Abstract
On vascular damage, coagulation is initiated by extravascular tissue factor (TF). Intravascular TF, which is present on circulating cell-derived vesicles, is noncoagulant under physiologic conditions but prothrombotic under pathologic conditions. Human saliva triggers coagulation, but the mechanism and physiologic relevance are unknown. Because saliva is known to contain TF, we hypothesized that this TF may also be associated with cell-derived vesicles to facilitate coagulation when saliva directly contacts blood. The saliva-induced shortening of the clotting time of autologous plasma and whole blood from healthy subjects (n = 10) proved TF-dependent. This TF was associated with various types of cell-derived vesicles, including microparticles and exosomes. The physiologic function was shown by adding saliva to human pericardial wound blood collected from patients undergoing cardiac surgery. Addition of saliva shortened the clotting time from 300 ± 96 to 186 ± 24 seconds (P = .03). Our results show that saliva triggers coagulation, thereby reducing blood loss and the risk of pathogens entering the blood. We postulate that our reflex to lick a wound may be a mechanism to enable TF-exposing vesicles, present in saliva, to aid in the coagulation process and thus protect the organism from entering pathogens. This unique compartmentalization may be highly conserved because also animals lick their wounds.
Introduction
The clotting of blood is a complex and well-regulated process, requiring coagulation factors, calcium ions, a membrane surface exposing phosphatidylserine, and tissue factor (TF), the initiator of coagulation activation. TF is mainly present outside the vasculature under physiologic conditions, and this TF contacts the blood only on vascular injury. Within the blood, a low concentration of noncoagulant “blood-borne” TF is present under physiologic conditions. This intravascular TF is associated with circulating cell-derived microparticles1 and may become coagulant by disulfide isomerization.2-4
Under pathologic conditions, high levels of coagulant TF-exposing microparticles can be present within the blood. The presence of monocyte-derived microparticles exposing coagulant TF has been associated with disseminated intravascular coagulation,5 and TF-exposing microparticles in human wound blood during open heart surgery triggered TF-dependent thrombus formation in a rat venous stasis model, directly demonstrating that TF-exposing microparticles can trigger coagulation in vivo.6,7 More recently, the presence of microparticles exposing coagulant TF has been associated with the high incidence of venous thromboembolism in cancer patients.8-10
Intravascular TF-exposing microparticles may initiate thrombus formation directly or indirectly. Whereas TF-exposing microparticles can directly initiate coagulation and thrombus formation, cancer cell–derived microparticles exposing TF and P-selectin glycoprotein ligand-1 were shown recently to induce thrombus formation by binding to activated platelets at a site of vascular injury in vivo.11 The prothrombotic state in this mouse model was reduced by antibodies against P-selectin, implicating that circulating microparticles initiate coagulation after binding to activated platelets or endothelial cells exposing P-selectin.12,13
Blood and other body fluids contain several different types of cell-derived vesicles.14 The most widely studied types of vesicles are microparticles and exosomes. Microparticles are released from the plasma membrane by a “budding” process and typically range in size between 100 nm and 1 μm. Exosomes are smaller than microparticles and range in size between 30 and 90 nm and become released when membranes of multivesicular bodies fuse with the plasma membrane. Typically, exosomes contain heat-shock proteins and often, but not always, expose tetraspanins, such as CD63,15 although CD63 can also be exposed on, for example, microparticles from degranulated platelets. Furthermore, several body fluids, including saliva, contain P4 particles, also known as membrane particles,14 which originate from epithelial cells and are of similar size as exosomes. These particles were reported to expose prominin-1 (CD133) but lack CD63.16-18 Throughout this manuscript, we will use the term exosomes for both exosomes and P4 (membrane) particles in saliva, unless stated otherwise.
Blood is not our only body fluid that can contains TF-exposing coagulant microparticles. Previously, we demonstrated that synovial fluid from arthritic patients also contains such microparticles.19,20 When we have a wound, for instance, a bleeding finger, our reflex is to put this finger in our mouth. In this way, blood is directly exposed to saliva. This reflex has previously been explained by the presence of antimicrobial and wound-healing compounds, such as histatin, in saliva.21 We hypothesized that there may be an alternative explanation for this reflex. For more than 70 years, human saliva has been known to contain a compound that efficiently triggers coagulation.22 Indeed, already in 1960, it was observed that “human saliva accelerates the prothrombin time as much as snake venom does.”23,24 In the 1990s, the TF antigen was shown to be present in saliva and other human body fluids, but its coagulant activity was not investigated.25 This raised the question of whether TF in saliva is also capable of initiating coagulation and thus can be an additional source of extravascular TF that promotes blood clotting, thereby reducing blood loss and preventing microorganisms from entering the blood.
Methods
Antibodies and reagents
Antifactor VII (clone CLB VII-1), antifactor XII (clone CLB OT2), and anti-CD63 were obtained from Sanquin. Anti-TF (anti-CD142) 4502 was obtained from American Diagnostica. Fluorescein isothiocyanate (FITC)–labeled IgG1, phycoerythrin (PE)–labeled IgG1, allophycocyanin (APC)–labeled IgG1, and monoclonal antibodies against TF (anti-CD142–PE) and B cells (anti-CD20–FITC) were obtained from BD Biosciences. Anti-CD63–FITC and anti-CD66b–FITC were from Immunotech, anti-CD61–FITC and FITC-labeled anti-CD235a (glycophorin A) were from Dako Denmark, Ema-FITC (epithelial membrane antigen) from Biomeda, anti-CD133/1 pure, anti-CD133/1-PE, and anti-CD133/1-APC were from Miltenyi Biotec. The following final dilutions of antibodies were used: IgG1-FITC (1:100), IgG1-PE (1:100), IgG1-APC (1:200), anti-CD142–PE (1:10), anti-CD20–FITC (1:50), anti-CD66b–FITC (1:100), anti-CD61–FITC (1:300), anti-CD235a–FITC (1:400), anti–CD-133/1-PE (1:100), anti–CD-133/1-APC (1:50), and anti-CD63–FITC (1:25).
Collection of blood and saliva
Citrate-anticoagulated blood (0.32%) was collected from 10 healthy subjects with informed consent after overnight fasting by venapuncture without a tourniquet through a 19-gauge needle using a Vacutainer system (5 males and 5 females; age 40 ± 16 years). Concurrently, saliva was collected from the same subjects and immediately placed on melting ice. To remove cells, saliva and blood were centrifuged (1550g, 20 minutes) at 4°C (saliva) or 20°C (blood) to prepare cell-free saliva and plasma. Saliva and plasma were both placed on melting ice and used for experiments or snap-frozen as 250 μL in liquid nitrogen for 15 minutes and stored at −80°C until use.
Isolation of cell-derived microparticles and exosomes
Cell-free saliva and plasma samples (250 μL) were centrifuged for 30 minutes at 18 890g and 4°C to isolate microparticles. In turn, to obtain exosomes, the microparticle-depleted supernatant (225 μL) was centrifuged for 1 hour at 154 000g and 4°C in 1.5-mL polyallomer microfuge tubes in a Optima Max ultracentrifuge using a TLA-55 rotor from Beckman Instruments. The exosome-depleted supernatants (200 μL) from saliva and plasma were collected, and supernatants as well as isolated fractions of microparticles (25 μL) and exosomes (25 μL) were stored on melting ice until use.
Measurement of TF, coagulation factors II, V, VII, VIII, and X, and fibrinogen
To determine the levels of TF (antigen), coagulation factors, and fibrinogen in human plasma and saliva, we used commercially available enzyme-linked immunosorbent assay kits. TF kits were obtained from American Diagnostics, kits for coagulation factors II and VIII were from Dako Denmark, and kits for coagulation factors V, VII, IX, X, and fibrinogen were from Hyphen BioMed. All assays were performed as described by the manufacturers.
Measurement of the coagulant properties of saliva in the fibrin generation test
Autologous cell-free plasma, containing microparticles and exosomes (70 μL), was incubated with cell-free saliva (20 μL; ie, saliva still containing microparticles and exosomes) for 5 minutes at 37°C in a 96-well plate (n = 5). Similarly, autologous vesicle-depleted human plasma (1 hour at 154 000g; 70 μL) was incubated with cell-free saliva (20 μL) or cell-free saliva depleted also from microparticles and exosomes (1 hour at 154 000g; 20 μL), without (3 μL saline) or with antibodies (1 mg/mL) against factor VII(a) or XII(a) (3 μL) for 5 minutes at 37°C in a 96-well plate (n = 10). The clotting was initiated by addition of CaCl2 (15 μL; 0.1M). Fibrin (clot) formation was monitored by measuring the optical density (λ = 405 nm) of the plasma on a Spectramax microplate reader (Molecular Devices) at 37°C for 1 hour.
In control experiments, we compared the activity and specificity of antifactor VII(a) and anti-TF in vesicle-depleted normal plasma to inhibit thromborel- and kaolin-induced fibrin generation. Both antifactor VII(a) and anti-TF (4502) completely inhibited thromborel-induced fibrin generation but did not inhibit kaolin-induced fibrin generation. Furthermore, both antifactor VII(a) and anti-TF also completely inhibited fibrin generation induced by saliva or isolated fractions of vesicles from saliva in autologous vesicle-depleted plasma (n = 3; data not shown). In the experiments described herein, we used antifactor VII(a) because this antibody can be used at a lower concentration (1.0 μg/mL) than anti-TF (7.8 μg/mL) to completely inhibit TF-initiated coagulation. With regard to the specificity of antifactor XII, we have previously demonstrated the specificity of this antibody in thrombin generation experiments26 and found similar results in the fibrin generation test (data not shown).
Measurement of the coagulant properties of microparticles and exosomes from saliva in the fibrin generation test
Fractions of isolated microparticles or exosomes (6 μL) were added to vesicle-depleted plasma (84 μL) and preincubated without or with antibodies similar to the experiments described in the previous paragraph. Coagulation was initiated and monitored as described for the fibrin generation test.
Thromboelastography
Into empty ROTEM cups, saline (3 μL), antifactor VII (3 μL), or antifactor XII (3 μL) was added. Subsequently, either saline (20 μL; control) plus CaCl2 (20 μL; 0.1M) or cell-free saliva (20 μL) plus CaCl2 (20 μL; 0.1M) was added, and clotting was started by addition of whole blood (300 μL). Clot formation was measured on a ROTEM (Phentapharm).
PAGE and Western blot
Western blots were stained for TF. Isolated fractions of microparticles and exosomes were resuspended in 25 μL of reducing sample buffer, containing sodium dodecyl sulfate and 6% β-mercapthoethanol. All samples were boiled for 5 minutes. Samples (15 μL) were loaded on an 8% to 16% gradient gel (Bio-Rad) and blotted to polyvinylidene difluoride membrane (Millipore). Membranes were incubated with 5% protifar (Nutricia) for 1 hour to reduce nonspecific staining, followed by incubation with a mouse anti-TF (clone HTF-1, BD Biosciences PharMingen). A secondary antibody goat antimouse-horseradish peroxidase (1:30 000; Dako Denmark) was used. To visualize the bands, membranes were incubated with a 5-fold diluted peroxidase substrate (LumiLight; Roche Diagnostics) for 5 minutes, followed by analysis of luminescence using a LAS3000 luminescent image analyzer (Fuji).
Transmission electron microscopy
Vesicles were resuspended in 0.1% paraformaldehyde (weight/volume). Fixed suspensions were used within 5 days. After fixation for at least 18 hours, the fractions were either negatively stained or labeled with immunogold. Grids were carbon coated before use. Vesicles were adsorbed on grids by letting formvar-carbon coated grids float on drops of 10-μL fraction suspensions on a sheet of parafilm at room temperature for 7 minutes. Subsequent washing and incubation steps were performed by transferring the grids from one drop to the next (see negative staining and immunogold labeling for further details). Stained vesicles on grids were visualized with electron microscopy operated at 80 kV (Fei, Tecnai-12) using a Veleta 2000 × 2000 side-mounted CCD camera and Imaging Solutions software (Olympus). After vesicles were absorbed onto grids, the grids were either transferred onto drops of 1.75% uranyl acetate (wt/vol) for negative staining alone or used for immunogold labeling followed by negative staining. For the latter procedure, grids were washed with phosphate-buffered saline (PBS), incubated with glycine (50mM glycine in PBS) for 15 minutes, washed again, and blocked with Aurion blocking solution (Aurion) for 30 minutes. Subsequently, the grids were washed with 0.1% BSA-c washing buffer (weight/volume; Aurion), incubated with the indicated primary antibodies for 1 hour, washed with washing buffer, and then incubated overnight with ultra-small gold-labeled secondary (Fab-goat anti–mouse) antibody (Aurion). Finally, the grids were washed with washing buffer and PBS, incubated with 2% glutaraldehyde (weight/volume) for 5 minutes, washed with PBS, and washed with 1× ECS solution (Aurion) before silver enhancement (40 minutes) with enhancement mixture (Aurion). Finally, the grids were washed with 1× ECS solution before negative staining.
Flow cytometry
To estimate the concentrations of vesicles, isolated fractions of microparticles (25 μL) and exosomes (25 μL) were diluted with 100 μL of citrate-containing PBS. From these suspensions and from the saliva or plasma depleted from vesicles (1 hour; 154 000g), 5-μL aliquots were labeled by addition of one or more CD-specific monoclonal antibodies or the corresponding isotype-matched control antibodies (5 μL per antibody). The samples were incubated in the dark for 15 minutes at room temperature. After incubation, citrate-containing PBS was added to a final volume of 955 μL (microparticles) or 355 μL (exosomes). Samples were analyzed for one minute in a fluorescence automated cell sorter (FACSCalibur) with CellQuest Version 4.02 software (BD Biosciences). Both forward scatter and sideward scatter were set at logarithmic gain. Vesicles were identified on the basis of their size and density and on their ability to bind cell-type specific antibodies. The gate settings were confirmed using beads with a maximum diameter of 1.0 μm. Labeling with cell-specific monoclonal antibodies was corrected for identical concentrations of isotype-matched control antibodies by subtracting the amount of isotype-matched positive events from the total positive events. The concentrations of microparticles and exosomes in saliva and plasma were estimated as described earlier.27 It should be mentioned that the analysis of cell-derived vesicles, although the best method of choice at present, is stretching the capability of the flow cytometer, the size of these vesicles being near or below the detection limit of the technique. Thus, the side scatter will not accurately reflect the size of the vesicles and not all vesicles will be detected.
Statistics
Data were analyzed by 2-tailed Student t test (GraphPad Prism software Version 5.0). A probability value of less than .05 was considered to be statistically significant. All data are shown as mean ± SD.
Results
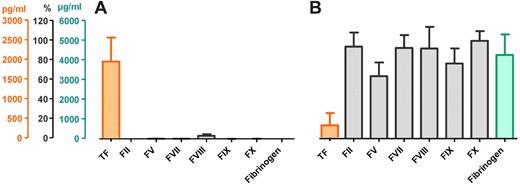
Saliva contains TF but no coagulation factors or fibrinogen
Cell-free saliva from healthy subjects (n = 10) contained high levels of TF (1956 ± 610 pg/mL), but all other components required for coagulation were below the detection limit of the various assays (prothrombin [factor II], factors V, VII, IX, X, and fibrinogen) or barely detectable (factor VIII; Figure 1A). On the other hand, plasma contained a relatively low concentration of “blood-borne” TF (320 ± 311 pg/mL), but the concentrations of all other coagulation factors and fibrinogen were within their normal range (Figure 1B).
Concentrations of TF, coagulation factors, and fibrinogen in human saliva and plasma. Orange represents concentrations of TF (pg/mL); gray, coagulation factors (% of reference pool plasma); and green, fibrinogen (μg/mL) in saliva (A) and plasma (B; n = 10).
Concentrations of TF, coagulation factors, and fibrinogen in human saliva and plasma. Orange represents concentrations of TF (pg/mL); gray, coagulation factors (% of reference pool plasma); and green, fibrinogen (μg/mL) in saliva (A) and plasma (B; n = 10).
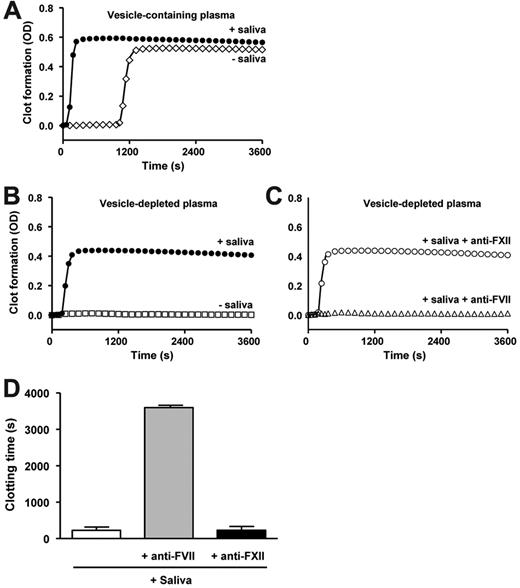
Saliva triggers factor VII-mediated coagulation of human plasma
We tested the ability of saliva to induce clot formation of autologous (vesicle-containing) plasma (n = 5). Addition of saliva shortened the clotting time from 1274 ± 197 seconds to 176 ± 42 seconds (P < .0001; Figure 2A). Because human plasma from healthy subjects contains low levels of coagulant cell-derived vesicles,26 we depleted plasma from all vesicles by ultracentrifugation to rule out a role for plasma vesicles in this model system. Figure 2B and C show representative curves obtained within a single experiment using plasma and saliva from one healthy subject. When vesicle-depleted plasma was recalcified, no plasma clot formation was observed for at least 3600 seconds (Figure 2B), indicating that this plasma was indeed free of vesicles. On addition of cell-free saliva, the clotting time of vesicle-depleted plasma shortened to 228 ± 95 seconds (P < .0001; Figure 2B-D). The saliva-induced shortening of the clotting time was completely abolished in the presence of antifactor VII(a) (> 3600 seconds, P < .0001) but unaffected by antifactor XII(a) (233 ± 101 seconds, P = .587; Figure 2C-D).
Saliva triggers the coagulation of plasma. Human plasma containing vesicles (A) was incubated without (◇) or with saliva (●). Plasma depleted from endogenous vesicles before the addition of saliva (B-D) was incubated with (B) saliva (●), or without saliva (□), or with (C) saliva preincubated with antifactor XII (○) or antifactor VII (Δ). Coagulation/clot formation was initiated by addition of calcium chloride (t = 0) and monitored for 1 hour. (B-C) Graphs were obtained in a single experiment and are representative of 6 other independent experiments. (D) The effects of antifactor XII and antifactor VII on the saliva-induced shortening of the plasma clotting time have been summarized (n = 7).
Saliva triggers the coagulation of plasma. Human plasma containing vesicles (A) was incubated without (◇) or with saliva (●). Plasma depleted from endogenous vesicles before the addition of saliva (B-D) was incubated with (B) saliva (●), or without saliva (□), or with (C) saliva preincubated with antifactor XII (○) or antifactor VII (Δ). Coagulation/clot formation was initiated by addition of calcium chloride (t = 0) and monitored for 1 hour. (B-C) Graphs were obtained in a single experiment and are representative of 6 other independent experiments. (D) The effects of antifactor XII and antifactor VII on the saliva-induced shortening of the plasma clotting time have been summarized (n = 7).
Saliva contains cell-derived vesicles that initiate TF/factor VII–mediated coagulation activation of human plasma
To investigate whether TF is associated with subpopulations of cell-derived vesicles in saliva, we subjected cell-free saliva to ultracentrifugation. After ultracentrifugation, the supernatant saliva was unable to shorten the clotting time of the vesicle-depleted plasma (Table 1), indicating that the coagulant activity of saliva is completely associated with cell-derived vesicles. Subsequently, we isolated microparticles and exosomes from cell-free saliva by differential centrifugation. Microparticles from saliva shortened the clotting time (Figure 3A), which was unaffected by antifactor XII (Figure 3A; Table 1, P = .272) but completely inhibited by antifactor VII (Figure 3A; Table 1; P < .0001). Similarly, exosomes shortened the clotting time (Figure 3B), and this shortening was unaffected by antifactor XII (Figure 3B; Table 1; P = .284) and completely inhibited by antifactor VII (Figure 3B; Table 1; P = .016). Thus, cell-derived vesicles present in saliva initiate TF/factor VII–dependent coagulation.
TF/factor VII–initiated coagulation activation of saliva is associated with cell-derived vesicles from saliva
| Monoclonal antibody . | Supernatant . | Microparticles . | Exosomes . |
|---|---|---|---|
| Saliva | |||
| None | > 3600 | 283 ± 126 | 2202 ± 1251 |
| FVII | > 3600 | > 3600 (P < .0001) | > 3600 (P = .016) |
| FXII | > 3600 | 270 ± 135 (P = .272) | 1940 ± 1152 (P = .284) |
| Plasma | |||
| None | > 3600 | 2675 ± 849 | > 3600 |
| FVII | > 3600 | 2838 ± 617 (P = .464) | > 3600 |
| FXII | > 3600 | 2422 ± 660 (P = .403) | > 3600 |
| Monoclonal antibody . | Supernatant . | Microparticles . | Exosomes . |
|---|---|---|---|
| Saliva | |||
| None | > 3600 | 283 ± 126 | 2202 ± 1251 |
| FVII | > 3600 | > 3600 (P < .0001) | > 3600 (P = .016) |
| FXII | > 3600 | 270 ± 135 (P = .272) | 1940 ± 1152 (P = .284) |
| Plasma | |||
| None | > 3600 | 2675 ± 849 | > 3600 |
| FVII | > 3600 | 2838 ± 617 (P = .464) | > 3600 |
| FXII | > 3600 | 2422 ± 660 (P = .403) | > 3600 |
Saliva fractions (ie, isolated microparticles, exosomes, and vesicle-depleted saliva [Supernatant]) were tested for their ability to initiate clotting of autologous vesicle-depleted plasma, in the absence or presence of antibodies against factor VII or XII. For comparison, we also tested the ability of isolated fractions of microparticles and exosomes from plasma to initiate clotting (n = 7). Data are shown as clotting time (seconds).
Cell-derived vesicles from saliva initiate TF/factor VII–mediated coagulation activation. Human plasma, depleted from vesicles by ultracentrifugation, was incubated with microparticles (A,C) or exosomes (B,D) isolated from autologous saliva (A-B) or plasma (C-D), without (●) or with antifactor XII (○) or antifactor VII (▵). Coagulation/clot formation was initiated by addition of calcium chloride (t = 0) and monitored for 1 hour. The graphs shown were obtained within one single experiment and are representative of 6 other independent experiments.
Cell-derived vesicles from saliva initiate TF/factor VII–mediated coagulation activation. Human plasma, depleted from vesicles by ultracentrifugation, was incubated with microparticles (A,C) or exosomes (B,D) isolated from autologous saliva (A-B) or plasma (C-D), without (●) or with antifactor XII (○) or antifactor VII (▵). Coagulation/clot formation was initiated by addition of calcium chloride (t = 0) and monitored for 1 hour. The graphs shown were obtained within one single experiment and are representative of 6 other independent experiments.
For comparison, we also isolated microparticles and exosomes from the corresponding plasma samples. Plasma microparticles shortened the clotting time from more than 3600 seconds to 2675 ± 849 seconds (Figure 3C; P = .028). The extent of inhibition by antifactor VII and antifactor XII differed between subjects, but no net inhibition was observed when the overall data were analyzed (Table 1; P = .464 and P = .403, respectively). Addition of plasma exosomes did not affect the clotting time (Figure 3D).
TF in saliva is associated with microparticles and exosomes
To further investigate the association between TF and vesicles in saliva, we fractionated cell-free saliva in microparticles, exosomes, and vesicle-depleted saliva, and determined the presence of TF. Figure 4A shows that TF antigen is associated with microparticles and exosomes but is absent in the corresponding vesicle-depleted saliva. There was a considerable variation between subjects. The association between TF and cell-derived vesicles was further established by flow cytometry (Figure 4B) and transmission electron microscopy (TEM; Figure 4C).
TF is associated with cell-derived vesicles in saliva. Microparticles and exosomes were isolated from cell-free saliva (n = 10). (A) Western blot showing the presence of TF in fractions of microparticles and exosomes, but below the detection limit in the corresponding supernatant saliva. Data from 3 persons are shown. (B) Representative fluorescence histograms of microparticles (left), exosomes (middle), and supernatant (right) stained with control antibody (filled) or anti-TF (open). (C) TEMs of isolated microparticles (> 100 nm diameter, top left) and exosomes (< 100 nm, top right) stained with anti-TF followed by immunogold labeling. Representative controls of vesicles labeled with control antibody (IgG) plus immunogold staining (bottom left), or immunogold staining alone (goat anti–mouse [GAM], bottom right).
TF is associated with cell-derived vesicles in saliva. Microparticles and exosomes were isolated from cell-free saliva (n = 10). (A) Western blot showing the presence of TF in fractions of microparticles and exosomes, but below the detection limit in the corresponding supernatant saliva. Data from 3 persons are shown. (B) Representative fluorescence histograms of microparticles (left), exosomes (middle), and supernatant (right) stained with control antibody (filled) or anti-TF (open). (C) TEMs of isolated microparticles (> 100 nm diameter, top left) and exosomes (< 100 nm, top right) stained with anti-TF followed by immunogold labeling. Representative controls of vesicles labeled with control antibody (IgG) plus immunogold staining (bottom left), or immunogold staining alone (goat anti–mouse [GAM], bottom right).
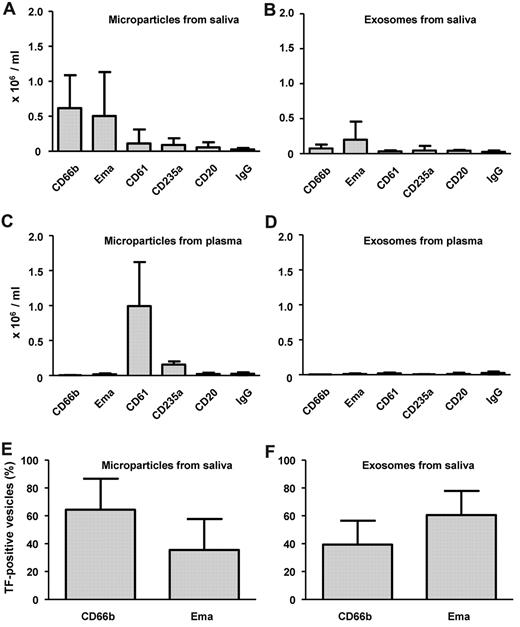
The cellular origin of cell-derived vesicles in saliva and plasma
In preliminary experiments, we determined the origin of the cells present in saliva. Both epithelial cells and granulocytes could be identified. Figure 5A and B show that most cell-derived vesicles in saliva originate from these cell types, whereas relatively very low numbers expose CD61 (platelets, possibly also granulocytes), CD235a (erythrocytes), or CD20 (B-lymphocytes). On the other hand, microparticles in plasma originated predominantly from platelets (CD61+) and to a lesser extent from erythrocytes (CD235a+; Figure 5C). The levels of exosomes in plasma were very low, and we were unable to establish their cellular origin (Figure 5D).
Cellular origin of TF-exposing vesicles in saliva and plasma. Microparticles (A,C,E) and exosomes (B,D,F) were isolated from saliva (A-B,E-F) and plasma (C-D) by differential centrifugation, labeled, and analyzed by flow cytometry as outlined in “Flow cytometry.” To establish the cellular origin, antibodies were used directed against CD66b (granulocytes), Ema (epithelial membrane antigen, epithelial cells), CD61 (integrin β3, platelets, possibly granulocytes in saliva), CD235a (glycophorin A, erythrocytes), and CD20 (B lymphocytes), or control antibody (IgG). Microparticles (E) and exosomes (F) from saliva were double-labeled with anti-TF plus antibodies against either CD66b or Ema, and analyzed by flow cytometry. (A-D) Data are number of microvesicles per milliliter. (E-F) Data are expressed as percentage of the total number of TF-exposing microparticles or TF-exposing exosomes (n = 10).
Cellular origin of TF-exposing vesicles in saliva and plasma. Microparticles (A,C,E) and exosomes (B,D,F) were isolated from saliva (A-B,E-F) and plasma (C-D) by differential centrifugation, labeled, and analyzed by flow cytometry as outlined in “Flow cytometry.” To establish the cellular origin, antibodies were used directed against CD66b (granulocytes), Ema (epithelial membrane antigen, epithelial cells), CD61 (integrin β3, platelets, possibly granulocytes in saliva), CD235a (glycophorin A, erythrocytes), and CD20 (B lymphocytes), or control antibody (IgG). Microparticles (E) and exosomes (F) from saliva were double-labeled with anti-TF plus antibodies against either CD66b or Ema, and analyzed by flow cytometry. (A-D) Data are number of microvesicles per milliliter. (E-F) Data are expressed as percentage of the total number of TF-exposing microparticles or TF-exposing exosomes (n = 10).
The cellular origin of TF-exposing vesicles in saliva
We double-labeled the 2 most abundant types of microparticles and exosomes present in saliva with anti-TF. The absolute (100%) values for TF-exposing microparticles and exosomes were 1.35 ± 1.42 × 106/mL and 0.35 ± 0.32 × 106/mL, respectively (Figure 5E-F). TF-exposing vesicles mainly originated from granulocytes and epithelial cells. Because of the fact that antibodies against Ema and CD66b are only commercially available with FITC labels, triple-labeling of TF-exposing vesicles with both these antibodies was not possible; therefore, we cannot exclude that part of these vesicles are of mixed lineage (ie, fusion of vesicles) as has been described earlier.28
TF in saliva may be associated with exosomes and P4 (membrane) particles
Because there was a considerable overlap with regard to the cellular origin of microparticles and exosomes by flow cytometry (Figure 5E-F), we first investigated whether the differential centrifugation to separate both types of vesicles was successful. Therefore, we studied the relative presence of CD63 and CD133 in the fractions of microparticles and exosomes (Figure 6A), as CD63 and CD133 are markers of exosomes and P4 (membrane) particles, respectively. Relatively few microparticles stained for CD133 or CD63 (Figure 6A; 2.6% ± 2.3% and 12. 7% ± 6.7%, respectively). In contrast, 40.6% ± 15.2% and 64.6% ± 9.6% of the vesicles isolated by ultracentrifugation from the microparticle-depleted saliva stained for CD133 and CD63, respectively, indicating a 15-fold (CD133) and 5-fold (CD63) enrichment compared with microparticles (both P < .0001). The presence of (CD133-exposing) P4 (membrane) particles and (CD63-exposing) exosomes was confirmed by TEM (Figure 6B and C, respectively). When we quantified the frequency of vesicles with a diameter less than 100 nm that stained for CD133 or CD63, there was a 11-fold and 6-fold enrichment in these populations compared with the microparticles (> 100 nm). These data show that exosomes and P4 (membrane) particles are enriched in the fraction of vesicles isolated by ultracentrifugation compared with the fraction of microparticles isolated by high-speed centrifugation.
Association of TF in saliva with exosomes and P4 (membrane) particles. Fractions of microparticles and exosomes/P4 (membrane) particles were labeled with antibodies against CD133 and CD63, and analyzed by flow cytometry (A). Representative TEM images of exosomes/P4 (membrane) particles staining for CD133 (B) or CD63 (C) are also shown. Exosomes/P4 (membrane) particles were triple-labeled with anti-TF, anti-CD133, and anti-CD63, and analyzed by flow cytometry, summarized in panel D (n = 10).
Association of TF in saliva with exosomes and P4 (membrane) particles. Fractions of microparticles and exosomes/P4 (membrane) particles were labeled with antibodies against CD133 and CD63, and analyzed by flow cytometry (A). Representative TEM images of exosomes/P4 (membrane) particles staining for CD133 (B) or CD63 (C) are also shown. Exosomes/P4 (membrane) particles were triple-labeled with anti-TF, anti-CD133, and anti-CD63, and analyzed by flow cytometry, summarized in panel D (n = 10).
Of the TF-exposing exosomes and P4 (membrane) particles (Figure 6D), a fraction stained for CD63 but was negative for CD133, suggesting that these vesicles are probably exosomes (32.6%). A small fraction stained for CD133 but was negative for CD63, and these vesicles are most probably P4 (membrane) particles (2.8%). In addition, a major fraction stained for CD133 and CD63, possibly reflecting complexes of P4 (membrane) particles and exosomes that may have been formed during sample preparation (47.9%) or in vivo. The remainder of the TF-exposing vesicles seemed to be neither exosomes nor P4 particles because these vesicles did not stain for either CD63 or CD133 (16.8%).
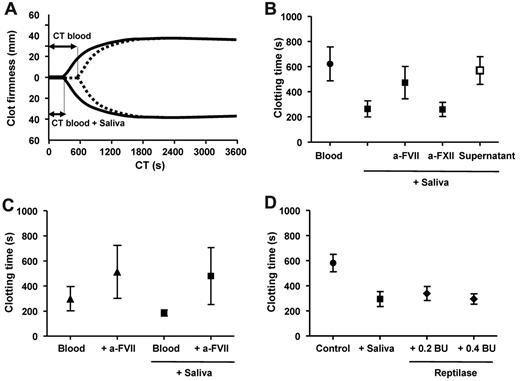
Biologic activity of saliva
To establish whether or not saliva has a true physiologic role as an additional extravascular source of TF, we used a whole blood clotting model (ROTEM thromboelastography). Figure 7A shows representative curves with blood collected from a healthy subject, incubated without or with cell-free saliva. Clotting was initiated by addition of CaCl2. The addition of saliva dramatically shortened the clotting time of whole blood. The overall data with 10 volunteers are summarized in Figure 7B, confirming that also in whole blood the saliva-triggered shortening of the clotting time is entirely TF/factor VII–dependent and factor XII–independent. When blood was incubated with vesicle-depleted saliva (supernatant in Figure 7B), no shortening of the clotting time was observed, confirming that removal of cell-derived vesicles completely abolishes the ability of saliva to trigger whole blood clot formation.
Saliva triggers coagulation activation of whole blood. (A) Blood was incubated without (dotted line) or with (solid line) cell-free autologous saliva. Clot formation was initiated by addition of calcium chloride (t = 0) and monitored by thromboelastography for 1 hour. (B) Summary of clotting times from blood incubated without saliva, with cell-free saliva in the absence or presence of antifactor VII or antifactor XII, or with vesicle-depleted saliva (supernatant; n = 10). (C) Human wound blood was incubated without or with cell-free saliva, both in the absence or presence of antifactor VII (n = 5). (D) Blood was incubated with cell-free saliva or reptilase, snake venom, which directly converts fibrinogen into insoluble fibrin.
Saliva triggers coagulation activation of whole blood. (A) Blood was incubated without (dotted line) or with (solid line) cell-free autologous saliva. Clot formation was initiated by addition of calcium chloride (t = 0) and monitored by thromboelastography for 1 hour. (B) Summary of clotting times from blood incubated without saliva, with cell-free saliva in the absence or presence of antifactor VII or antifactor XII, or with vesicle-depleted saliva (supernatant; n = 10). (C) Human wound blood was incubated without or with cell-free saliva, both in the absence or presence of antifactor VII (n = 5). (D) Blood was incubated with cell-free saliva or reptilase, snake venom, which directly converts fibrinogen into insoluble fibrin.
To further investigate the role of saliva as an extravascular source of TF, we also collected wound (pericardial) blood from patients undergoing cardiac surgery. We used this blood as a model system to test the ability of saliva or fractions thereof to initiate or facilitate clotting of wound blood, which contains high levels of endogenous coagulant TF, capable of triggering coagulation in vitro and thrombus formation in vivo.6,7 To investigate the ability of saliva to shorten the clotting time of this human wound blood, pericardial blood was collected from 5 patients undergoing cardiac surgery. The clotting time of the wound blood was 300 ± 96 seconds (Figure 7C), which was further shortened by addition of cell-free saliva to 186 ± 24 seconds (P = .031). Both the clotting time of pericardial blood without saliva or with saliva returned to normal levels in the presence of antifactor VII (514 ± 211 seconds, P = .016 and 480 ± 227 seconds, P = .033, respectively). The clotting time of wound blood in the absence of saliva was considerably shorter than the clotting time of blood from control subjects (622 ± 135 seconds, Figure 7B; P = .0004), which is the result of the presence of the coagulant TF-exposing vesicles in this blood. Taken together, these data show that TF in saliva may be a physiologically relevant extravascular source of TF that can shorten the clotting time of human wound blood.
Finally, we compared the coagulant activity of TF in saliva with that of a snake venom from the Common Lancehead Bothrops atrox. This venom, reptilase, activates the final steps of the clotting cascade by converting soluble fibrinogen into insoluble fibrin. As shown in Figure 7D, saliva and reptilase (0.2 and 0.4 BU) similarly shortened the clotting time of whole blood from healthy subjects (P = .0003 and .0002, respectively), confirming the remarkable coagulant activity of human saliva.
Discussion
Saliva has often been associated with wound healing and cleaning because it contains growth factors and histatin, which promote wound healing,21,29,30 and has an antimicrobial activity.31,32 Studies going back to the 1930s, however, showed that saliva also promotes the clotting of blood, but the underlying mechanism has remained hitherto obscure.22
The results presented in our study show that TF in saliva is associated with microparticles and exosomes originating from a variety of cell types. Based on our data, we postulate that the reflex to put a bleeding finger into the mouth or to lick a wound may be an elegant mechanism by which spatially separated essential coagulation components are brought together.21,30 Therefore, this reflex may not be solely induced by the antimicrobial and wound-healing properties of saliva, or as a reflex to keep the environment clean of blood or to release the pain, but may also be because of the ability of saliva to promote coagulation.
Our present study shows that various types of TF-exposing vesicles seem to coexist in human saliva. Saliva contains microparticles, exosomes, and P4 (membrane) particles exposing TF, but to which extent these different types of vesicles contribute to the observed TF-dependent coagulant activity will require additional studies because the applied isolation procedure of differential centrifugation did not completely separate the various types of vesicles. A relatively large population of vesicles stained for CD133 and CD63, which may be clusters of P4 particles and exosomes, because we frequently observed such clusters by TEM. Given the very small size of exosomes and P4 (membrane) particles, such complexes cannot be safely distinguished from single vesicles by flow cytometry. Furthermore, the detection of single vesicles by flow cytometry is challenging, and future studies will be essential to gain more insight into the complexity of coexisting types of vesicles of mixed cellular origin in body fluids, such as saliva or plasma. Very recently, we have summarized the limitations of flow cytometry and other optical and nonoptical technologies to detect the size distribution, number, biochemical composition, and cellular origin of vesicles in such biologic fluids.33 Further improvement and validation of detection techniques of vesicles are essential to provide a basis for obtaining reliable information about purity, recovery, and cross-contamination of the various types of vesicles.
The question then arises: why does saliva contain TF-exposing vesicles? Based on our present findings, we propose that the TF-exposing vesicles in saliva facilitate hemostasis as one of the first steps in the process of wound healing. We hypothesize that saliva and possibly other body fluids that are located at the interface between the “milieu extérieur” and “milieu intérieur” may provide an important additional source of extravascular TF to promote hemostasis, thereby reducing blood loss and the risk of pathogens entering the blood, in this way contributing to the innate immunity and host defense. To obtain further evidence to support this hypothesis, we collected urine from healthy subjects. In line with the experiments performed with saliva, addition of cell-free urine, urine microparticles, or urine exosomes from healthy subjects to (vesicle-depleted) plasma shortened the clotting time from more than 3600 seconds to 708 ± 242 seconds, 175 ± 81 seconds, and 402 ± 74 seconds, respectively (n = 7; P = .0001 for all). This shortening was reversed by antifactor VII and ultracentrifugation but unaffected by antifactor XII. Thus, the presence of coagulant TF-exposing vesicles is not restricted to human saliva but also holds true for urine and possibly other body fluids. Taken together, saliva, urine, and possibly other body fluids may provide an additional source of extravascular TF under normal, physiologic conditions.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. J. F. van Noorden, J. Stap, J. van Marle (Department of Cell Biology and Histology, Academic Medical Center, Amsterdam, The Netherlands), and C. M. Hau for Western blot experiments.
Authorship
Contribution: R.J.B. and R.N. designed the study, interpreted results, and wrote the manuscript; L.M.v.T. performed the electron microscopy experiments; R.J.B. and M.C.L.S. performed experiments; A.S. interpreted results and contributed to writing the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: René J. Berckmans, Department of Clinical Chemistry, B1-237, Academic Medical Center Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: r.j.berckmans@amc.uva.nl.


![Figure 4. TF is associated with cell-derived vesicles in saliva. Microparticles and exosomes were isolated from cell-free saliva (n = 10). (A) Western blot showing the presence of TF in fractions of microparticles and exosomes, but below the detection limit in the corresponding supernatant saliva. Data from 3 persons are shown. (B) Representative fluorescence histograms of microparticles (left), exosomes (middle), and supernatant (right) stained with control antibody (filled) or anti-TF (open). (C) TEMs of isolated microparticles (> 100 nm diameter, top left) and exosomes (< 100 nm, top right) stained with anti-TF followed by immunogold labeling. Representative controls of vesicles labeled with control antibody (IgG) plus immunogold staining (bottom left), or immunogold staining alone (goat anti–mouse [GAM], bottom right).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/11/10.1182_blood-2010-06-290460/4/m_zh89991167950004.jpeg?Expires=1765900321&Signature=NvcA8mPollFmAtbCoEcN9zi8a~MlDuVK19SdqxWt7MIUtC89BQoNYst0Xmb8p6pEN49QbxrythS8yU~cfkJTJGl5~5r4nk~IpZyZ5OUNGEDqaTE-xWopKJFFLMGreoWU-MLpNnODR0gw~4hEGByJEeEiRpJMDr1ImwZBqL97OQS4cIHGtUPeA9nLVr1vOp1sn2L8-D4HMsoxjKT9fjIC1Kyob7t9Hk6n0ChZ7XGErwMiE9Wh1GPkiL0bL1FYtopOaj6OnFLGxizIZ5Fjt2lkIorjyzWFgy6eZAE0iXW4bS8V6H4tfqnvFKIPMYOZQ0jUBRPyMRgxJ3ZUc~xEiLAYHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal