Abstract
Severe combined immunodeficiency (SCID) carries a poor prognosis without definitive treatment by hematopoietic stem cell transplantation. The outcome for transplantation varies and is dependent on donor status and the condition of the child at the time of transplantation. Diagnosis at birth may allow for better protection of SCID babies from infection and improve transplantation outcome. In this comparative study conducted at the 2 designated SCID transplantation centers in the United Kingdom, we show that SCID babies diagnosed at birth because of a positive family history have a significantly improved outcome compared with the first presenting family member. The overall improved survival of more than 90% is related to a reduced rate of infection and significantly improved transplantation outcome irrespective of donor choice, conditioning regimen used, and underlying genetic diagnosis. Neonatal screening for SCID would significantly improve the outcome in this otherwise potentially devastating condition.
Introduction
Severe combined immunodeficiencies (SCIDs) are a genetically heterogeneous group of inherited defects characterized by severe abnormalities of immune system development and function.1,2 Affected infants present in the first few months of life with severe and recurrent infections; without definitive treatment, the condition is invariably fatal. The genetic defects in approximately 90% of the different forms of SCID have now been identified; and despite genetic heterogeneity, all patients are characterized by abnormalities of thymopoiesis and T-cell maturation and function.
Definitive treatment is available for SCID predominantly by allogeneic hematopoietic stem cell transplantation (HSCT)3,4 but also in specific forms of SCID (SCID-X1 and ADA-SCID) by gene therapy5-8 and by enzyme replacement therapy for adenosine deaminase (ADA) SCID.9 Detailed analysis of a large European SCID cohort showed that survival after HSCT was dependent on a number of factors.3 Patients transplanted before 6 months of age had a significantly better outcome. Matched sibling donor and matched unrelated donor transplants in SCID had approximately 90% and 80% 3-year survival rates, respectively, whereas mismatched transplants predominantly from parental donors showed a 65% success rate. The outcomes in the T−B− and ADA-deficient forms of SCID were also shown to be less successful, especially after mismatched donor transplants. Also important was the presence of pneumonitis before transplantation, with patients who have had such infections having a significantly poorer outcome (P < .0001).
Over the last 5 years, considerable interest has been generated by the ability to screen for SCID babies at birth. Detection of recent thymic emigrants by quantitative polymerase chain reaction of DNA extracted from neonatal dried blood spots can detect all SCID forms regardless of genetic diagnosis.10,11 The potential advantage of SCID newborn screening would be to protect infants at the time of birth from respiratory pathogens, including Pneumocystis jiroveci, and respiratory viruses (eg, adenovirus and parainfluenzae), and to undertake a transplantation at an earlier age. Myers et al12 showed that this strategy may be effective in 21 patients diagnosed at birth, but in this series all patients received an unconditioned procedure with the majority (19 of 21) receiving a T cell-depleted haploidentical procedure. To date, there have been no formal comparative data to show that newborn diagnosis would improve survival in patients regardless of the type of donor or conditioning regimen used. We therefore performed a retrospective study into the outcomes in a cohort of SCID patients who had been diagnosed antenatally or at birth because of a diagnosis of SCID in a previous sibling or family member. We compared this with the outcome in the first presenting person in the family. This report shows that, compared with the presenting sibling, SCID babies diagnosed at birth have a significantly decreased number of infections, are transplanted earlier, and have a dramatically improved survival outcome after HSCT regardless of the donor match, conditioning regimen, and SCID type.
Methods
Patient details and outcomes were gathered from databases held at the Great Ormond Street Hospital National Health Service Trust and Newcastle General Hospital, United Kingdom. Patients undergoing transplantation gave consent for data entry onto databases and use in studies in accordance with the Declaration of Helsinki. Data collection was approved by the Great Ormond Street Hospital and Newcastle General Hospital ethics committees.
Results and discussion
Data were collected from the 2 designated centers for transplantation of SCID in the United Kingdom. From 1982 to 2010, 60 SCID patients were diagnosed antenatally or at birth (termed sibling cohort) because of a diagnosis of SCID in 48 previous family members, most commonly a sibling (termed proband cohort) who presented between 1979 and 2009. Multiple subsequent births within families account for the difference in numbers between probands and siblings. Four of the sibling cohorts had been diagnosed antenatally, and the median age of diagnosis in the remaining sibling cohort was 0 days (range, 0-29 days). By contrast, the median age of diagnosis in the proband group was 143.5 days (range, 1-455 days; P < .001).
A diagnosis of SCID warrants intervention with prophylactic antibiotics and immunoglobulin replacement therapy. Specifically, all the sibling cohort was started on Septrin (GlaxoSmithKline) principally as antipneumocystis but also antibacterial prophylaxis once the risk of sulphonamide-induced jaundice had subsided. The proband group would not have received any prophylactic medication; and as a result, the rate of infectious complications in this group was high. Although data were unavailable for 19 of the 48 patients, in the remaining 29 probands, 89% (n = 26) had at least one infection, with 25 of 29 having multiple infections. The most common pathogen was P jiroveci, which was seen in 12 infants followed by fungal infection with Candida and together a total of 65 pathogens were identified in the 29 patients. By contrast, in the sibling cohort, where data were available on 57 of 60 patients, 17% (n = 10) had a total of 12 infections and no cases of pneumocystis were documented. Furthermore, there were no cases of parainfluenza virus and reduced numbers of other respiratory viruses in the sibling cohort.
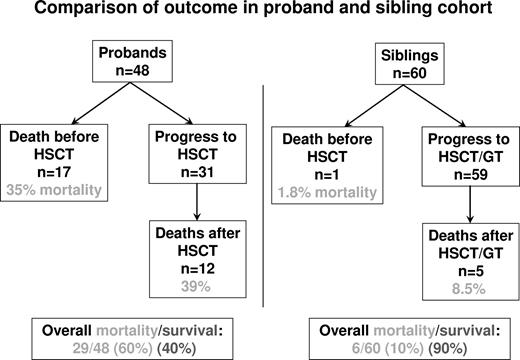
The outcomes of the 2 cohorts are summarized in Figure 1. Of the 48 probands, 17 (35.4%) died before allogeneic HSCT; and in all cases where data were available, the cause of death was an infectious complication. Of the sibling cohort, only one of the 60 patients died before HSCT, again of infection, and this was in a family who refused transplantation for the child. All surviving infants from both cohorts went onto allogeneic HSCT, apart from one child in the sibling cohort who received gene therapy for ADA-SCID because of the lack of a well-matched donor.
Flow chart showing survival outcomes in the 48 proband and 60 sibling cohorts.
The overall data for HSCT from the 2 cohorts show significant differences. Of the 31 probands who underwent HSCT, 12 died (38.7% transplantation-related mortality) and 19 survived the procedure (61.3%). By comparison, of the 59 sibling cohort who went through HSCT or gene therapy (GT), 5 died (8.5% mortality), giving a transplantation survival rate of 91.5% (P < .001). The significantly improved survival in the sibling cohort was analyzed in further detail to determine whether any differences in conditioning or donor availability may have made major contributions to improved survival outcome (Table 1).
Analysis of survival outcome in proband and sibling cohorts following different conditioning regimes, donor sources, and molecular diagnosis
| . | Proband cohort . | Mortality . | Sibling cohort . | Mortality . | Significance (P) . |
|---|---|---|---|---|---|
| Conditioning | |||||
| Unconditioned | 11 | 5 | 20 | 2 | < .05* |
| RIC | 7 | 2 | 20 | 0 | < .05* |
| Myeloablative | 12 | 5 | 18 | 3 | NS |
| Donor | |||||
| MSD | 6 | 0 | 11 | 1 | NS |
| MFD | 6 | 4 | 8 | 0 | < .05* |
| MUD | 2 | 0 | 5 | 1 | NS |
| mMUD | 1 | 0 | 3 | 0 | NS |
| Cord | 0 | 0 | 6 | 0 | NS |
| Haplo | 16 | 8 | 24 | 3 | < .01* |
| GT-autologous | 0 | 0 | 1 | 0 | NS |
| Genetic defect | |||||
| γ-c | 10 | 6 | 14 | 1 | < .01* |
| JAK3 | 3 | 2 | 3 | 1 | NS |
| IL-7RA | 1 | 0 | 3 | 1 | NS |
| RAG1/2 | 6 | 5 | 10 | 1 | < .01* |
| Artemis | 2 | 1 | 4 | 0 | NS |
| Omenn | 2 | 2 | 1 | 0 | NS |
| ADA | 8 | 5 | 8 | 1 | < .05* |
| Undefined | 16 | 8 | 17 | 1 | < .05* |
| . | Proband cohort . | Mortality . | Sibling cohort . | Mortality . | Significance (P) . |
|---|---|---|---|---|---|
| Conditioning | |||||
| Unconditioned | 11 | 5 | 20 | 2 | < .05* |
| RIC | 7 | 2 | 20 | 0 | < .05* |
| Myeloablative | 12 | 5 | 18 | 3 | NS |
| Donor | |||||
| MSD | 6 | 0 | 11 | 1 | NS |
| MFD | 6 | 4 | 8 | 0 | < .05* |
| MUD | 2 | 0 | 5 | 1 | NS |
| mMUD | 1 | 0 | 3 | 0 | NS |
| Cord | 0 | 0 | 6 | 0 | NS |
| Haplo | 16 | 8 | 24 | 3 | < .01* |
| GT-autologous | 0 | 0 | 1 | 0 | NS |
| Genetic defect | |||||
| γ-c | 10 | 6 | 14 | 1 | < .01* |
| JAK3 | 3 | 2 | 3 | 1 | NS |
| IL-7RA | 1 | 0 | 3 | 1 | NS |
| RAG1/2 | 6 | 5 | 10 | 1 | < .01* |
| Artemis | 2 | 1 | 4 | 0 | NS |
| Omenn | 2 | 2 | 1 | 0 | NS |
| ADA | 8 | 5 | 8 | 1 | < .05* |
| Undefined | 16 | 8 | 17 | 1 | < .05* |
RIC indicates reduced-intensity conditioning; MSD, matched sibling donor; MFD, matched family donor; MUD, matched unrelated donor; mMUD, mismatched unrelated donor; Cord, cord blood; Haplo, haploidentical; GT, gene therapy; ADA, adenosine deaminase; and NS, no statistical difference.
Statistically significant difference.
When conditioning regimens are considered, it was found that, even in unconditioned transplants, the mortality was 50% in the proband cohort. This suggests that preexisting infections were responsible for death. Only 2 of 20 patients undergoing unconditioned transplantation in the sibling cohort died. No deaths were seen in 20 reduced intensity transplants in the sibling cohort, whereas 2 of 7 probands died. Although not a statistically significant difference, in the proband cohort 41.6% (5 of 12) receiving myeloablative transplants died, whereas only 16% (3 of 18) died in the sibling cohort. Outcomes in relation to the type of donor used for transplantation highlight important findings. Even though there were proportionately more haploidentical transplantations undertaken in the sibling cohort than in the proband cohort, the former showed a significantly improved outcome. In the probands, 50% mortality (8 of 16) was seen after haploidentical transplantation, whereas only 12.5% mortality (3 of 24; P < .01) was seen in the sibling cohort. This difference cannot be explained by differing conditioning regimens as 5 of 16 probands did not receive conditionings in comparison to 4 of 24 siblings and myeloablative procedures were undertaken in 9 of 16 probands and 8 of 24 siblings. Differences were also observed in those undergoing matched family donor transplantations with a mortality of 67% (4 of 6) in the proband cohort, of which 4 were unconditioned, compared with none in the sibling cohort, of which 5 were unconditioned. No differences were seen for the other donor groups.
The underlying genetic diagnosis was also analyzed and shows important differences. There was a high mortality rate in the proband X-SCID cohort (6 of 10) but only one death in the sibling cohort. Of the siblings, 9 successfully underwent haploidentical transplantations; 4 of these were performed without conditioning. In patients with ADA deficiency, there was again a high mortality rate in probands with no deaths in the sibling cohort. The success in the ADA sibling cohort may in part be attributed to the early use of PEG-ADA, which will have protected infants from the metabolic consequences of ADA deficiency.
Studies have also shown that there has been an improvement in SCID transplantation outcome over time as supportive care and transplantation-related techniques have improved.3,13 We therefore performed a subcohort analysis of probands/siblings that were transplanted within 10 years of each other. In this subcohort analysis, 54% (13 of 24) probands survived compared with 93% (29 of 31) siblings, suggesting that, even if transplanted within 10 years of each other, there is still a significant improvement in outcome in SCIDs diagnosed at birth (Table 2).
Analysis of survival outcome in proband/sibling sets transplanted within 10 years of each other
| . | Total . | Survival . | Significance (P) . |
|---|---|---|---|
| Proband cohort | 24 | 13 (54%) | < .01* |
| Sibling cohort | 31 | 29 (93%) |
| . | Total . | Survival . | Significance (P) . |
|---|---|---|---|
| Proband cohort | 24 | 13 (54%) | < .01* |
| Sibling cohort | 31 | 29 (93%) |
Statistically significant difference.
Previous studies have shown an improvement in early T-cell recovery in newborn transplantations but no improvement in B-cell reconstitution.16 In our series, total T-cell numbers at 1 year after HSCT were no different between the 2 groups with a mean of 2418 CD3+ cells/mm3 (range, 209-5583 cells/mm3) in 14 evaluable probands compared with a mean of 2465 CD3+ cells/mm3 (range, 87-7956 cells/mm3) in 45 evaluable siblings. Similarly, there was no significant difference in humoral immune reconstitution with 15 of 18 evaluable probands able to stop immunoglobulin replacement compared with 43 of 51 evaluable siblings (data not shown). Thus, in contrast to previous studies, we did not see improved T-cell recovery in the siblings, although our analysis was not as detailed because of the unavailability of retrospective data and overall B-cell recovery was similar to that previously observed. Newborn transplantations in our study did not improve immune recovery, but neither was there any adverse effect. The differences between the 2 studies may relate to the patient population studied (predominantly X-SCID in the Myers et al cohort12 ) and the nature of the transplantations performed.
For the proband cohort, the overall mortality, including deaths before and after transplantation, was 60.4%, whereas a 10% overall mortality was observed in the sibling series (P < .001). These data show that the improved survival is a result of improved survival both before and after HSCT and after HSCT is seen irrespective of donor choice, conditioning regimen used, or underlying diagnosis. It is highly probable that the improved survival relates to the ability to make an early diagnosis, thereby protecting infants from infection and secondary organ damage and improving nutritional status, and therefore allowing an improved ability to withstand HSCT. These comparative data argue strongly for the more widespread use of neonatal screening for SCID, which may allow an extremely favorable prognosis for this previously devastating condition.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
H.B.G. is supported in part by the Great Ormond Street Hospital National Institute for Health Research Biomedical Research Center and Great Ormond Street Hospital Children's Charity.
Authorship
Contribution: L.B., J.X.-B., Z.A., A.R.G., and M.S. gathered and analyzed the data and reviewed the manuscript; P.V., E.G.D., and A.C. reviewed and advised on the manuscript; and H.B.G. conceived the project, directed data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Bobby Gaspar, Molecular Immunology Unit, UCL Institute of Child Health, 30 Guilford St, London WC1N 1EH, United Kingdom; e-mail: h.gaspar@ich.ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal