49 | 12.2 | 2/11 | 18.2 | 8/22 | 36.4 |
| Rituximab-based | 47/82 | 57.3 | 33/49 | 67.3 | 4/11 | 36.4 | 10/22 | 45.5 |
| Allogeneic stem cell transplantation | 7/82 | 8.5 | 6/49 | 12.1 | 0/11 | 0 | 1/22 | 4.5 |
| CR after RS induction treatment | 23/83 | 27.7 | 10/49 | 20.4 | 6/12 | 50.0 | 7/22 | 31.8 |
ALC indicates absolute lymphocyte count; Hb, hemoglobin; ULN, upper limit of normal; ZAP70, ζ-associated protein 70; IPI, International Prognostic Index; GC, germinal center; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; NHL, non-Hodgkin lymphoma; and CR, complete remission.
All RS were classified as DLBCL. CD5 was expressed in 40 of 83 RS samples (48.2%), and expression of CD10 was observed at transformation in 4 of 82 RS samples (4.9%), whereas it was consistently negative in all CLL samples (Table 1). According to cell of origin,31,32 the overwhelming majority of RS (76 of 82, 92.7%) displayed a non–germinal center phenotype. EBV infection was documented in 5 of 85 RS (5.9%; Table 1). Following the 98% cutoff value for identity to the germline, the IGHV genes were unmutated in 55 of 85 RS (64.7%) RS. Stereotyped VH CDR3 occurred in 31 of 85 cases (36.4%; Table 2).
Molecular characteristics of the RS series
| Characteristic . | Whole series . | Clonally related . | Clonally unrelated . | Undetermined clonal relationship . | ||||
|---|---|---|---|---|---|---|---|---|
| n/N . | % . | n/N . | % . | n/N . | % . | n/N . | % . | |
| Genetic features | ||||||||
| TP53 disruption* | 40/85 | 47.1 | 30/50 | 60.0 | 3/13 | 23.1 | 7/22 | 31.8 |
| c-MYC aberrations | 16/61 | 26.2 | 10/36 | 27.8 | 3/10 | 30.0 | 3/15 | 20.0 |
| Deletions | ||||||||
| 17p13 deletion | 28/86 | 32.6 | 20/50 | 40.0 | 3/13 | 23.1 | 5/23 | 21.7 |
| 11q22-q23 deletion | 8/53 | 15.1 | 4/31 | 12.9 | 2/11 | 18.2 | 2/11 | 18.2 |
| 13q14 deletion | 7/53 | 13.2 | 7/33 | 21.3 | 0/8 | 0 | 0/12 | 0 |
| 6q21 deletion | 4/50 | 8.0 | 2/31 | 6.5 | 0/7 | 0 | 2/12 | 16.7 |
| Translocations | ||||||||
| c-MYC translocation | 10/61 | 16.4 | 8/36 | 22.2 | 1/10 | 10.0 | 1/15 | 6.7 |
| BCL3 translocation | 1/46 | 2.2 | 1/26 | 3.8 | 0/9 | 0 | 0/11 | 0 |
| BCL6 translocation | 1/59 | 1.7 | 1/35 | 2.9 | 0/10 | 0 | 0/14 | 0 |
| BCL2 translocation | 0/60 | 0 | 0/35 | 0 | 0/9 | 0 | 0/16 | 0 |
| Amplifications | ||||||||
| c-MYC amplification | 6/61 | 9.8 | 2/36 | 5.6 | 2/10 | 20.0 | 2/15 | 13.3 |
| REL amplification | 4/39 | 10.3 | 3/24 | 12.5 | 0/6 | 0 | 1/9 | 11.1 |
| BCL2 amplification | 5/59 | 8.5 | 3/34 | 8.8 | 1/9 | 11.1 | 1/16 | 6.3 |
| BCL11A amplification | 2/25 | 8.0 | 2/17 | 11.8 | 0/3 | 0 | 0/5 | 0 |
| MYCN amplification | 2/54 | 3.7 | 2/31 | 6.5 | 0/8 | 0 | 0/15 | 0 |
| Trisomy 12 | 9/61 | 14.8 | 5/35 | 14.3 | 2/11 | 18.2 | 2/15 | 13.3 |
| Mutations | ||||||||
| TP53 mutation* | 31/85 | 36.4 | 23/50 | 46.0 | 2/13 | 15.4 | 6/22 | 27.3 |
| RhoH/TTF mutation | 4/28 | 14.3 | 4/25 | 16.0 | 0/1 | 0 | 0/2 | 0 |
| PIM1 mutation | 3/28 | 10.7 | 3/25 | 12.0 | 0/1 | 0 | 0/2 | 0 |
| PAX5 mutation | 2/28 | 7.1 | 2/25 | 8.0 | 0/1 | 0 | 0/2 | 0 |
| c-MYC mutation | 1/28 | 3.6 | 1/25 | 4.0 | 0/1 | 0 | 0/2 | 0 |
| BCL6 exon 1 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CARD11 mutation | 4/78 | 5.1 | 2/44 | 4.5 | 0/12 | 0 | 2/22 | 9.1 |
| PRDM1 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| TNFAIP3/A20 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CD79A mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CD79B mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| EZH2 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| Immunogenetic features | ||||||||
| IGHV identity ≥ 98% | 55/85 | 64.7 | 36/50 | 72.0 | 5/13 | 38.5 | 14/22 | 63.6 |
| Stereotyped VH CDR3* | 31/85 | 36.4 | 25/50 | 50.0 | 1/13 | 7.6 | 5/22 | 22.7 |
| Characteristic . | Whole series . | Clonally related . | Clonally unrelated . | Undetermined clonal relationship . | ||||
|---|---|---|---|---|---|---|---|---|
| n/N . | % . | n/N . | % . | n/N . | % . | n/N . | % . | |
| Genetic features | ||||||||
| TP53 disruption* | 40/85 | 47.1 | 30/50 | 60.0 | 3/13 | 23.1 | 7/22 | 31.8 |
| c-MYC aberrations | 16/61 | 26.2 | 10/36 | 27.8 | 3/10 | 30.0 | 3/15 | 20.0 |
| Deletions | ||||||||
| 17p13 deletion | 28/86 | 32.6 | 20/50 | 40.0 | 3/13 | 23.1 | 5/23 | 21.7 |
| 11q22-q23 deletion | 8/53 | 15.1 | 4/31 | 12.9 | 2/11 | 18.2 | 2/11 | 18.2 |
| 13q14 deletion | 7/53 | 13.2 | 7/33 | 21.3 | 0/8 | 0 | 0/12 | 0 |
| 6q21 deletion | 4/50 | 8.0 | 2/31 | 6.5 | 0/7 | 0 | 2/12 | 16.7 |
| Translocations | ||||||||
| c-MYC translocation | 10/61 | 16.4 | 8/36 | 22.2 | 1/10 | 10.0 | 1/15 | 6.7 |
| BCL3 translocation | 1/46 | 2.2 | 1/26 | 3.8 | 0/9 | 0 | 0/11 | 0 |
| BCL6 translocation | 1/59 | 1.7 | 1/35 | 2.9 | 0/10 | 0 | 0/14 | 0 |
| BCL2 translocation | 0/60 | 0 | 0/35 | 0 | 0/9 | 0 | 0/16 | 0 |
| Amplifications | ||||||||
| c-MYC amplification | 6/61 | 9.8 | 2/36 | 5.6 | 2/10 | 20.0 | 2/15 | 13.3 |
| REL amplification | 4/39 | 10.3 | 3/24 | 12.5 | 0/6 | 0 | 1/9 | 11.1 |
| BCL2 amplification | 5/59 | 8.5 | 3/34 | 8.8 | 1/9 | 11.1 | 1/16 | 6.3 |
| BCL11A amplification | 2/25 | 8.0 | 2/17 | 11.8 | 0/3 | 0 | 0/5 | 0 |
| MYCN amplification | 2/54 | 3.7 | 2/31 | 6.5 | 0/8 | 0 | 0/15 | 0 |
| Trisomy 12 | 9/61 | 14.8 | 5/35 | 14.3 | 2/11 | 18.2 | 2/15 | 13.3 |
| Mutations | ||||||||
| TP53 mutation* | 31/85 | 36.4 | 23/50 | 46.0 | 2/13 | 15.4 | 6/22 | 27.3 |
| RhoH/TTF mutation | 4/28 | 14.3 | 4/25 | 16.0 | 0/1 | 0 | 0/2 | 0 |
| PIM1 mutation | 3/28 | 10.7 | 3/25 | 12.0 | 0/1 | 0 | 0/2 | 0 |
| PAX5 mutation | 2/28 | 7.1 | 2/25 | 8.0 | 0/1 | 0 | 0/2 | 0 |
| c-MYC mutation | 1/28 | 3.6 | 1/25 | 4.0 | 0/1 | 0 | 0/2 | 0 |
| BCL6 exon 1 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CARD11 mutation | 4/78 | 5.1 | 2/44 | 4.5 | 0/12 | 0 | 2/22 | 9.1 |
| PRDM1 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| TNFAIP3/A20 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CD79A mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| CD79B mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| EZH2 mutation | 0/78 | 0 | 0/44 | 0 | 0/12 | 0 | 0/22 | 0 |
| Immunogenetic features | ||||||||
| IGHV identity ≥ 98% | 55/85 | 64.7 | 36/50 | 72.0 | 5/13 | 38.5 | 14/22 | 63.6 |
| Stereotyped VH CDR3* | 31/85 | 36.4 | 25/50 | 50.0 | 1/13 | 7.6 | 5/22 | 22.7 |
IGHV indicates immunoglobulin heavy chain variable region gene; and VH CDR3, immunoglobulin heavy chain complementarity-determining region 3.
Molecular features with significantly different prevalence between clonally related and clonally unrelated RS.
TP53 disruption and c-MYC abnormalities are the most frequent genetic lesions in RS
TP53 mutations (n = 35 mutation events) were documented in 31 of 85 RS (36.4%; Table 2). Four cases carried 2 mutations on independent alleles. All TP53 mutations predicted functional consequences according to the International Agency for Research on Cancer TP53 Mutation Database.30 Only 6 of 35 mutations (17.1%) were localized within codons that have been previously described as mutational hotspots in de novo DLBCL (Lys132, 0 mutations; Arg175, 0 mutations; Arg213, 3 mutations; Arg248, 1 mutation; Arg273, 2 mutations; Arg282, 0 mutations).43
Deletion of 17p13 was documented in 28 of 86 RS (32.6%). By combining results of 17p13 deletion and TP53 mutation in cases for which both data were available, 40 of 85 RS (47.1%) carried TP53 disruption through mutation or deletion or both. Among clonally related RS, analysis of CLL/RS pairs showed that TP53 disruption was acquired at transformation in 10 of 18 assessable cases (55.6%).
c-MYC abnormalities were documented in 16 of 61 patients (26.2%) and included c-MYC translocations (10 of 61, 16.4%) and c-MYC amplification (6 of 61, 9.8%). At variance with de novo DLBCL, c-MYC abnormalities in RS did not display concurrent BCL2 or BCL6 translocations.44 However, similar to other models of transformation from indolent to aggressive B-cell malignancy,45 half of the c-MYC abnormalities (8 of 16, 50.0%) paired with TP53 disruption in the same RS sample. Analysis of clonally related CLL/RS pairs disclosed that c-MYC abnormalities were acquired at transformation in 3 of 4 assessable cases.
RS lacked most genetic lesions that are recurrent in de novo DLBCL (Table 2).46 CARD11 mutations were restricted to 4 of 78 RS (5.1%) and were acquired at transformation in all assessable CLL/RS pairs. All cases were devoid of mutations targeting other genes of the nuclear factor-κB pathway (TNFAIP3/A20, 0 of 78; CD79A, 0 of 78; CD79B, 0 of 78). BCL6 translocation was rare (1 of 59). BCL2 translocation (0 of 60) as well as mutations of PRDM1 (0 of 78), BCL6 exon 1 (0 of 78), and EZH2 (0 of 78) were consistently absent in RS. Finally, aberrant somatic hypermutation (ASHM) of proto-oncogenes, which targets more than one-half de novo DLBCL,46 occurred at a low frequency in RS (aberrant somatic hypermutation targeting > 2 proto-oncogenes, 2 of 28, 7.1%).
TP53 disruption is an independent predictor of survival in RS
In this cohort, TP53 disruption emerged as a predictor of RS survival. Median survival for patients with TP53 mutations was 10.0 months (95% CI, 6.6-13.3 months) versus 27.0 months (95% CI, 8.5-45.4 months) for patients without TP53 mutations (P = .008; Figure 1A). In addition to TP53 mutations, TP53 deletion was also a predictor of RS survival. Median survival for RS with 17p13 deletion was 10.0 months (95% CI, 3.0-17.0 months) versus 35.8 months (95% CI, 7.1- 64.6 months) for patients without 17p13 deletion (P < .001; Figure 1B). Median survival for RS with TP53 disruption by mutation or deletion or both was 9.4 months (95% CI, 4.6- 14.2 months) versus 47.1 months (95% CI, 6.9-87.3 months) for patients without TP53 disruption (P < .001, q = .001; Table 3; Figure 1C). Compared with RS without TP53 disruption, survival was uniformly poor in all RS subgroups harboring any type of TP53 disruption (P < .05 for pairwise comparisons).
Kaplan-Meier estimates of RS survival according to TP53 status. (A) RS with TP53 mutations showed a significantly shorter survival (Events/N, 24/31; median, 10.0 months; 95% CI, 6.6-13.3 months) compared with RS devoid of TP53 mutations (Events/N, 30/52; median, 27.0 months; 95% CI, 8.5-45.4 months; P = .008). (B) RS with 17p13 deletion showed a significantly shorter survival (Events/N, 23/28; median, 10.0 months; 95% CI, 3.0-17.0 months) compared with RS devoid of 17p13 deletion (Events/N, 31/55; median, 35.8 months; 95% CI, 7.1-64.6 months; P < .001). (C) RS with TP53 disruption by deletion and/or mutation showed a significantly shorter survival (Events/N, 32/40; median, 9.4 months, 95% CI: 4.6-14.2 months) compared with RS without TP53 disruption (Events/N, 22/43; median, 47.1 months; 95% CI, 6.9-87.3 months).
Kaplan-Meier estimates of RS survival according to TP53 status. (A) RS with TP53 mutations showed a significantly shorter survival (Events/N, 24/31; median, 10.0 months; 95% CI, 6.6-13.3 months) compared with RS devoid of TP53 mutations (Events/N, 30/52; median, 27.0 months; 95% CI, 8.5-45.4 months; P = .008). (B) RS with 17p13 deletion showed a significantly shorter survival (Events/N, 23/28; median, 10.0 months; 95% CI, 3.0-17.0 months) compared with RS devoid of 17p13 deletion (Events/N, 31/55; median, 35.8 months; 95% CI, 7.1-64.6 months; P < .001). (C) RS with TP53 disruption by deletion and/or mutation showed a significantly shorter survival (Events/N, 32/40; median, 9.4 months, 95% CI: 4.6-14.2 months) compared with RS without TP53 disruption (Events/N, 22/43; median, 47.1 months; 95% CI, 6.9-87.3 months).
Variables associated with RS survival by univariate analysis
| Characteristic . | HR . | LCI . | UCI . | P . | q . |
|---|---|---|---|---|---|
| Clinical feature | |||||
| Age > 60 y | 2.41 | 1.30 | 4.44 | .005* | .019* |
| Male | 0.69 | 0.40 | 1.21 | .199 | .412 |
| ECOG PS > 1 | 4.54 | 2.52 | 8.20 | < .001* | < .001* |
| Ann Arbor III-IV | 1.92 | 0.59 | 6.18 | .274 | .485 |
| Binet B-C | 1.97 | 0.95 | 4.10 | .068 | .174 |
| Extranodal sites > 1 | 1.28 | 0.71 | 2.30 | .403 | .579 |
| Tumor size > 5 cm | 2.62 | 1.45 | 4.71 | .001* | .006* |
| ALC ≥ 5.0 × 109/L | 1.17 | 0.67 | 2.03 | .579 | .699 |
| Platelet count < 100 × 109/L | 2.20 | 1.23 | 3.91 | .007* | .023* |
| LDH > 1.5 ULN | 2.52 | 1.41 | 4.48 | .002* | .009* |
| CD38 expression | 0.81 | 0.38 | 1.66 | .544 | .699 |
| ZAP70 expression | 1.31 | 0.46 | 3.75 | .604 | .699 |
| Prior therapies > 1 | 1.00 | 0.57 | 1.76 | .979 | .979 |
| Rituximab-containing regimen | 0.80 | 0.46 | 1.38 | .312 | .513 |
| Allogeneic stem cell transplantation | 0.19 | 0.04 | 0.78 | .027* | .078* |
| CR after RS induction treatment | 0.21 | 0.04 | 0.46 | < .001* | .001* |
| Pathologic feature | |||||
| CD5 expression | 1.12 | 0.65 | 1.93 | .669 | .699 |
| Non-GC phenotype | 1.30 | 0.40 | 4.18 | .659 | .699 |
| EBV infection | 2.84 | 0.84 | 9.60 | .091 | .209 |
| Molecular features | |||||
| TP53 disruption | 2.90 | 1.67 | 5.04 | < .001* | .001* |
| c-MYC aberrations | 1.20 | 0.59 | 2.43 | .612 | .699 |
| IGHV identity ≥ 98% | 1.29 | 0.72 | 2.34 | .384 | .579 |
| Stereotyped VH CDR3 | 1.41 | 0.81 | 2.46 | .215 | .412 |
| Characteristic . | HR . | LCI . | UCI . | P . | q . |
|---|---|---|---|---|---|
| Clinical feature | |||||
| Age > 60 y | 2.41 | 1.30 | 4.44 | .005* | .019* |
| Male | 0.69 | 0.40 | 1.21 | .199 | .412 |
| ECOG PS > 1 | 4.54 | 2.52 | 8.20 | < .001* | < .001* |
| Ann Arbor III-IV | 1.92 | 0.59 | 6.18 | .274 | .485 |
| Binet B-C | 1.97 | 0.95 | 4.10 | .068 | .174 |
| Extranodal sites > 1 | 1.28 | 0.71 | 2.30 | .403 | .579 |
| Tumor size > 5 cm | 2.62 | 1.45 | 4.71 | .001* | .006* |
| ALC ≥ 5.0 × 109/L | 1.17 | 0.67 | 2.03 | .579 | .699 |
| Platelet count < 100 × 109/L | 2.20 | 1.23 | 3.91 | .007* | .023* |
| LDH > 1.5 ULN | 2.52 | 1.41 | 4.48 | .002* | .009* |
| CD38 expression | 0.81 | 0.38 | 1.66 | .544 | .699 |
| ZAP70 expression | 1.31 | 0.46 | 3.75 | .604 | .699 |
| Prior therapies > 1 | 1.00 | 0.57 | 1.76 | .979 | .979 |
| Rituximab-containing regimen | 0.80 | 0.46 | 1.38 | .312 | .513 |
| Allogeneic stem cell transplantation | 0.19 | 0.04 | 0.78 | .027* | .078* |
| CR after RS induction treatment | 0.21 | 0.04 | 0.46 | < .001* | .001* |
| Pathologic feature | |||||
| CD5 expression | 1.12 | 0.65 | 1.93 | .669 | .699 |
| Non-GC phenotype | 1.30 | 0.40 | 4.18 | .659 | .699 |
| EBV infection | 2.84 | 0.84 | 9.60 | .091 | .209 |
| Molecular features | |||||
| TP53 disruption | 2.90 | 1.67 | 5.04 | < .001* | .001* |
| c-MYC aberrations | 1.20 | 0.59 | 2.43 | .612 | .699 |
| IGHV identity ≥ 98% | 1.29 | 0.72 | 2.34 | .384 | .579 |
| Stereotyped VH CDR3 | 1.41 | 0.81 | 2.46 | .215 | .412 |
HR indicates hazard ratio; LCI, 95% lower confidence interval; UCI, 95% upper confidence interval; ALC, absolute lymphocyte count; ULN, upper limit of normal; ZAP70, ζ-associated protein 70; CR, complete response; GC, germinal center; IGHV, immunoglobulin heavy chain variable region gene; and VH CDR3, immunoglobulin heavy chain complementarity-determining region 3.
Statistically significant.
To assess the independent value of TP53 disruption in predicting RS outcome, we proceeded to test the prognostic role of clinical variables in our RS cohort. Among clinical variables at RS diagnosis, age > 60 years (P = .005, q = .019), ECOG PS > 1 (P < .001, q < .001), tumor size > 5 cm (P = .001, q = .006), platelet count < 100 × 109/L (P = .007, q = .023), and LDH > 1.5 ULN (P = .002, q = .009) were associated with poor survival (Table 3). Concerning RS therapies, the variables associated with improved survival were (1) achievement of CR after RS induction treatment (P < .001, q = .001) and (2) allogeneic SCT as therapy after remission (P = .027, q = .078). None of the pathologic features of DLBCL diagnostic biopsies correlated with RS prognosis (Table 3).
By multivariate analysis, TP53 disruption was selected in the final model as an independent predictor of RS survival (hazard ratio [HR], 2.27; 95% CI, 1.28-4.05; P = .004), along with achievement of CR after RS induction treatment (HR, 0.21; 95% CI, 0.08-0.51; P < .001) and poor ECOG PS > 1 (HR, 4.58; 95% CI, 2.05-8.40; P < .001; Table 4). After adjusting for clinical covariates in the multivariate analysis, RS lacking TP53 disruption had a 57% reduction in risk of death compared with RS harboring TP53 disruption. Clinical covariates accounted for only 22% of the overall risk of death determined by TP53 disruption.
Variables associated with RS survival by multivariate analysis
| Characteristic . | Final model* . | Bootstrap parameters, mean . | Bootstrap selection, % . | |||||
|---|---|---|---|---|---|---|---|---|
| HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | ||
| ECOG PS > 1 | 4.58 | 2.05 | 8.40 | < .001 | 5.82 | 2.90 | 11.79 | 96.4 |
| CR after RS induction treatment | 0.21 | 0.08 | 0.51 | < .001 | 0.20 | 0.08 | 0.52 | 95.1 |
| TP53 disruption | 2.27 | 1.28 | 4.05 | .004 | 2.66 | 1.43 | 4.96 | 94.4 |
| Characteristic . | Final model* . | Bootstrap parameters, mean . | Bootstrap selection, % . | |||||
|---|---|---|---|---|---|---|---|---|
| HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | ||
| ECOG PS > 1 | 4.58 | 2.05 | 8.40 | < .001 | 5.82 | 2.90 | 11.79 | 96.4 |
| CR after RS induction treatment | 0.21 | 0.08 | 0.51 | < .001 | 0.20 | 0.08 | 0.52 | 95.1 |
| TP53 disruption | 2.27 | 1.28 | 4.05 | .004 | 2.66 | 1.43 | 4.96 | 94.4 |
HR indicates hazard ratio; LCI, 95% lower confidence interval; UCI, 95% upper confidence interval; and CR, complete response.
Bias-corrected c-index of the final model: 0.801.
The final model for RS survival was internally validated with a bootstrapping resampling technique. The first step of validation showed that TP53 disruption, achievement of CR after RS induction treatment, and poor ECOG PS > 1 were the sole covariates selected at high frequency (> 90%) as independent predictors of RS survival in each one of the 1000 bootstrap samples that were generated (Table 4). This step of the analysis validated that TP53 disruption, achievement of CR after RS induction treatment, and poor ECOG PS > 1 are the most important variables affecting RS survival in this series. The second step of the validation analysis showed that the HRs produced from the original series were very close to those produced from the 1000 bootstrap samples (Table 4). This step validated the stability of the final model for RS survival prediction on the basis of TP53 disruption, achievement of CR after RS induction treatment, and poor ECOG PS > 1.
TP53 disruption, induction treatment response, and ECOG PS stratify patients with RS into distinct survival categories
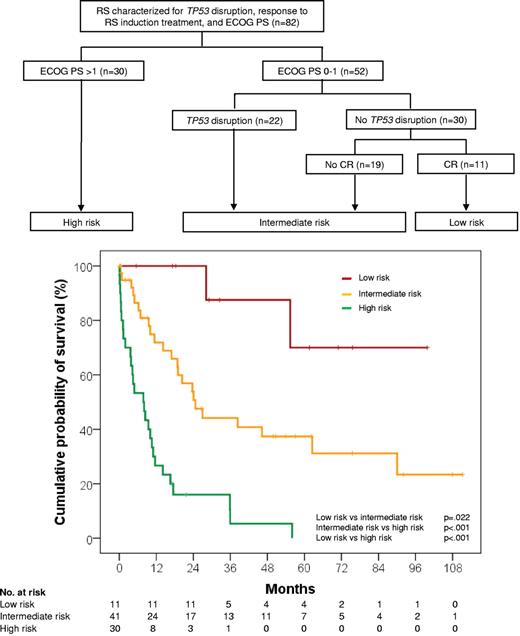
By recursive-partitioning analysis, TP53 status, response to RS induction treatment, and ECOG PS were used to build an algorithm for classifying patients with RS according to risk of death (Figure 2).
Classification of RS into risk-of-death categories and Kaplan-Meier estimates of RS survival according to those categories. The high-risk group includes patients presenting with a poor ECOG PS > 1, irrespective of TP53 status and type of response to RS induction treatment (Events/N, 28/30; median survival, 7.8 months; 95% CI, 2.5-13.1 months). The intermediate-risk group includes patients presenting with a good ECOG PS ≤ 1 but harboring TP53 disruption or not achieving CR after RS induction treatment despite being wild type on TP53 (Events/N, 23/41; median survival, 24.6 months; 95% CI, 15.8-33.4 months). The low-risk group includes patients who presented with a good ECOG PS ≤ 1, had no TP53 disruption, and achieved CR after RS induction treatment (Events/N, 2/11; median survival, not reached; 5-year survival, 70%; 95% CI, 34.4%-100%).
Classification of RS into risk-of-death categories and Kaplan-Meier estimates of RS survival according to those categories. The high-risk group includes patients presenting with a poor ECOG PS > 1, irrespective of TP53 status and type of response to RS induction treatment (Events/N, 28/30; median survival, 7.8 months; 95% CI, 2.5-13.1 months). The intermediate-risk group includes patients presenting with a good ECOG PS ≤ 1 but harboring TP53 disruption or not achieving CR after RS induction treatment despite being wild type on TP53 (Events/N, 23/41; median survival, 24.6 months; 95% CI, 15.8-33.4 months). The low-risk group includes patients who presented with a good ECOG PS ≤ 1, had no TP53 disruption, and achieved CR after RS induction treatment (Events/N, 2/11; median survival, not reached; 5-year survival, 70%; 95% CI, 34.4%-100%).
Patients presenting with a poor ECOG PS > 1 had a high risk of death, irrespective of TP53 status and type of response to RS induction treatment (median survival, 7.8 months; 95% CI, 2.5-13.1 months). Patients presenting with a good ECOG PS ≤ 1, but harboring TP53 disruption or not achieving CR after RS induction treatment despite being TP53 wild type, had an intermediate risk of death (median survival, 24.6 months; 95% CI, 15.8-33.4 months). Patients who presented with a good ECOG PS ≤ 1, had no TP53 disruption, and achieved CR after RS induction treatment displayed a low risk of death (median survival, not reached; 5-year survival, 70%; 95% CI, 34.4%-100%; Figure 2).
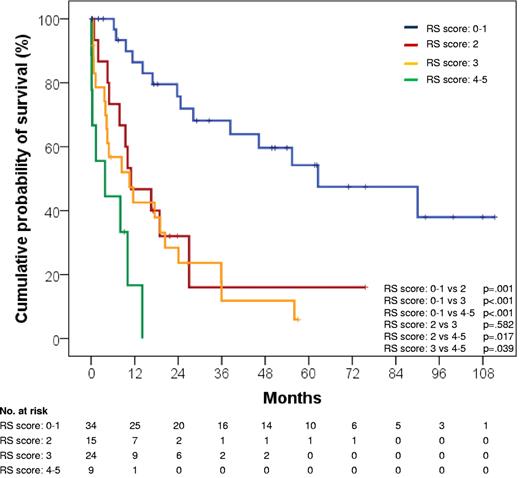
In this cohort, the prognostic model that included TP53 status, response to RS induction treatment, and ECOG PS allowed to better discriminate RS survival compared with the RS score that is based solely on clinical parameters,23 namely ECOG PS, LDH, platelet count, tumor size, and number of prior therapies (Figure 3). In fact, more than one-half (58.5%) RS that are assigned by our model to the intermediate risk group would otherwise be classified as favorable risk according to the RS score (Table 5).23 In addition, the model based on TP53 status, response to RS induction treatment, and ECOG PS had a significantly higher probability of correctly predicting RS survival (c-index = .809) compared with the clinical RS score (c-index = .750; P < .001).
Kaplan-Meier estimates of RS survival according to the RS score. The RS score is calculated by adding one point for each of the following risk factors: ECOG PS > 1, LDH > 1.5 ULN, platelet count < 100 × 109/L, tumor size > 5 cm, and number of prior CLL therapies > 1. RS score 0-1: events/N, 14/34; median survival, 62.5 months; 95% CI, 19.4-105.6 months. RS score 2: events/N, 11/15; median survival, 11.0 months; 95% CI, 1.9-20.0 monhts. RS score 3: events/N, 20/24; median survival, 10.5 months; 95% CI, 0.7-20.3 months. RS score 4-5: events/N, 8/9; median survival, 3.8 months; 95% CI, 0-11.1 months.
Kaplan-Meier estimates of RS survival according to the RS score. The RS score is calculated by adding one point for each of the following risk factors: ECOG PS > 1, LDH > 1.5 ULN, platelet count < 100 × 109/L, tumor size > 5 cm, and number of prior CLL therapies > 1. RS score 0-1: events/N, 14/34; median survival, 62.5 months; 95% CI, 19.4-105.6 months. RS score 2: events/N, 11/15; median survival, 11.0 months; 95% CI, 1.9-20.0 monhts. RS score 3: events/N, 20/24; median survival, 10.5 months; 95% CI, 0.7-20.3 months. RS score 4-5: events/N, 8/9; median survival, 3.8 months; 95% CI, 0-11.1 months.
Distribution of the risk group according to the RS score
| Risk group . | 0-1 points . | 2 points . | 3 points . | 4-5 points . |
|---|---|---|---|---|
| Low risk, n/N (%) | 10/11 (90.9) | 0/11 | 1/11 (9.1) | 0/11 |
| Intermediate risk, n/N (%) | 24/41 (58.5) | 8/41 (19.5) | 8/41 (19.5) | 1/41 (2.4) |
| High risk, n/N (%) | 0/30 | 7/30 (23.3) | 15/30 (50.0) | 8/30 (26.7) |
| Risk group . | 0-1 points . | 2 points . | 3 points . | 4-5 points . |
|---|---|---|---|---|
| Low risk, n/N (%) | 10/11 (90.9) | 0/11 | 1/11 (9.1) | 0/11 |
| Intermediate risk, n/N (%) | 24/41 (58.5) | 8/41 (19.5) | 8/41 (19.5) | 1/41 (2.4) |
| High risk, n/N (%) | 0/30 | 7/30 (23.3) | 15/30 (50.0) | 8/30 (26.7) |
Clonal relationship identifies distinct RS subsets with different molecular features and different prognosis
RS was clonally related to the CLL phase in 50 of 63 cases (79.3%) and clonally unrelated in 13 of 63 cases (20.6%). In 23 cases, clonal relationship remained undetermined because paired CLL samples were not available.
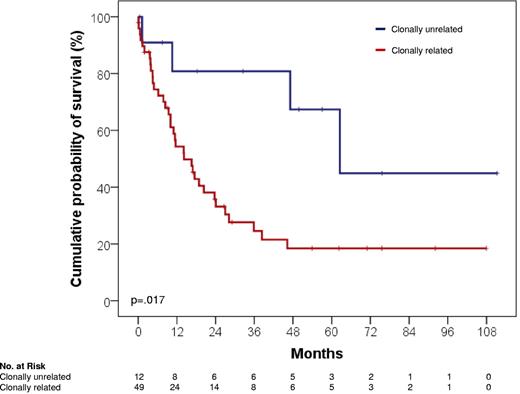
The biology and clinical course of clonally unrelated RS significantly differed from that of clonally related cases. From a molecular standpoint, clonally unrelated RS was characterized by a significantly lower prevalence of TP53 disruption (3 of 13, 23.1%) compared with clonally related cases (30 of 50, 60.0%; P = .018; Table 2). From an immunogenetic standpoint, clonally unrelated RS was characterized by a significantly lower prevalence of stereotyped VH CDR3 (1 of 13, 7.6%) compared with clonally related cases (25 of 50, 50.0%; P = .009; Table 2). The difference in stereotyped VH CDR3 frequency occurred irrespective of the IGHV gene mutational status.
The prognostic role of clonal relationship in RS was explored in cases for which this information was available (n = 63). Despite similar clinical features at presentation (P > .05 in all instances; Table 1), clonally unrelated RS was characterized by a significantly longer survival (median, 62.5 months; 95% CI, 34.0-91.0 months) compared with clonally related cases (median, 14.2 months; 95% CI, 7.7-20.6 months; P = .017; Figure 4). The survival difference was observed even though no case of clonally unrelated RS was consolidated with allogeneic SCT (Table 1).
Kaplan-Meier curves for RS survival from diagnosis according to clonal relationship. Clonally unrelated RS showed a significantly longer survival (Events/N, 4/12; median, 62.5 months; 95% CI, 34.0-91.0 months) compared with clonally related RS (Events/N, 35/49; median, 14.2 months; 95% CI, 7.7-20.6 months; P = .017).
Kaplan-Meier curves for RS survival from diagnosis according to clonal relationship. Clonally unrelated RS showed a significantly longer survival (Events/N, 4/12; median, 62.5 months; 95% CI, 34.0-91.0 months) compared with clonally related RS (Events/N, 35/49; median, 14.2 months; 95% CI, 7.7-20.6 months; P = .017).
Discussion
The results of this study indicate that RS is characterized by molecular and immunogenetic heterogeneity that might be important for clinical outcome. The original contributions of this study in the field of RS include the demonstration that (1) TP53 disruption is one of the major factors affecting RS survival and (2) clonally unrelated RS is clinically and biologically distinct from clonally related RS and is characterized by an outcome similar to that of de novo DLBCL.
In this cohort, TP53 disruption and c-MYC abnormalities emerged as the 2 most frequent recurrent genetic lesions in RS. Analysis of sequential samples documented that TP53 disruption and c-MYC abnormalities are frequently acquired at transformation, thus pointing to the pathogenetic relevance of these genetic lesions for the acquisition of an aggressive large cell phenotype. Acquisition of TP53 mutations and c-MYC aberrations represent a dual-hit genetic mechanism shared by other models of transformation from indolent to aggressive lymphomas,45,47,48 suggesting that, to a certain extent, the clinicopathologic transformation of B-cell malignancies follows similar pathways in different disease contexts. The high prevalence of TP53 disruption in RS probably reflects the selection of a chemorefractory clone under the pressure of previous CLL treatments49 and might explain the poor outcome of this disease and the limited sensitivity to conventional drugs.
Despite morphologic and phenotypic similarities with non–germinal center de novo DLBCL,24 the molecular profile of RS is not reminiscent of that lymphoma. In fact, most genetic features that are recurrent in de novo DLBCL, including alterations of the BCL6/PRDM1 and of the nuclear factor-κB pathways, are rare or absent in RS.46 Moreover, the high frequency of unmutated IGHV genes observed in RS is in sharp contrast with the immunogenetic features of de novo DLBCL that carry mutated IGHV genes in virtually all cases.50 Such molecular and immunogenetic differences between RS and de novo DLBCL might reflect differences in the microenvironment in which the lymphoma develops, as well as differences in host factors potentially relevant for B-cell malignancies, including the degree of immunocompetence and the host genetic background of patients with CLL.
The prognosis of RS is generally considered poor.3,4 However, a fraction of patients with RS may survive years after RS development.23 The RS score, which is currently the only tool to predict RS prognosis, fails to sort out cases projected to experience a long survival.23 In the present study, TP53 status, type of response after RS induction treatment, and ECOG PS emerged as independent predictors of RS survival and allowed to segregate RS into risk categories. The low-risk category shows an expected 5-year survival probability of 70% that in all but one patient was achieved only with conventional chemotherapy without allogeneic SCT, thus questioning the role of allogeneic SCT in this group of patients.23 Intermediate-risk patients, marked by TP53 disruption and failure to achieve CR after RS induction therapy, show an expected median survival of only 2 years, even though one-third of the patients received allogeneic SCT. This observation provides the rationale for clinical trials to test pretransplantation induction treatments with agents circumventing TP53 disruption.51-54 Patients belonging to the high-risk category, marked by ECOG PS > 1, show a poor outcome, with a median survival of only a few months. In these patients, the application of aggressive treatment is frequently hampered by patient frailty due to disease aggressiveness and to the sequelae of multiple lines of ineffective treatment that have a negative effect on performance status. To avoid deterioration of performance status, a close clinical monitoring for early recognition of RS development should be applied to patients with CLL at high risk of RS transformation because of their clinicobiologic profile.6-9
Several lines of evidence document that clonally unrelated and clonally related RS are distinct disorders both biologically and clinically. In terms of pathogenesis, the frequency of TP53 disruption in clonally unrelated RS is low and overall similar to that of de novo DLBCL,43 whereas TP53 inactivation occurs in more than one-half of clonally related RS. In addition, stereotyped VH CDR3, an immunogenetic feature of CLL denoting the pathogenetic role of antigens,55 is frequent in clonally related RS9 but rare in clonally unrelated cases. In terms of clinical outcome, our study shows that patients with clonally unrelated RS survive longer than clonally related cases and display a survival probability in the range of de novo DLBCL.24 At variance with our results, a previous investigation22 failed to show a difference in survival between clonally related and clonally unrelated RS. This discrepancy might be related to the different number of patients analyzed by Mao et al22 (19 clonally related and 5 clonally unrelated RS cases) compared with the current study (50 clonally related and 13 clonally unrelated RS cases). The lower number of cases analyzed by Mao et al22 might have underpowered the study to detect a statistically significant difference in survival between clonally related and unrelated RS.
Overall, these observations suggest that clonally unrelated RS should be considered as a secondary DLBCL arising de novo in the context of CLL rather than a true RS. Therefore, we propose that the diagnosis of RS should be restricted to clonally related cases. Given the molecular and immunogenetic heterogeneity of the disease, patients with RS should be offered an accurate work-up that focuses on the clonal relationship with the preceding CLL phase and on the occurrence of TP53 disruption. As suggested by the results of this study, knowledge of the biologic features of RS in the individual patient might have important implications for outcome prediction and clinical management.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michaela Cerri, PhD, for help in sequencing analysis and Antonio Ramponi, MD, for pathologic review of cases.
This study was supported by Progetto FIRB-Programma “Futuro in Ricerca” 2008 and PRIN 2008, MIUR, Rome, Italy; AIRC, Special Program Molecular Clinical Oncology, 5 × 1000, No. 10007, Milan, Italy; Progetto Giovani Ricercatori 2008, Ministero della Salute, Rome, Italy; Ricerca Sanitaria Finalizzata, Regione Piemonte, Torino, Italy; Oncosuisse grant OCS-02296-08-2008; Helmut Horten Foundation, San Salvatore Foundation, Fondazione per la Ricerca e la Cura sui Linfomi, Lugano, Switzerland; and Novara-Associazione Italiana contro le Leucemie, Linfomi e Mielomi (AIL) Onlus, Novara. V.S. is being supported by the Associazione Franca Capurro per Novara Onlus, Novara. E.C. is recipient of a European Society for Medical Oncology (ESMO) Fellowship Grant.
Authorship
Contribution: D.R. and G.G. designed the study, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; V.S. and S.R. performed mutation analysis and interpreted data; C.D. performed FISH analysis and interpreted data; L.L., K.S., L.A., M.L., G.B.R, Z.Y.X.-M., C.V., J.C., E.C., F.F., R.M., C.B., and T.P. collected clinical data; M.P., L.M.L., S.A.P., V.G., F.B., K.H.Y contributed to data analysis and interpretation; and R.F. contributed to data interpretation and to drafting the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Davide Rossi, Division of Hematology, Department of Clinical and Experimental Medicine, Amedeo Avogadro University of Eastern Piedmont, Via Solaroli 17, 28100 Novara, Italy; e-mail: rossidav@med.unipmn.it