Abstract
Inflammatory signals induced during infection regulate T-cell expansion, differentiation, and memory formation. Toll-like receptors (TLRs) are inflammatory mediators that allow innate immune cells to recognize and respond to invading pathogens. In addition to their role in innate immune cells, we have found that signals delivered through the TLR adapter protein myeloid differentiation protein 88 (MyD88) play a critical, T cell–intrinsic role in supporting the survival and accumulation of antigen-specific effector cells after acute viral infection. However, the importance of MyD88-dependent signals in regulating the generation and maintenance of memory T cells remained unclear. To address this, we used a novel, inducible knockout system to examine whether MyD88 is required for optimal memory CD8 T-cell generation and responses after lymphocytic choriomeningitis virus infection. We show that whereas MyD88 is critical for initial T-cell expansion, it is not required for the subsequent differentiation and stable maintenance of a memory T-cell population. Furthermore, in contrast to naive CD8 T cells, memory CD8 T cells do not depend on MyD88 for their secondary expansion. Our findings clarify the importance of MyD88 during distinct phases of the antiviral T-cell response and establish differential dependence on MyD88 signaling as a novel characteristic that distinguishes naive from memory CD8 T cells.
Introduction
During acute viral infection, naive CD8 T cells undergo an initial phase of expansion and differentiation into functional effectors that play an integral role in viral clearance. The expansion phase is typically followed by a period of contraction, during which time the majority of effector T cells undergo apoptosis, and the remaining virus-specific T cells persist as a stable memory population that differs both phenotypically and functionally from the naive T-cell population. These memory CD8 T cells can rapidly differentiate into secondary effectors on subsequent reinfection and confer long-lasting, protective immunity. Thus, developing a better understanding of the signals that regulate the generation, maintenance, and reactivation of memory T cells is central to rational vaccine design.
Myeloid differentiation protein 88 (MyD88) is an adaptor protein that is required for signal transduction through most Toll-like receptors (TLRs), as well as the interleukin-1 receptor (IL-1R) family.1 TLR ligands that are released during infection are known agonists of MyD88-dependent pathways in innate immune cells, inducing activation, costimulatory molecule up-regulation, and inflammatory cytokine production, which serve to promote effective adaptive immune responses.2,3 In addition to this indirect role, we and others have shown previously that MyD88 plays a critical, T cell–intrinsic role in regulating the survival and accumulation of antigen-specific effector T cells after acute viral infection.4-6 Specifically, in the absence of MyD88, the number of effector CD8 T cells at the peak of the response and the number of antigen-specific memory T cells that persist are greatly reduced. It is well-established that the magnitude of the initial clonal burst significantly influences the size of the resulting memory T-cell compartment,7 suggesting that the reduced number of memory cells that develop in the absence of MyD88 may be a secondary outcome of the role of MyD88 in controlling naive T-cell expansion. However, there is abundant evidence to suggest a direct role for MyD88 in regulating memory T-cell generation. Memory T cells have been found to express higher levels of TLRs than naive T cells and are more responsive to TLR stimulation in vitro.8,9 In vitro TLR2 stimulation has also been shown to enhance memory CD8 T-cell generation,10 and recent work has further shown that TLR2 ligands can act directly on memory CD8 T cells in the absence of specific antigen to promote proliferation and effector cytokine production.11 These findings raise the possibility that MyD88-dependent signals may play an important role in regulating the homeostasis and responses of memory T cells that are generated during physiologic infections.
To evaluate this possibility, we used a novel, inducible knockout mouse system to examine whether MyD88 is required for optimal memory T-cell generation and responses after lymphocytic choriomeningitis virus (LCMV) infection. We confirm our earlier results showing that MyD88 expression is required for primary T-cell expansion after LCMV infection; however, we also show that in contrast to the generation of effector cells, the generation and maintenance of functional memory CD8 T cells proceeds independently of MyD88. Furthermore, we show that MyD88 is also not required for optimal expansion of memory CD8 T cells after secondary LCMV challenge. Our results identify reduced reliance on MyD88-dependent signals as a factor that distinguishes memory from naive T cells.
Methods
Mice
C57BL/6 (B6) mice were purchased from The Jackson Laboratory. Myd88−/− mice were kindly provided by S. Akira (Osaka University, Osaka, Japan), backcrossed at least 10 generations onto the B6 background, and maintained as a breeding colony in our facility. Myd88flox/flox mice have been described previously,12 and were backcrossed at least 7 generations onto the B6 background. Myd88flox/flox mice were crossed with CD4-Cre mice13 to generate Myd88ΔT mice with a T cell–specific deficiency in MyD88. Myd88flox/flox mice were also crossed with R26RYFP mice to generate mice in which Cre recombinase–mediated deletion is detectable by the yellow fluorescent protein (YFP) reporter.14 These mice were crossed with Myd88+/− CreT2 transgenic mice expressing a tamoxifen-inducible Cre recombinase15 to generate Myd88flox/−R26RYFPCreT2 (cKO) mice and Myd88flox/+R26RYFPCreT2 (cHet) controls. R26RYFP and CreT2 mice were purchased from The Jackson Laboratory and CD4 Cre mice were purchased from Taconic. These strains were backcrossed at least 10 generations onto the B6 background and maintained as breeding colonies in our facilities. All mice were housed in the animal facilities at the University of Pennsylvania (Philadelphia, PA) under specific pathogen-free conditions, and animal procedures were approved by the institutional animal care and use committee of each institution.
Tamoxifen-induced MyD88 deletion
Tamoxifen (Sigma-Aldrich) was prepared by mixing in 1 g/mL of ethanol. This mixture was then diluted to 20 mg/mL with corn oil and dissolved by incubating at 37°C for several hours with constant mixing. To induce deletion, cKO and cHet mice were treated with 200 μg of tamoxifen per gram body weight for 5 consecutive days by oral gavage. Mice were bled 5 days after the last day of treatment; successful treatment was confirmed by the presence of YFP+ cells.
LCMV infection
To induce acute LCMV infection, mice were injected with 2 × 105 plaque-forming units of LCMV-Armstrong (LCMV-Arm) via intraperitoneal injection. Secondary reinfection was induced by injecting 2 × 106 plaque-forming units of LCMV Clone 13 (LCMV-CL13) intravenously into mice that had been infected with LCMV-Arm 50-60 days earlier.
Flow cytometry and immunoblotting
Allophycocyanin-conjugated major histocompatibility complex class I tetramers of H-2Db complexed with LCMV GP33-41, NP396-404, and GP276-286 were prepared as described previously.16 All antibodies were purchased from eBiosciences or BD Biosciences. Surface and intracellular staining was performed according to the manufacturers' protocols, and cells were analyzed using FACSCanto or LSRII flow cytometers (BD Biosciences) or sorted using a FACSAria cell sorter (BD Biosciences). Data analysis was performed using FlowJo software (TreeStar).
To determine MyD88 expression, cell populations were sorted from the spleens and peripheral lymph nodes of mice as indicated in the figure legends, lysed in SDS-PAGE/lysis buffer (Bio-Rad), resolved on a 10% Bis-Tris SDS-PAGE gel (Life Technologies), transferred to a Hybond enhanced chemiluminescence nitrocellulose membrane (Amersham Biosciences), and probed with rabbit anti–mouse MyD88 antibody (2127; ProSci). Detection was conducted with horseradish peroxidase–conjugated secondary antibodies (Amersham Biosciences) and enhanced chemiluminescence reagent (Amersham Biosciences).
Statistical analysis
Groups were compared using a Student t test (2-tailed distribution, equal variance) and differences with a P value < .05 were considered statistically significant.
Results
MyD88 expression in T cells regulates primary expansion after acute LCMV infection
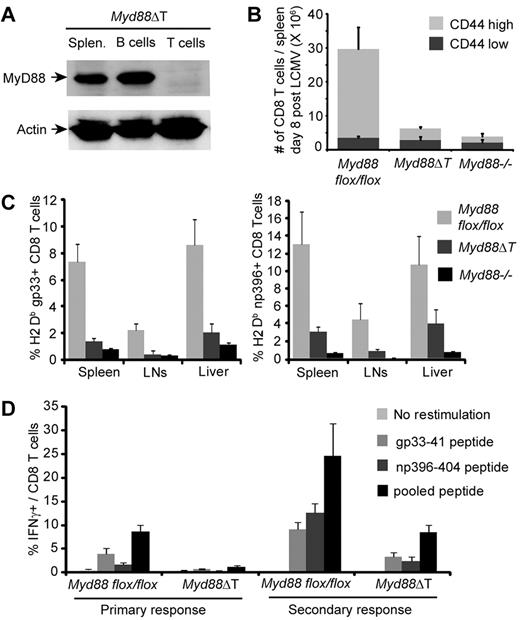
Whereas we and others have previously used adoptive transfers and mixed bone marrow chimeras to study in vivo the role of MyD88 in naive T cells,4-6 we believed that determination of its role in memory T-cell responses would be best accomplished via conditional, temporally regulated deletion. This could potentially be done using a recently described Myd88flox/flox mouse.12 To validate the use of this system, we first verified that conditional deletion of MyD88 in naive T cells using Myd88flox/flox mice produced results consistent with our earlier findings of reduced effector CD8 T-cell survival and accumulation using germline Myd88−/− mice.6 We therefore crossed Myd88flox/flox mice with mice expressing Cre recombinase under the CD4 promoter. In the resulting mice, which we will subsequently refer to as Myd88ΔT mice, MyD88 expression is selectively deleted in all αβT cells on the basis of Cre expression starting at the CD4+CD8+ double-positive stage of thymocyte development (Figure 1A).
Myd88ΔT mice mount greatly reduced CD8 T-cell responses to LCMV infection. (A) Splenocytes were isolated from a Myd88ΔT mouse, T and B cells were FACS purified, and MyD88 expression was examined by Western blot. (B-C) Myd88flox/flox, Myd88ΔT, and Myd88−/− mice were examined 8 days after infection with LCMV-Arm. Data represent the means ± SD of 4 mice per group. (B) The number of splenic CD8 T cells was determined. (C) The frequency of H2-Db-gp33–specific (left panel) and H2-Db-np396–specific (right panel) cells among gated CD8 T cells from the indicated tissues was measured using tetramers. (D) Naive Myd88flox/flox and Myd88ΔT mice and mice that had been primed with LCMV-Arm 60 days earlier were infected with LCMV-CL13. Six days after CL13 infection, splenocytes were restimulated with the indicated peptides and antigen-specific IFNγ production was assessed. Data represent the means ± SD of at least 3 mice per group.
Myd88ΔT mice mount greatly reduced CD8 T-cell responses to LCMV infection. (A) Splenocytes were isolated from a Myd88ΔT mouse, T and B cells were FACS purified, and MyD88 expression was examined by Western blot. (B-C) Myd88flox/flox, Myd88ΔT, and Myd88−/− mice were examined 8 days after infection with LCMV-Arm. Data represent the means ± SD of 4 mice per group. (B) The number of splenic CD8 T cells was determined. (C) The frequency of H2-Db-gp33–specific (left panel) and H2-Db-np396–specific (right panel) cells among gated CD8 T cells from the indicated tissues was measured using tetramers. (D) Naive Myd88flox/flox and Myd88ΔT mice and mice that had been primed with LCMV-Arm 60 days earlier were infected with LCMV-CL13. Six days after CL13 infection, splenocytes were restimulated with the indicated peptides and antigen-specific IFNγ production was assessed. Data represent the means ± SD of at least 3 mice per group.
We found greatly reduced CD8 T-cell expansion in Myd88ΔT mice in response to acute LCMV infection, resulting in dramatically reduced numbers of CD44highCD8 T cells in the spleens of infected Myd88ΔT mice compared with Myd88flox/flox littermates that did not express Cre-recombinase (P < .01; Figure 1B). Strikingly, CD8 T-cell numbers in Myd88ΔT mice were reduced almost to the extent of complete Myd88−/− mice, supporting our earlier findings suggesting that the contribution of MyD88 to T-cell expansion during LCMV infection is largely T-cell intrinsic.6 Consistent with our earlier findings, we found that the reduced number of CD44highCD8 T cells was correlated with a reduced frequency of LCMV-specific effector cells in all tissues examined, as identified by tetramer staining (P < .05; Figure 1C). Assessment of antigen-specific IFNγ production indicated a reduced frequency of effector CD8 T cells in Myd88ΔT mice during both the primary response and the secondary memory response to LCMV infection (Figure 1D). Overall, these findings confirm our earlier results showing that MyD88 expression in T cells is critical for T-cell responses to LCMV infection, and establish that conditional deletion of MyD88 using Myd88flox/flox mice represents an appropriate model for examining the role of MyD88 during LCMV infection.
A system to temporally delete MyD88
Our results indicated that MyD88 regulates secondary T-cell responses to LCMV infection. However, it is unclear whether this reflects a direct role for MyD88 in regulating memory T-cell responses or this it was an indirect consequence of the importance of MyD88 in regulating primary responses to LCMV infection. To address this question, we developed a system in which we could circumvent the defects in memory T-cell formation associated with the absence of MyD88 during the primary response to LCMV infection. We crossed Myd88flox/flox mice with CreT2 transgenic mice, allowing tamoxifen-inducible deletion of MyD88 expression. To better identify cells that underwent Cre-mediated deletion, we further crossed these mice with R26RYFP reporter mice in which the activity of Cre recombinase induces the expression of YFP. To improve the efficiency of Cre-mediated deletion of MyD88, we used experimental mice in which only a single allele of MyD88 needed to be deleted because the other was knocked out in the germline (cKO). As controls we used mice in which one allele of MyD88 was floxed and the other was WT (cHet).
We found that tamoxifen treatment induced high expression of YFP in 25%-50% of splenocytes in both cKO and cHet mice, and that the proportion of YFP-expressing cells in the CD4 and CD8 T-cell compartments was comparable to the proportion in the overall splenocyte population (Figure 2A). To determine whether YFP serves as an accurate reporter of MyD88 deletion, we sorted the YFP+ and YFP− fractions from tamoxifen-treated cKO and cHet mice or untreated controls, and performed a Western blot to assess MyD88 protein levels. We found that the majority of YFP+ splenocytes in cKO mice had deleted MyD88, whereas there was minimal deletion in the YFP− compartment (Figure 2B). In contrast, MyD88 expression was comparable in YFP+ and YFP− splenocytes from cHet mice, suggesting that the loss of a single Myd88 allele does not appreciably reduce MyD88 protein expression. Whereas this showed that tamoxifen treatment effectively induced MyD88 deletion in bulk YFP+ cKO splenocytes, we also wanted to confirm that deletion occurred specifically in the T-cell compartment. Therefore, we sorted YFP+ and YFP− CD4 and CD8 T cells from cKO mice and examined MyD88 expression by Western blot. Consistent with the overall splenocyte population, we found that MyD88 was largely deleted in the YFP+ CD4 and CD8 T cells, but was only minimally deleted in the YFP− population (Figure 2C).
Tamoxifen treatment induces deletion of MyD88 in YFP+ cells in cKO but not cHet mice. (A) cHet and cKO mice were treated with tamoxifen or left untreated, and YFP expression in the indicated splenocyte populations was determined 5 days later. (B) YFP+ and YFP− splenocytes were FACS purified from tamoxifen-treated or untreated cHet and cKO mice, and MyD88 expression was determined by Western blot. (C) YFP+ and YFP− CD4 and CD8 T cells were FACS purified from treated or untreated cKO mice, and MyD88 expression was determined by Western blot.
Tamoxifen treatment induces deletion of MyD88 in YFP+ cells in cKO but not cHet mice. (A) cHet and cKO mice were treated with tamoxifen or left untreated, and YFP expression in the indicated splenocyte populations was determined 5 days later. (B) YFP+ and YFP− splenocytes were FACS purified from tamoxifen-treated or untreated cHet and cKO mice, and MyD88 expression was determined by Western blot. (C) YFP+ and YFP− CD4 and CD8 T cells were FACS purified from treated or untreated cKO mice, and MyD88 expression was determined by Western blot.
To further confirm that YFP+ cells in cKO mice reproduce the phenotype that we had observed in germline Myd88−/− T cells and Myd88ΔT mice, we infected tamoxifen-treated cKO and cHet mice with LCMV. As predicted, we observed significantly reduced expansion of the YFP+ CD8 T cells in cKO relative to cHet mice after LCMV infection (P < .01; Figure 3A). In contrast, we did not observe a significant difference in the expansion of the YFP− CD8 T cells in cKO relative to cHet mice, consistent with the limited deletion of MyD88 that we had observed in YFP− cKO T cells (Figure 2C). In further support of our finding that YFP− and YFP+ cells in cHet mice express comparable levels of MyD88 (Figure 2B), there was no significant difference between the expansion of YFP+ and YFP− CD8 T cells in cHet mice (Figure 3A), suggesting that heterozygous expression of MyD88 is sufficient to regulate T-cell expansion in response to LCMV infection. The reduced CD8 T-cell expansion in cKO mice corresponded to a significantly reduced percentage of LCMV-specific effector cells in the YFP+ but not in the YFP− CD8 T-cell population, as determined by antigen-specific IFNγ production (Figure 3B) and tetramer staining (Figure 3C). These results demonstrate that tamoxifen treatment results in conditional deletion of MyD88 in cKO but not in cHet mice, and that YFP expression serves as an accurate reporter for MyD88 expression in cKO cells.
YFP+ T cells in cKO mice exhibit reduced expansion in response to LCMV infection. cKO and cHet mice were treated with tamoxifen and infected with LCMV-Arm 5 days later. Splenocytes were examined 8 days after infection. (A) The frequency of CD8 T cells in the YFP+ (left panel) and YFP− (right panel) splenocyte populations was determined. (B) Splenocytes were restimulated with the indicated peptides and the frequency of IFNγ-producing YFP+ (left panel) and YFP− (right panel) CD8 T cells was determined by intracellular staining. (C) The frequency of LCMV-specific YFP+ CD8 T cells was determined using tetramers. A representative flow cytometry plot is shown and all graphs represent the means ± SD of at least 4 mice.
YFP+ T cells in cKO mice exhibit reduced expansion in response to LCMV infection. cKO and cHet mice were treated with tamoxifen and infected with LCMV-Arm 5 days later. Splenocytes were examined 8 days after infection. (A) The frequency of CD8 T cells in the YFP+ (left panel) and YFP− (right panel) splenocyte populations was determined. (B) Splenocytes were restimulated with the indicated peptides and the frequency of IFNγ-producing YFP+ (left panel) and YFP− (right panel) CD8 T cells was determined by intracellular staining. (C) The frequency of LCMV-specific YFP+ CD8 T cells was determined using tetramers. A representative flow cytometry plot is shown and all graphs represent the means ± SD of at least 4 mice.
CD8 T-cell contraction and memory differentiation after LCMV infection is MyD88 independent
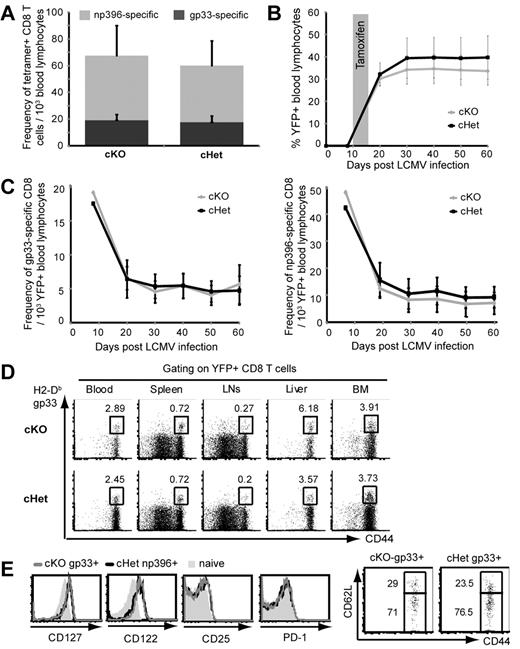
Having shown that the YFP+ T cells in cKO and cHet mice closely replicate the phenotype of germline Myd88−/− and Myd88+/− T cells after LCMV infection, we used matched cKO and cHet control mice to examine whether MyD88 plays a role in the contraction and differentiation of memory T cells independently of its role in initial expansion. After infection with LCMV-Arm, we found no significant differences in the frequency of LCMV-specific CD8 T cells in the blood of cKO and cHet mice 8 days after infection (Figure 4A), confirming that Myd88 is haplosufficient for regulating initial T-cell expansion. Mice were treated with tamoxifen beginning on day 10 after infection to delete MyD88 after the peak of the initial CD8 T-cell response. Serial bleeds showed that tamoxifen treatment induced a comparable frequency of YFP+ cells in the blood of both cKO and cHet mice, and that these cells were maintained stably after infection (Figure 4B), indicating that expression of YFP as a neoantigen in cells that deleted MyD88 did not induce their rejection.
MyD88 is not required for the development and maintenance of memory T cells after LCMV infection. (A) The frequency of tetramer-specific CD8 T cells in the blood of cKO and cHet mice was determined 8 days after LCMV infection. (B) cKO and cHet mice were treated with tamoxifen 10 days after LCMV infection, and the frequency of YFP+ cells was measured in blood lymphocytes by serial bleeds. (C) The frequency of H2-Db-gp33–specific (left panel) and H2-Db-np396–specific (right panel) cells within YFP+ blood lymphocytes of cKO and cHet mice was determined by tetramer staining. Data represent the means ± SD of at least 8 mice. (D) The frequency of H2-Db-gp33–specific cells within the YFP+ CD8 T cells from the indicated tissues of cKO and cHet mice was assessed by tetramer staining 60 days after infection. (E) The expression of the indicated phenotypic markers was determined on gated gp33-specific YFP+ cells from the spleens of cKO and cHet mice 60 days after infection.
MyD88 is not required for the development and maintenance of memory T cells after LCMV infection. (A) The frequency of tetramer-specific CD8 T cells in the blood of cKO and cHet mice was determined 8 days after LCMV infection. (B) cKO and cHet mice were treated with tamoxifen 10 days after LCMV infection, and the frequency of YFP+ cells was measured in blood lymphocytes by serial bleeds. (C) The frequency of H2-Db-gp33–specific (left panel) and H2-Db-np396–specific (right panel) cells within YFP+ blood lymphocytes of cKO and cHet mice was determined by tetramer staining. Data represent the means ± SD of at least 8 mice. (D) The frequency of H2-Db-gp33–specific cells within the YFP+ CD8 T cells from the indicated tissues of cKO and cHet mice was assessed by tetramer staining 60 days after infection. (E) The expression of the indicated phenotypic markers was determined on gated gp33-specific YFP+ cells from the spleens of cKO and cHet mice 60 days after infection.
We examined the contraction and maintenance of LCMV-specific CD8 T cells after deletion of MyD88 by measuring their frequency in peripheral blood. Because we had found that MyD88 was only deleted effectively in the YFP+ population of cKO mice (Figure 2), we limited our analysis to YFP+ T cells. We found that the populations of both gp33-specific and np396-specific YFP+CD8 T cells contracted with similar kinetics and were maintained at a comparable frequency in the blood of cKO and cHet mice for up to 60 days after infection (Figure 4C).
One of the attributes that distinguishes memory T cells from naive T cells is their migration through peripheral tissues and bone marrow. Because in vitro studies have suggested that TLR stimulation may influence the expression of chemokines and integrins that regulate trafficking,17 we examined whether the loss of MyD88 influenced the trafficking of memory T cells. We found a comparable frequency of gp33-specific cells within the YFP+ lymphocyte population in the blood, spleen, lymph nodes, liver, and bone marrow of cKO and cHet mice 60 days after LCMV infection (Figure 4D). This showed that the comparable frequency of LCMV-specific memory cells detected in the blood of cKO and cHet mice reflected a comparable representation in other compartments, suggesting that MyD88 is not required for normal homing and circulation of memory cells.
Consistent with their stable maintenance, we found that YFP+ LCMV-specific memory CD8 T cells in cKO mice expressed comparable levels of CD127, CD122, and CD25 to those in cHet mice, suggesting normal recognition of the homeostatic cytokines IL-7, IL-2, and IL-15 (Figure 4E). In addition, we found that YFP+ LCMV-specific memory cells in both cKO and cHet mice expressed comparable levels of CD62L, indicating that MyD88 is not required for differentiation into effector memory or central memory subsets. We also noted that both cKO and cHet memory cells had down-regulated PD-1 expression to the levels of naive cells, further supporting a memory-cell phenotype rather than a functionally exhausted phenotype.
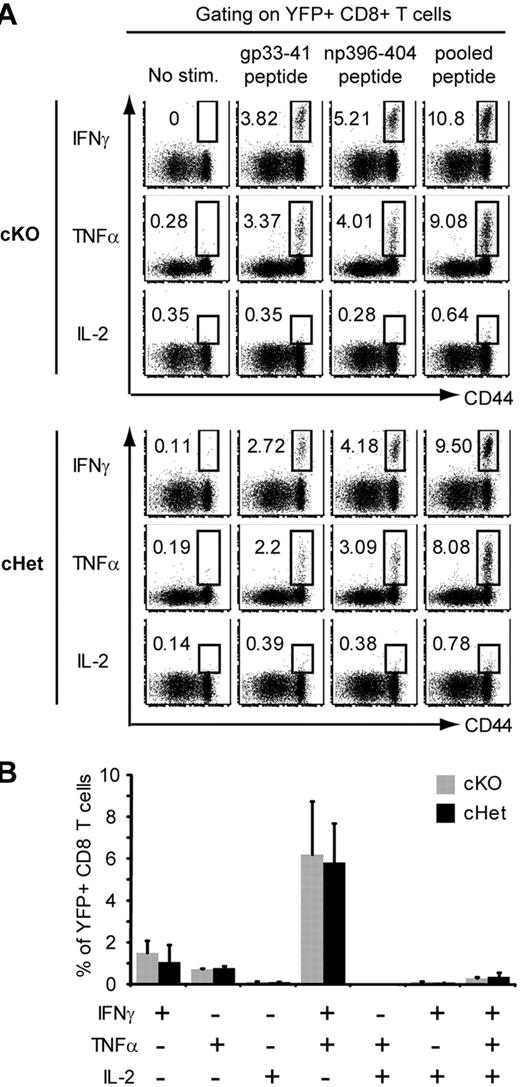
Because normal memory T cells are able to rapidly acquire effector functions in response to stimulation, we examined whether the loss of MyD88 altered memory T-cell cytokine production. Sixty days after LCMV infection, we found that a comparable frequency of YFP+ CD8 T cells in the splenocytes of cKO and cHet mice produced IFNγ, tumor necrosis factorα (TNFα), and IL-2 after a brief restimulation with LCMV-derived peptides (Figure 5A). Furthermore, there was no significant difference in the overall distribution of memory cells that produced various combinations of these cytokines (Figure 5B), suggesting that multifunctional memory cells develop independently of MyD88. Overall, these findings indicate that MyD88 regulates primary T-cell expansion, but once expansion has occurred (ie, by day 10 after infection), MyD88 is no longer required for the differentiation, maintenance, and migration of functional LCMV-specific memory CD8 T cells.
MyD88 is not required for the rapid production of effector cytokines by memory T cells. cKO and cHet mice were infected with LCMV and then treated with tamoxifen as in Figure 4. Splenocytes were isolated 60 days after LCMV infection and restimulated with the indicated LCMV-derived peptides. (A) IFNγ, TNFα, and IL-2 production by YFP+ CD8 T cells was assessed by intracellular staining. (B) The frequency of YFP+ CD8 T cells producing the indicated combinations of cytokines in response to stimulation with pooled LCMV peptide was determined. Data represent the means ± SD of 3 mice.
MyD88 is not required for the rapid production of effector cytokines by memory T cells. cKO and cHet mice were infected with LCMV and then treated with tamoxifen as in Figure 4. Splenocytes were isolated 60 days after LCMV infection and restimulated with the indicated LCMV-derived peptides. (A) IFNγ, TNFα, and IL-2 production by YFP+ CD8 T cells was assessed by intracellular staining. (B) The frequency of YFP+ CD8 T cells producing the indicated combinations of cytokines in response to stimulation with pooled LCMV peptide was determined. Data represent the means ± SD of 3 mice.
MyD88 does not regulate memory T-cell responses to secondary LCMV infection
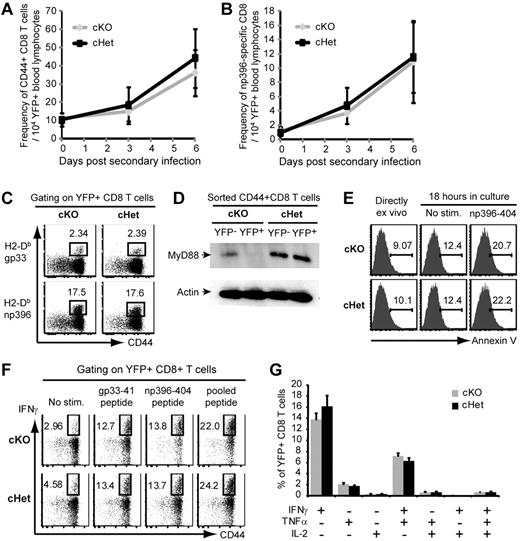
Our results thus far indicated that T-cell expression of MyD88 was required during initial expansion after LCMV infection, but was dispensable for the subsequent development of functional memory cells. We next examined whether MyD88 plays a role in controlling the survival and expansion of memory cells after secondary infection. LCMV-immune cKO and cHet mice that had been infected with LCMV-Arm 60 days earlier and treated with tamoxifen after the primary response were secondarily infected with a high dose of LCMV-CL13. Reinfection induced a rapid expansion of YFP+ CD8 T cells in both cKO and cHet mice (Figure 6A). We detected minimal expansion of the gp33-specific population in the blood of either cKO and cHet mice (data not shown), but did find robust expansion of the np396-specific population (Figure 6B), which has been reported to dominate secondary responses.18 Consistent with the pattern in overall CD44high CD8 T cells, there were no significant differences in the frequencies of YFP+ np396-specific CD8 T cells in the blood of cKO and cHet mice; this was mirrored by a comparable frequency of LCMV-specific CD8 T cells in the spleens of cKO and cHet mice 6 days after infection (Figure 6C).
Memory T-cell expansion in response to secondary LCMV infection is MyD88 independent. cKO and cHet mice were infected with LCMV-Arm, treated as in Figure 4, and reinfected with LCMV-CL13 60 days after primary infection. (A-B) The frequency of CD44+ (A) and H2-Db-np396–specific (B) YFP+ CD8 T cells was measured in the peripheral blood at the indicated time points. Data are the means ± SD of 9 mice per group. (C) The frequency of antigen-specific YFP+ CD8 T cells in the spleens of cKO and cHet mice was assessed 6 days after reinfection by tetramer staining. Data are representative of 9 mice per group. (D) YFP+ and YFP− CD44+CD8 T cells were sorted from the spleens of reinfected cKO and cHet mice and MyD88 expression was measured by Western blot. (E) The viability of gated np396-specific YFP+ CD8 T cells from cKO and cHet mice was assessed by annexin V staining either directly ex vivo or after the indicated 18 hours of in vitro culture as indicated. Data are representative of 3 mice per group. (F) Splenocytes were restimulated with the indicated LCMV-derived peptides, and IFNγ production by YFP+ CD8 T cells was assessed by intracellular staining. (G) The frequency of YFP+ CD8 T cells producing the indicated combinations of IFNγ, TNFα, and IL-2 in response to stimulation with pooled LCMV peptide was determined. Data represent the means ± SD of 3 mice per group.
Memory T-cell expansion in response to secondary LCMV infection is MyD88 independent. cKO and cHet mice were infected with LCMV-Arm, treated as in Figure 4, and reinfected with LCMV-CL13 60 days after primary infection. (A-B) The frequency of CD44+ (A) and H2-Db-np396–specific (B) YFP+ CD8 T cells was measured in the peripheral blood at the indicated time points. Data are the means ± SD of 9 mice per group. (C) The frequency of antigen-specific YFP+ CD8 T cells in the spleens of cKO and cHet mice was assessed 6 days after reinfection by tetramer staining. Data are representative of 9 mice per group. (D) YFP+ and YFP− CD44+CD8 T cells were sorted from the spleens of reinfected cKO and cHet mice and MyD88 expression was measured by Western blot. (E) The viability of gated np396-specific YFP+ CD8 T cells from cKO and cHet mice was assessed by annexin V staining either directly ex vivo or after the indicated 18 hours of in vitro culture as indicated. Data are representative of 3 mice per group. (F) Splenocytes were restimulated with the indicated LCMV-derived peptides, and IFNγ production by YFP+ CD8 T cells was assessed by intracellular staining. (G) The frequency of YFP+ CD8 T cells producing the indicated combinations of IFNγ, TNFα, and IL-2 in response to stimulation with pooled LCMV peptide was determined. Data represent the means ± SD of 3 mice per group.
Whereas these data suggested effective expansion of MyD88-deficient memory T cells, it was possible that the secondary T-cell response observed in cKO mice was because of the outgrowth of a population of YFP+ CD8 T cells that had failed to delete MyD88. We therefore sorted the YFP+ and YFP− fractions from the CD44highCD8 T-cell populations in cKO and cHet mice and examined MyD88 expression. We found that the majority of YFP+ cells in cKO mice still lacked MyD88 expression, indicating that the memory T-cell response in cKO mice did not merely reflect preferential expansion of MyD88-sufficient cells (Figure 6D).
Because we have previously reported increased apoptosis in Myd88−/− effector cells during primary expansion in response to LCMV,6 we also examined the survival of LCMV-specific T cells after secondary expansion. Consistent with their comparable expansion, we found no notable differences in the apoptosis of YFP+ np396-specific cells in cKO and cHet mice either directly ex vivo or after in vitro culture (Figure 6E).
We also considered whether MyD88 might potentially be involved in the differentiation of memory cells into functional secondary effectors. Ex vivo restimulation with LCMV-derived peptides indicated a comparable frequency of IFNγ-producing cells within the YFP+ CD8 T-cell compartments of cKO and cHet mice (Figure 6F). Whereas we observed only minimal expansion of gp33-specific cells in response to secondary LCMV infection using H2-Db tetramers (data not shown), we found a considerable expansion in the population of cells that produced IFNγ in response to restimulation with gp33-41 peptide in both cKO and cHet mice. This likely indicates preferential expansion of cells recognizing the epitope in the context of H2-Kb during the secondary immune response to LCMV. In addition to the comparable frequency of IFNγ-producing cells, we also found a similar frequency and distribution of cells that produced combinations of IFNγ, TNFα, and IL-2 in cKO and cHet mice (Figure 6G). These data indicate that memory T cells do not require MyD88-dependent signals to achieve maximal expansion or differentiation in response to secondary LCMV infection.
Discussion
Memory T cells form a central component of protective immunity, and therefore there has been great interest in understanding the factors that regulate their development and maintenance. Using adoptive transfer systems, we and others have previously shown that MyD88 expression in T cells is critical for regulating the size of the resulting memory population after acute viral infection.4-6 Our initial experiments with T cell–specific Myd88-deficient mice confirmed these observations, showing that in the absence of MyD88 expression in T cells, LCMV infection induced significantly smaller populations of effector and memory cells. We further confirmed these results using cKO mice in which tamoxifen treatment deletes MyD88 expression before viral infection. Given that tamoxifen treatment of cKO mice results in deletion of MyD88 in multiple immune compartments, the fact that we observed significant differences in the expansion of YFP− and YFP+ T-cell populations in cKO mice further supports a T cell–intrinsic role for MyD88. In addition, the finding that there was no significant difference between the expansion of the YFP− T-cell population in cKO and cHet mice suggests that expression of MyD88 by part of the innate immune compartment is sufficient to support effective T-cell expansion during LCMV infection. This is consistent with our previously published studies showing that expression of MyD88 by half of the immune system in mixed bone marrow chimeras was sufficient to support T-cell responses against LCMV.6
These results, together with the finding that direct stimulation of memory T cells with TLR ligands can promote cytokine dependent proliferation,11 raised the possibility that direct MyD88-dependent signaling may be an important component of memory T-cell homeostasis. However, using a novel mouse model in which MyD88 was conditionally deleted after the initial phase of T-cell expansion, we showed that the virus-specific T-cell population that is generated during a physiologic LCMV infection does not require MyD88 for subsequent memory T-cell differentiation or maintenance. This suggests that the reduced population of Myd88−/− memory T cells that we and others have reported after viral infection primarily reflects an indirect consequence of impaired effector expansion rather than a direct role for MyD88-dependent signals in memory T-cell homeostasis.
It was recently shown that the maintenance of memory T cells in a state of rapid responsiveness depends on the provision of adequate costimulatory signals through members of the TNF superfamily.19 Given that MyD88-dependent signals can potentially serve as a T cell–costimulatory pathway10,20 and that TLR ligands can directly promote IFNγ secretion from memory T cells,11 we hypothesized that the loss of MyD88 may influence memory T-cell cytokine production. However, our results clearly show that cKO cells rapidly produce effector cytokines in response to ex vivo peptide stimulation, indicating that MyD88-dependent signals are not required to maintain memory T cells in a responsive state.
Our results raised the possibility that the MyD88-dependent survival signals in effector T cells are triggered by ligands that are only present during active LCMV infection, and that the lack of a role for MyD88 in memory cells merely reflects the fact that the relevant ligands are no longer present because the virus has been cleared. Alternatively, it was possible that once naive cells had differentiated into LCMV-specific memory cells, signals through alternate pathways could compensate for the absence of MyD88-dependent signals. To distinguish between these possibilities, we reinfected mice with LCMV and found that, in contrast to naive CD8 T cells, memory T-cell expansion in response to LCMV infection occurred independently of MyD88. This is consistent with earlier studies showing that memory T cells induced in Myd88−/− mice by vaccination with gp33-expressing adenovirus could be shown to expand effectively in response to LCMV infection.5 Given that tamoxifen treatment results in the deletion of MyD88 in multiple immune compartments (Figure 2), these results also indicate that the loss of MyD88 from a portion of the innate immune compartment does not significantly affect secondary T-cell responses to LCMV infection.
In conclusion, our studies clearly show that whereas MyD88-dependent signals play a critical role in supporting the survival and expansion of naive T cells during primary LCMV infection, these signals are not directly required for the subsequent differentiation and maintenance of memory T cells. Furthermore, memory T cells do not require MyD88-dependent signals to support expansion in response to secondary infection. These findings identify differential dependence on MyD88 as a novel factor that distinguishes naive from memory CD8 T cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Haina Shin, Evann Corbo, and Eleni Argyropoulou for insightful discussion and technical assistance.
This work was supported by National Institutes of Health grant AI-62789.
National Institutes of Health
Authorship
Contribution: A.H.R. designed, performed, and analyzed experiments and wrote the manuscript; R.Z. and C.D.B. performed experiments; B.H. and A.L.D. generated and contributed the Myd88flox/flox mouse; J.S.M. and E.J.W. provided critical experimental reagents and contributed to the experimental design; and L.A.T. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.H.R is Lineberger Comprehensive Cancer Center, University of North Carolina-Chapel Hill, Chapel Hill, NC. The current affiliation for R.Z. and L.A.T. is Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA. The current affiliation for C.D.B. is Department of Internal Medicine, University of Iowa, Iowa City, IA.
Correspondence: Laurence Turka, Beth Israel Deaconess Medical Center, 607 CLS, 330 Brookline Ave, Boston, MA 02215. e-mail: lturka@bidmc.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal