Abstract
Human hematolymphoid mice have become valuable tools for the study of human hematopoiesis and uniquely human pathogens in vivo. Recent improvements in xenorecipient strains allow for long-term reconstitution with a human immune system. However, certain hematopoietic lineages, for example, the myeloid lineage, are underrepresented, possibly because of the limited cross-reactivity of murine and human cytokines. Therefore, we created a nonobese diabetic/severe combined immunodeficiency/interleukin-2 receptor-γ–null (NOD-SCID IL2Rγnull) mouse strain that expressed human stem cell factor, granulocyte-macrophage colony-stimulating factor, and interleukin-3, termed NSG-SGM3. Transplantation of CD34+ human hematopoietic stem cells into NSG-SGM3 mice led to robust human hematopoietic reconstitution in blood, spleen, bone marrow, and liver. Human myeloid cell frequencies, specifically, myeloid dendritic cells, were elevated in the bone marrow of humanized NSG-SGM3 mice compared with nontransgenic NSG recipients. Most significant, however, was the increase in the CD4+FoxP3+ regulatory T-cell population in all compartments analyzed. These CD4+FoxP3+ regulatory T cells were functional, as evidenced by their ability to suppress T-cell proliferation. In conclusion, humanized NSG-SGM3 mice might serve as a useful model to study human regulatory T-cell development in vivo, but this unexpected lineage skewing also highlights the importance of adequate spatiotemporal expression of human cytokines for future xenorecipient strain development.
Introduction
Humanized mice are amenable small-animal models that have been transplanted with human cells or tissues (and/or equipped with human transgenes). In particular, animals conditioned to support engraftment of human immune cells have emerged as powerful tools for analysis of human hematopoiesis and the study of pathogens with unique human tropism. From the earliest attempts to engraftment of human immune cells in mice in the late 1980s, the field has progressed substantially, and improved, highly immunocompromised xenorecipient strains now allow for high-level engraftment of human immune cells. Currently, the most advanced strains are the nonobese diabetic, severe combined immunodeficiency (NOD-SCID) mouse with either truncated (NOG) or complete (NSG) disruptions in the interleukin-2 (IL-2) receptor common γ-chain (IL2Rγnull) and BALB/c Rag2−/− IL2Rγnull (BRG) mice.1 Injection of human hematopoietic stem cells (HSCs) isolated from human cord blood2-5 or fetal liver tissue5-7 results in robust engraftment of a human hematolymphoid system. Such human immune system (HIS) mice have opened new opportunities to analyze human immunity in vivo and to study pathogens with unique human tropism, including Epstein-Barr virus, HIV, and dengue virus.8 However, current humanized mouse models have several shortcomings that must be overcome to advance toward a robust and predictive model for human immune responses. Specifically, the total amount of human cells in HIS mice is below the desired levels. HSCs are insufficiently maintained, and differentiation into particular lineages, such as erythromyeloid cells, is impaired.9 Furthermore, the inadequate formation of higher-order lymphoid structures may be central to the limited immune response in HIS mice.1
Modifications to the humanization protocol and xenorecipients have resulted in improved human hematopoiesis in specific compartments. For example, cotransplantation of small pieces of human fetal liver and thymus together with the injection of HSCs into irradiated NOD-SCID mice led to improved T-cell selection in so-called BLT (bone marrow/liver/thymus) mice.10 Human leukocyte antigen (HLA) class I–expressing humanized NSG mice generate functional human T-cell subsets with HLA-restricted T-cell responses against Epstein-Barr virus7,11 and dengue virus.12 Limited biologic cross-reactivity between murine and human orthologs of cytokines has been proposed as a contributing factor to inadequate representation of certain human hematopoietic lineages in humanized mice.8,9 In fact, administration of recombinant interleukin-15/interleukin-15 receptor fusion protein or transient expression of IL-15 and Flt-3/Flk-2L boosts natural killer cell frequencies in HIS mice.13-15 Administration of human IL-7 enhances thymic human T-cell development without affecting peripheral T-cell homeostasis.16 Similarly, transient expression of human granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4, macrophage colony-stimulating factor, or erythropoietin and IL-3 results in significantly enhanced reconstitution of dendritic cells, monocytes/macrophages, or erythrocytes, respectively.13
In this study, we describe the development and characterization of the NSG-SGM3 strain, an immunodeficient strain that expresses transgenes for human SCF/KIT ligand (KITLG), GM-CSF/colony-stimulating factor 2 (CSF2), and IL-3. It was recently shown that acute myeloid leukemia xenograft efficiency is significantly improved in NOD-SCID IL2Rγ mice that constitutively express human SCF, GM-CSF, and IL-3.17 In accordance with previous studies using NOD/SCID-SGM3 mice, the reconstitution of human immunity in NSG-SGM3 recipients through transplantation of purified human HSCs resulted in a significant increase of human myeloid cells in the bone marrow compared with NSG recipients. Specifically, we detected elevated numbers of myeloid dendritic cells (DCs). However, the most striking phenotype was a selective increase in the frequency of human CD4+ T cells in all organs analyzed compared with nontransgenic NSG mice. Within the CD4+ T-cell population, we observed a significant increase of regulatory T cells (Treg) but not T helper 1, 2, or 17 cells (Th1, Th2, and Th17). Such in vivo developed Treg cells expressed the lineage-specific transcription factor FoxP3 (forkhead box P3), CD25, and cytotoxic T-lymphocyte antigen 4 (CTLA-4) and were able to suppress the proliferation of polyclonally activated T cells. Treg cell expansion most likely occurs in the periphery after thymic T-cell development, because the frequency of single-positive CD4+FoxP3+ thymocytes was comparable in both mouse strains. Furthermore, human CD3+ T cells did not express detectable surface levels of CD116, CD123, or c-kit, the respective receptors for GM-CSF, IL-3, and SCF, which indicates that the unexpected preferential lineage skewing in humanized NSG-SGM3 is more likely to be mediated via an indirect mechanism. In summary, our analysis of human HSC transplantation into NSG-SGM3 mice highlights the challenges of modeling human hematopoiesis in humanized mice but also provides a novel platform with which to study human Treg cell biology in vivo.
Methods
Mice
NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice were obtained from The Jackson Laboratory and raised under specific pathogen–free conditions at Rockefeller University. NOD.Cg-PrkdcscidTg(hSCF/hGM-CSF/hIL3) (NOD/SCID-SGM3) mice18 were kindly provided by Dr James Mulloy (University of Cincinnati, Cincinnati, OH) and crossed onto the NSG background and homozygous transgene expression was established. Transgenic offspring were identified by gene-specific polymerase chain reaction, and homozygosity was confirmed by breeding with NSG mice, which resulted in the expected Mendelian distribution of 100% transgenic offspring. Homozygous NSG-SGM3 mice were then bred with NSG mice and the hemizygous offspring used for the generation of human immune system mice. Mice were bred and maintained under defined flora with irradiated food and acidified water at the Comparative Bioscience Center of the Rockefeller University according to guidelines established by the Institutional Animal Committee. All experiments in mice were performed at the Comparative Bioscience Center under protocols approved by the Institutional Animal Committee.
Purification of human HSCs and generation of HIS mice
All experiments were performed with authorization from the Institutional Review Board and the Institutional Animal Care and Use Committee at Rockefeller University. Human fetal livers (16-22 weeks of gestational age) were procured from Advanced Bioscience Resources, Inc and the Human Fetal Tissue Depository at Albert Einstein College of Medicine. Fetal liver was homogenized and incubated in digestion medium (Hanks balanced salt solution with 0.1% collagenase IV [Sigma-Aldrich], 40mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2M CaCl2, and 2 U/mL DNAse I [Roche]) for 30 minutes at 37°C. Human CD34+ HSCs were isolated with a CD34+ HSC isolation kit (StemCell Technologies) according to the manufacturer's protocol. One- to 5-day-old NSG and NSG-SGM3 mice were irradiated with 100 cGy, and 1.5-2 × 105 human CD34+ HSCs were injected intrahepatically 6 hours after irradiation. Four, 8, or 12 weeks after HSC transplantation, mice were killed for analysis of human immune system reconstitution. Male and female mice transplanted with CD34+ HSCs derived from various human donors were used in the present study.
Leukocyte isolation
Blood leukocytes were isolated from heparinized blood by Ficoll density gradient centrifugation (20 minutes, 930g). For isolation of intrahepatic leukocytes, the liver was perfused with 20 mL of cold phosphate-buffered saline (PBS; Gibco/Invitrogen), minced, and pressed through a cell strainer (100 μm; BD Biosciences). The homogenized liver was resuspended in cold RPMI 1640 (Gibco) and centrifuged for 10 minutes at 330g. The pellet was resuspended in digestion medium (see “Purification of human HSCs and generation of HIS mice”) and incubated at 37°C for 40 minutes. After digestion, the liver suspension was centrifuged for 10 minutes at 330g. The pellet was resuspended in RPMI 1640, gently overlaid onto a Ficoll gradient (Cellgro), and centrifuged for 20 minutes at 930g. Leukocytes were collected from the interphase and washed twice in PBS. For isolation of splenocytes, the spleen was homogenized through a cell strainer (100 μm; BD Biosciences) and digested for 20 minutes at 37°C in digestion medium (see “Purification of human HSCs and generation of HIS mice”). After digestion, leukocytes were isolated by density gradient centrifugation as described above. To isolate bone marrow cells, tibia and femur were flushed with PBS. The bone marrow was pressed through a cell strainer to obtain a single cell suspension, and leukocytes were isolated as described above. For analysis of human granulocytes, the bone marrow was homogenized and stained directly for surface-marker expression as described in “Antibody staining and flow cytometry.” For analysis of thymocytes, the thymus was homogenized through a cell strainer (70 μm; BD Biosciences) to obtain a single cell suspension.
Antibody staining and flow cytometry
The following anti–human antibodies were used: (1) CD45, Pacific Orange and PE-Cy5.5; CD14, Alexa Fluor 700; CD19, Pacific Blue; CD3, APC-Alexa Fluor 750 and Pe-TxR; CD38, Pe-TxR; and HLA-DR, Qdot 605, all from Invitrogen; (2) CD8, APC and fluorescein isothiocyanate (FITC); CD15, FITC; CD4, phycoerythrin (PE); CD56, PE-Cy5.5; CD117, PE-Cy7; CD33, PerCP-Cy5.5; CD86, PE; CD123, APC; CD133, APC; CD34, FITC; CD16, Pacific Blue; CD25, PE; interleukin-10 (IL-10), PE; and interferon-γ (IFN-γ), FITC, all from BD Biosciences; (3) CD11c, APC-Alexa Fluor 750; CD1c, Pacific Blue; CD127, PE-Cy7; CD45RO, PerCP-eFluor 710; CTLA-4, PE; interleukin 17-A (IL-17A), Alexa Fluor 647; and IL-4, PE, all from eBioscience; (4) CD116, PE; CD45RA, APC-Alexa Fluor 750; CD4, Pacific Blue; CD25, PE-Cy5.5; and FoxP3, Alexa Fluor 647, all from Biolegend; and (5) FITC for BDCA3, from Miltenyi Biotec. Appropriate isotype controls were also purchased from each company. Anti-mouse CD45 PE-Cy7 was obtained from eBioscience.
For surface-marker staining, cells were blocked for 10 minutes at room temperature with anti-mouse Fc Block (BD Biosciences), washed twice with staining buffer (PBS and 1% fetal bovine serum), stained with the appropriate antibodies for 15 minutes at room temperature, washed again twice with staining buffer, and fixed with 4% paraformaldehyde. Intracellular FoxP3 staining was performed with the Biolegend FoxP3 Staining Kit according to the manufacturer's instructions. To analyze intracellular cytokine production, cells were stimulated with 10 ng/mL phorbol-12-myristate-13-acetate (Sigma-Aldrich) and 200 ng/mL ionomycin (Sigma-Aldrich) in the presence of GolgiPlug (BD Biosciences) and incubated for 5 hours at 37°C. After incubation, cells were blocked, stained for surface marker, permeabilized (Cytofix/Cytoperm; BD Biosciences), and stained for intracellular IL-4, IFN-γ, IL-17, and IL-10. For cell-counting experiments, counting beads (Invitrogen) were added to the samples directly before fluorescence-activated cell sorter analysis according to the manufacturer's instructions. Fluorescence-activated cell sorter analysis was performed with a BD LSRII flow cytometer (BD Biosciences), and data were analyzed with FlowJo Software (Tree Star).
In vitro suppression assays
Treg cells were isolated from HIS-SGM3 mice blood-, spleen-, and liver-derived pooled leukocytes with a human CD4+CD25+ Treg cell isolation kit (Miltenyi Biotec) according to the manufacturer's protocol. CD25-depleted autologous T cells were labeled with 1μM carboxyfluorescein succinimidyl ester (Molecular Probes/Invitrogen), stimulated with 0.1 μg/mL anti-CD3 (Biolegend) and 0.5 μg/mL anti-CD28 (BD Biosciences), and cocultured with either isolated CD4+CD25+ Treg cells or CD4+CD25− T cells on a 96-well plate at ratios of 1:1 and 2:1 in complete medium (RPMI 1640 with 10% fetal bovine serum, 1.5% HEPES, and 1% penicillin/streptomycin). After 4 days of culture, proliferation of carboxyfluorescein succinimidyl ester–labeled responder T cells was analyzed by flow cytometry.
Analysis of cytokine concentrations
The concentration of human cytokine levels in the serum and plasma of mice was determined with GM-CSF and SCF enzyme-linked immunosorbent assay construction kits (Antigenix America, Inc) or an IL-3 enzyme-linked immunosorbent assay kit (R&D Systems). The concentration of human transforming growth factor-β (TGF-β), IL-10, and IL-2 was determined by the performance of Cytometric Bead Arrays (BD Biosciences) on an LSR II flow cytometer (BD Biosciences).
Statistical analysis
An unpaired Student t test was used to evaluate statistically significant differences.
Results
Characterization of human hematopoiesis in humanized NSG-SGM3 mice
SCF, also known as steel factor (SF) or kit ligand (KITL), may serve as a guidance cue that directs HSCs to their stem cell niche, and it plays an important role in HSC maintenance.19 GM-CSF and IL-3 stimulate the differentiation of HSCs in myeloid progenitor cells, and IL-3 also stimulates proliferation of all cells in the myeloid lineage (ie, erythrocytes, thrombocytes, granulocytes, and monocytes). All of these cytokines are species specific,9 and expression of human SCF, IL-3, and GM-CSF in NOD-SCID mice (NS-SGM3) results indeed in a larger number of myeloid cells in the bone marrow, albeit at the cost of a loss of multipotent progenitor cells in the bone marrow.20,21 Given the overall positive effect on human myelopoiesis in these models, we crossed NS-SGM3 onto the NSG background, which resulted in NSG-SGM3 mice. As reported previously,20,21 transgenic cytokine expression led to elevated levels of human SCF, GM-CSF, and IL-3 in the serum of NSG-SGM3 mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To comprehensively analyze the effect of human cytokine expression on human hematopoiesis on this improved immunodeficient background, we injected sublethally irradiated neonatal NSG and NSG-SGM3 mice with human fetal liver–derived CD34+ HSCs. In both recipient strains, we achieved a similarly high human hematopoietic chimerism of approximately 50% in blood (Figure 1A), which remained stable from 8 to 12 weeks after HSC transplantation.
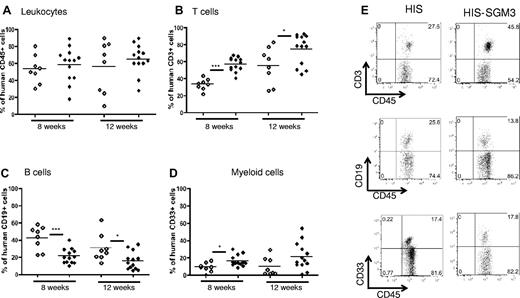
Peripheral human immune system reconstitution in HIS versus HIS-SGM3 mice. HIS mice and HIS-SGM3 mice were generated by transplantation of human CD34+ HSCs into newborn NSG (NOD/SCID/IL2Rγnull) mice and NSG mice transgenic for human GM-CSF, IL-3, and SCF (NSG-SGM3). Eight and 12 weeks after transplantation, blood was analyzed for human immune system reconstitution by flow cytometry. (A) Isolated leukocytes from HIS (◇) and HIS-SGM3 (♦) mice were counterstained with anti-mouse CD45 and anti-human CD45 antibodies to determine the overall human immune cell chimerism. Within the human CD45+ cell population, the frequency of T cells (B), B cells (C), and myeloid cells (D) in HIS versus HIS-SGM3 mice was determined. (E) Representative fluorescence-activated cell sorter dot plots. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001.
Peripheral human immune system reconstitution in HIS versus HIS-SGM3 mice. HIS mice and HIS-SGM3 mice were generated by transplantation of human CD34+ HSCs into newborn NSG (NOD/SCID/IL2Rγnull) mice and NSG mice transgenic for human GM-CSF, IL-3, and SCF (NSG-SGM3). Eight and 12 weeks after transplantation, blood was analyzed for human immune system reconstitution by flow cytometry. (A) Isolated leukocytes from HIS (◇) and HIS-SGM3 (♦) mice were counterstained with anti-mouse CD45 and anti-human CD45 antibodies to determine the overall human immune cell chimerism. Within the human CD45+ cell population, the frequency of T cells (B), B cells (C), and myeloid cells (D) in HIS versus HIS-SGM3 mice was determined. (E) Representative fluorescence-activated cell sorter dot plots. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001.
Comparable total numbers of human leukocytes were detected in liver, spleen, and bone marrow in both mouse strains 12 weeks after transplantation (Figure 2D). Here, we refer to humanized NSG mice as “HIS” mice and to humanized NSG-SGM3 mice as “HIS-SGM3” mice. In concordance with previous reports,20 frequencies of CD33+ myeloid cells were significantly elevated in HIS-SGM3 compared with HIS mice at week 8 but not at week 12, with peak levels of approximately 20% achieved in peripheral blood (Figure 1D-E). In all other tested organs (bone marrow, liver, spleen), CD33+ myeloid (Figure 2C-E) and CD14+ monocyte (data not shown) populations were equivalent in HIS and HIS-SGM3 mice. Because our leukocyte isolation protocol includes density gradient centrifugation, granulocytes are largely excluded from analysis. To compare granulocyte development, we also analyzed freshly isolated bone marrow directly for the presence of CD15+ granulocytes. As shown in supplemental Figure 2A-B, the levels of human granulocytes were slightly elevated in HIS-SGM3 compared with HIS mice (P = .05).
Human immune system reconstitution in spleen, liver, and bone marrow of HIS versus HIS-SGM3 mice. Twelve weeks after human CD34+ HSC engraftment, various organs from HIS and HIS-SGM3 mice were analyzed for human immune cell subset reconstitution by flow cytometry. Frequencies of human CD3+ T cells (A), CD19+ B cells (B), and CD33+ myeloid cells (C) in spleen, liver, and bone marrow of HIS (◇) and HIS-SGM3 (♦) mice are shown. (D) Total cell counts of indicated human immune cell subsets in the liver, spleen, and bone marrow. (E) Representative fluorescence-activated cell sorter dot plots for bone marrow and spleen. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. BM indicates bone marrow; error bars indicate SEM.
Human immune system reconstitution in spleen, liver, and bone marrow of HIS versus HIS-SGM3 mice. Twelve weeks after human CD34+ HSC engraftment, various organs from HIS and HIS-SGM3 mice were analyzed for human immune cell subset reconstitution by flow cytometry. Frequencies of human CD3+ T cells (A), CD19+ B cells (B), and CD33+ myeloid cells (C) in spleen, liver, and bone marrow of HIS (◇) and HIS-SGM3 (♦) mice are shown. (D) Total cell counts of indicated human immune cell subsets in the liver, spleen, and bone marrow. (E) Representative fluorescence-activated cell sorter dot plots for bone marrow and spleen. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. BM indicates bone marrow; error bars indicate SEM.
An analysis of human DC development revealed that all major subsets of human DCs were present in the bone marrow of HIS mice, specifically CD123high CD11cneg plasmacytoid DCs, BDCA-3+ CD11clow myeloid DCs, and BDCA-1+CD11chigh myeloid DCs (Figure 3A-B). Interestingly, myeloid CD1c (BDCA-1)+ DCs were increased approximately 3-fold in the bone marrow of HIS-SGM3 mice (Figure 3C-D). These myeloid DCs displayed an activated phenotype, as indicated by high expression of CD86 and HLA-DR (Figure 3E). In spleen, liver, and thymus, equal levels of plasmacytoid DCs and myeloid DCs were detected in HIS and HIS-SGM3 mice (supplemental Figure 2C-D).
HIS-SGM3 mice show increased levels of myeloid DCs and decreased levels of primary HSCs in the bone marrow. Bone marrow–derived leukocytes from HIS and HIS-SGM3 mice were stained for the expression of human CD45, CD123, CD11c, CD1c (BDCA-1), BDCA-4, HLA-DR, and CD86 to analyze the development of human DCs. (A) Representative fluorescence-activated cell sorter plots demonstrate the presence of CD123+ plasmacytoid DCs and BDCA-3+ or BDCA-1+ myeloid DCs in HIS mice. (B) Histogram shows CD11c expression on CD123+ (light gray outline), BDCA-4+ (black outline), and BDCA-1+ (shaded area) DCs. (C) Frequencies of CD1c+ (BDCA-1+) myeloid DCs. (D) Original fluorescence-activated cell sorter dot plots. (E) Histograms show CD86 and HLA-DR expression on CD1c+ myeloid DCs in HIS mice (shaded area) and HIS-SGM3 mice (black outline) compared with isotype control (light black outline). To compare the maintenance of primary human HSCs in the bone marrow of HIS and HIS-SGM3 mice, human leukocytes were analyzed for the expression of CD34, CD38, c-KIT, and CD133. (F) Group data of human CD34+CD38-c-KIT+ HSC frequencies. Unpaired Student t test: **P ≤ .005
HIS-SGM3 mice show increased levels of myeloid DCs and decreased levels of primary HSCs in the bone marrow. Bone marrow–derived leukocytes from HIS and HIS-SGM3 mice were stained for the expression of human CD45, CD123, CD11c, CD1c (BDCA-1), BDCA-4, HLA-DR, and CD86 to analyze the development of human DCs. (A) Representative fluorescence-activated cell sorter plots demonstrate the presence of CD123+ plasmacytoid DCs and BDCA-3+ or BDCA-1+ myeloid DCs in HIS mice. (B) Histogram shows CD11c expression on CD123+ (light gray outline), BDCA-4+ (black outline), and BDCA-1+ (shaded area) DCs. (C) Frequencies of CD1c+ (BDCA-1+) myeloid DCs. (D) Original fluorescence-activated cell sorter dot plots. (E) Histograms show CD86 and HLA-DR expression on CD1c+ myeloid DCs in HIS mice (shaded area) and HIS-SGM3 mice (black outline) compared with isotype control (light black outline). To compare the maintenance of primary human HSCs in the bone marrow of HIS and HIS-SGM3 mice, human leukocytes were analyzed for the expression of CD34, CD38, c-KIT, and CD133. (F) Group data of human CD34+CD38-c-KIT+ HSC frequencies. Unpaired Student t test: **P ≤ .005
As documented previously,20 the numbers of primitive HSCs characterized by a CD38− CD34+ c-KIT+ phenotype were reduced in the bone marrow of humanized NSG mice expressing SCF, GM-CSF, and IL-3 compared with nontransgenic control mice (Figure 3F-G). This finding can be explained by the ability of GM-CSF to mobilize HSCs.22
In summary, HIS-SGM3 mice are comparable to HIS mice in their capacity for human immune cell engraftment, with improved human myeloid cell development.
Increased frequencies of CD4+ lymphocytes in HIS-SGM3 mice
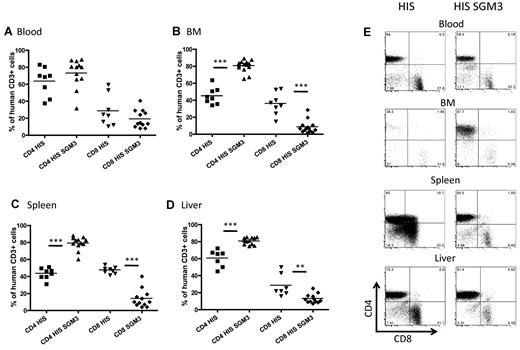
Interestingly, we detected the most significant differences in human hematopoiesis in HIS-SGM3 compared with HIS mice in the lymphocyte population. Indeed, B-cell frequencies decreased significantly in peripheral blood mononuclear cell fractions (from more than 40% to approximately 20%) at 8 weeks and had a slightly less pronounced decrease (from approximately 30% to 15%) at 12 weeks in HIS versus HIS-SGM3 mice, respectively (Figure 1C,E). Similar decreases in B-cell frequencies and numbers were observed in spleen and bone marrow of HIS-SGM3 mice (Figure 2B,D-E). This overall reduction in B cells was paralleled by a proportional increase in CD3+ T-cell frequencies and numbers in all compartments analyzed (Figures 1B,E and 2A,D,E). These significant changes in the lymphocyte populations had not been described previously.20 To further characterize the increased T-cell population in HIS-SGM3 mice, we first compared CD4+ to CD8+ T-cell ratios in both mouse strains. In peripheral blood, the ratio of CD4+ to CD8+ T cells was approximately 2:1 in both transgenic and control mice (Figure 4A,E). However, within the CD3+ T-cell population, the CD4+ T-cell subset increased significantly (from approximately 40% to 80%) in bone marrow and spleen, with a slightly less pronounced increase (from 60% to 80%) in the liver, whereas the CD8+ T-cell subset decreased proportionally in HIS-SGM3 and HIS mice, respectively (Figure 4B-E). Taken together, our results show that the transgenic expression of human SCF, IL-3, and GM-CSF significantly promotes the expansion of human CD4+ T cells in HIS mice.
Elevated levels of CD4+ T cells in HIS-SGM3 mice. Twelve weeks after human CD34+ HSC engraftment, HIS and HIS-SGM3 mice were analyzed for human CD4+ to CD8+ T-cell ratios in blood (A), bone marrow (BM) (B), spleen (C), and liver (D). (E) Original fluorescence-activated cell sorter plots. Cells were gated on human CD45+CD3+ T cells. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001.
Elevated levels of CD4+ T cells in HIS-SGM3 mice. Twelve weeks after human CD34+ HSC engraftment, HIS and HIS-SGM3 mice were analyzed for human CD4+ to CD8+ T-cell ratios in blood (A), bone marrow (BM) (B), spleen (C), and liver (D). (E) Original fluorescence-activated cell sorter plots. Cells were gated on human CD45+CD3+ T cells. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001.
Skewing toward the regulatory T-cell lineage within the increased pool of CD4+ T cells in HIS-SGM3 mice
Human CD4+ T cells can be divided phenotypically and functionally into at least 4 subpopulations: Th1, Th2, Th17, and Treg cells.23,24 Cells of the Th1 lineage, which evolved to enhance eradication of intracellular pathogens, are characterized by their production of IFN-γ. Cells of the Th2 lineage, which evolved to enhance elimination of parasitic infections (eg, helminths), are characterized by production of IL-4, IL-5, and IL-13.23 More recently, Th17 cells have been identified that primarily produce IL-17 and appear to have a crucial role in mediating autoimmunity and inducing tissue inflammation. Treg cells suppress immune responses and play an important role in the maintenance of immune system homeostasis. The expression of the lineage-specific transcription factor FoxP3 is one major phenotypic characteristic of Treg cells.25,26
To determine the relative distribution of the CD4+ T-cell subsets within the increased T-cell population, we quantified the numbers of IFN-γ+, IL-4+, IL-17+, or FoxP3+ CD4+ T cells in HIS and HIS-SGM3 mice. All CD4+ T-cell subsets could be detected in blood, liver, spleen, and bone marrow of HIS and HIS-SGM3 mice (Figure 5). Interestingly, however, CD4+FoxP3+ Treg cells were significantly increased (Figure 5A-B) but all T-helper subsets (Figure 5C) were similarly distributed in SGM3 transgenic versus nontransgenic mice. These data suggest that transgenic expression of human SCF, GM-CSF, and IL-3 leads to the specific development of human CD4+FoxP3+ T cells in HIS mice. It is unlikely, however, that the expansion of CD4+FoxP3+ Treg cells is the result of a direct action of these cytokines. CD3+ T cells in HIS and HIS-SGM3 mice did not express CD116, CD123, or c-Kit, the receptors of GM-CSF, IL-3, and SCF, respectively (supplemental Figure 3).
CD4+FoxP3+ regulatory T cells are selectively expanded in HIS-SGM3 mice. To determine the proportion of Treg cells within the CD3+CD4+ T-cell population, human leukocytes from 6 HIS and 6 HIS-SGM3 mice were analyzed for expression of transcription factor FoxP3 by flow cytometry. Frequencies of human CD4+FoxP3+ T cells in blood, spleen, liver, and bone marrow (BM) of HIS and HIS-SGM3 mice are shown in (A), with representative fluorescence-activated cell sorter plots from blood, spleen, and liver shown in (B). To determine the frequency of T-helper subsets Th1, Th2, and Th17 within the CD4+ T-cell population, cells were stimulated ex vivo with phorbol-12-myristate-13-acetate/ionomycin for 5 hours at 37°C and subsequently stained for intracellular IFN-γ (Th1), IL-4 (Th2), and IL-17 (Th17). (C) Group data of a total of 6 HIS and 6 HIS-SGM3 mice. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. Error bars indicate SEM.
CD4+FoxP3+ regulatory T cells are selectively expanded in HIS-SGM3 mice. To determine the proportion of Treg cells within the CD3+CD4+ T-cell population, human leukocytes from 6 HIS and 6 HIS-SGM3 mice were analyzed for expression of transcription factor FoxP3 by flow cytometry. Frequencies of human CD4+FoxP3+ T cells in blood, spleen, liver, and bone marrow (BM) of HIS and HIS-SGM3 mice are shown in (A), with representative fluorescence-activated cell sorter plots from blood, spleen, and liver shown in (B). To determine the frequency of T-helper subsets Th1, Th2, and Th17 within the CD4+ T-cell population, cells were stimulated ex vivo with phorbol-12-myristate-13-acetate/ionomycin for 5 hours at 37°C and subsequently stained for intracellular IFN-γ (Th1), IL-4 (Th2), and IL-17 (Th17). (C) Group data of a total of 6 HIS and 6 HIS-SGM3 mice. Unpaired Student t test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. Error bars indicate SEM.
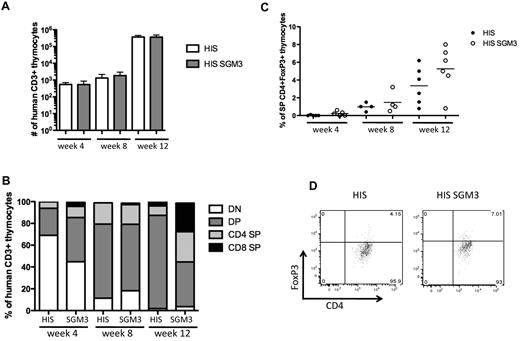
To examine whether the increase in Treg cells is caused by enhanced thymic generation of these cells in HIS-SGM3 mice, we comprehensively analyzed human thymocyte development in both mouse strains. Human CD3+ thymocyte numbers were low at week 4 and week 8 after human HSC transplantation but increased significantly at week 12 to comparable levels in HIS and HIS-SGM3 mice (Figure 6A). At week 4, the majority of CD3+ thymocytes displayed an immature CD4−CD8− double-negative phenotype, whereas at week 8, CD8+CD4+ double-positive thymocytes were predominant in both HIS and HIS-SGM3 mice (Figure 6B). Twelve weeks after HSC transplantation, lineage commitment to either CD8+ single-positive or CD4+ single-positive T cells was more advanced in the thymus of HIS-SGM3 mice than in nontransgenic HIS mice (Figure 6B).
Thymocyte development in HIS and HIS-SGM3 mice. Human thymocyte development was analyzed 4, 8, and 12 weeks after human HSC transplantation by staining thymus-derived cells for the expression of human CD45, CD3, CD4, CD8, and FoxP3. (A) Total numbers of human CD45+CD3+ thymocytes at weeks 4, 8, and 12 in HIS and HIS-SGM3 mice are shown. (B) Frequencies of human CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), CD4+ single-positive (CD4 SP), and CD8+ single-positive (CD8 SP) cells within the CD3+ thymocyte population at weeks 4, 8, and 12 are displayed. (C) The frequencies of single-positive CD3+CD4+FoxP3+ thymocytes at indicated time points in HIS and HIS-SGM3 mice are shown. (D) Original fluorescence-activated cell sorter plots showing FoxP3 expression in human CD4 SP thymocytes 12 weeks after HSC transplantation are depicted. Error bars indicate SEM.
Thymocyte development in HIS and HIS-SGM3 mice. Human thymocyte development was analyzed 4, 8, and 12 weeks after human HSC transplantation by staining thymus-derived cells for the expression of human CD45, CD3, CD4, CD8, and FoxP3. (A) Total numbers of human CD45+CD3+ thymocytes at weeks 4, 8, and 12 in HIS and HIS-SGM3 mice are shown. (B) Frequencies of human CD4−CD8− double-negative (DN), CD4+CD8+ double-positive (DP), CD4+ single-positive (CD4 SP), and CD8+ single-positive (CD8 SP) cells within the CD3+ thymocyte population at weeks 4, 8, and 12 are displayed. (C) The frequencies of single-positive CD3+CD4+FoxP3+ thymocytes at indicated time points in HIS and HIS-SGM3 mice are shown. (D) Original fluorescence-activated cell sorter plots showing FoxP3 expression in human CD4 SP thymocytes 12 weeks after HSC transplantation are depicted. Error bars indicate SEM.
Human FoxP3+CD4+ single-positive thymocytes were readily detectable only at week 12 after HSC transplantation at highly variable levels between individual mice (Figure 6C-D). HIS-SGM3 mice displayed slightly higher frequencies of FoxP3+CD4+ thymocytes than HIS mice (Figure 6B-C). In the thymus, however, we did not detect the consistent and highly significant elevated levels of FoxP3+CD4+ Treg cells that we observed in the periphery. Taken together, the equal numbers of human thymocytes and comparable levels of FoxP3+CD4+ cells in the thymus of both mouse strains suggest that the generation of Treg cells in HIS-SGM3 mice is more likely due to peripheral expansion than to thymic development.
We further evaluated whether the increase of Treg cells in HIS-SGM3 mice correlates with increased serum levels of human IL-2, IL-10, or TGF-β, 3 cytokines that play an important role in Treg cell expansion and homeostasis.27 Although we were able to detect minimal concentrations of these cytokines in the serum, no elevated levels were observed in HIS-SGM3 compared with HIS mice (data not shown). These results, however, do not exclude the possibility that IL-2, IL-10, or TGF-β is present at a higher concentration but only in local tissue environments, eg, in spleen or liver, of HIS-SGM3 mice.
CD4+FoxP3+ Treg cells developed in humanized NSG-SGM3 mice resemble human regulatory T cells in phenotype and function
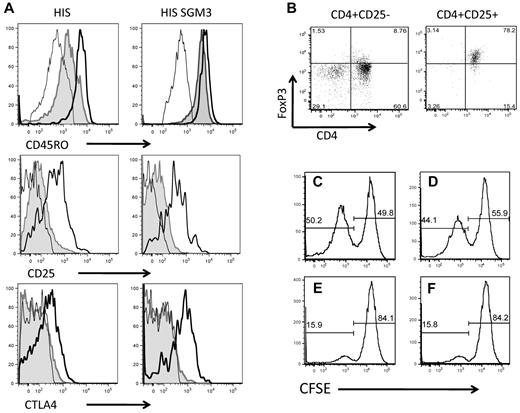
To determine whether the CD4+FoxP3+ Treg cells developed in HIS-SGM3 mice indeed resemble “bona fide” human regulatory T cells, we performed a phenotypic and functional analysis of these cells. Human CD4+FoxP3+ Treg cells, among others, are characterized by the constitutive expression of CD25, the IL-2 receptor α-chain, and CTLA-4.27 Functionally, they are characterized by their ability to suppress immune responses, eg, the proliferation of activated T cells. This suppressive activity can be mediated either through direct cell-cell contact-dependent mechanisms or through the secretion of anti-inflammatory cytokines such as IL-10.28 We found that CD4+FoxP3+ Treg cells in HIS mice and in HIS-SGM3 mice express high levels of CD45RO, CD25, and CTLA-4 (Figure 7A). Studies analyzing human peripheral blood mononuclear cells recently have defined T cells with this phenotype to be activated effector Treg cells with a potent in vitro suppressive capacity.29 Purification of CD4+ T cells derived from HIS-SGM3 mice according to CD25 expression resulted in an isolated CD4+FoxP3+ Treg cell population (Figure 7B). These purified cells significantly suppressed the proliferation of effector T cells in coculture experiments (Figure 7C-F). Indeed, effector T cells from HIS-SGM3 mice proliferated significantly, as indicated by carboxyfluorescein succinimidyl ester dilution, when stimulated with anti-CD3/CD28 alone (Figure 7C) or in the presence of isolated CD4+CD25− T cells (Figure 7D). In the presence of isolated CD4+CD25+FoxP3+ Treg cells, however, effector T-cell proliferation was strongly inhibited at effector-to-regulatory T-cell ratios of 1:1 and 2:1 (Figure 7E and F, respectively). CD4+FoxP3+ Treg cells from HIS and HIS-SGM3 mice did not produce IL-10 after stimulation with phorbol-12-myristate-13-acetate/ionomycin (data not shown), which indicates that their suppressive activity is rather mediated through cell-cell contact-dependent mechanisms. In summary, the present analysis of in vivo expanded human CD4+FoxP3+ Treg cells revealed that they phenotypically and functionally resemble those regulatory T cells that have been described to be present in human blood and tissue.27
Phenotypic and functional characteristics of CD4+FoxP3+ Treg cells expanded in HIS-SGM3 mice. Leukocytes isolated from blood, liver, and spleen of HIS and HIS-SGM3 mice were analyzed for the expression of human CD45, CD3, CD4, FoxP3, CD25, CD45RA, CD45RO, and CTLA-4 to determine the phenotype of in vivo expanded human Treg cells. (A) Representative histograms show CD45RO, CD25, and CTLA-4 expression on CD4+FoxP3− (shaded area) and CD4+FoxP3+ (black outline) T cells from the blood of HIS and HIS SGM3 mice compared with isotype control (light black outline). To test their suppressive capacity, CD4+FoxP3+ Treg cells from HIS-SGM3 mice were purified according to their CD25 expression (B) and cocultured with carboxyfluorescein succinimidyl ester (CFSE)–labeled autologous CD3+ T cells in the presence of anti-CD3/CD28. After 4 days of culture, the proliferation of human CD3+ T cells alone (C), in the presence of isolated CD4+CD25− T cells (D) (ratio 1:1), or in the presence of CD4+CD25+ Treg cells (E, ratio 1:1; F, ratio 2:1) was determined by flow cytometry. One of 4 individual experiments is shown.
Phenotypic and functional characteristics of CD4+FoxP3+ Treg cells expanded in HIS-SGM3 mice. Leukocytes isolated from blood, liver, and spleen of HIS and HIS-SGM3 mice were analyzed for the expression of human CD45, CD3, CD4, FoxP3, CD25, CD45RA, CD45RO, and CTLA-4 to determine the phenotype of in vivo expanded human Treg cells. (A) Representative histograms show CD45RO, CD25, and CTLA-4 expression on CD4+FoxP3− (shaded area) and CD4+FoxP3+ (black outline) T cells from the blood of HIS and HIS SGM3 mice compared with isotype control (light black outline). To test their suppressive capacity, CD4+FoxP3+ Treg cells from HIS-SGM3 mice were purified according to their CD25 expression (B) and cocultured with carboxyfluorescein succinimidyl ester (CFSE)–labeled autologous CD3+ T cells in the presence of anti-CD3/CD28. After 4 days of culture, the proliferation of human CD3+ T cells alone (C), in the presence of isolated CD4+CD25− T cells (D) (ratio 1:1), or in the presence of CD4+CD25+ Treg cells (E, ratio 1:1; F, ratio 2:1) was determined by flow cytometry. One of 4 individual experiments is shown.
Discussion
Limited biologic cross-reactivity among cytokines that are critical for hematopoietic development has been proposed as a major contributing factor to the misrepresentation of certain blood cell types in humanized mice.8,9,30 In fact, transient expression or exogenous administration of IL-3, IL-4, IL-7, IL-15, GM-CSF, and erythropoietin, for example, has been shown to boost human natural killer cell, T-cell, myeloid cell, and erythrocyte development in humanized mice.13-15,31 Although such transient-expression approaches allow for rapid testing of (combinations of) different human cytokines, trough levels decrease quickly after administration, and frequent reinjection is required. Thus, mice expressing transgenically human SCF, GM-CSF, and IL320,21 allow for analysis of the long-term effects of these cytokines on human hematopoiesis. Originally, aspects of human hematopoiesis were analyzed in SGM3 transgenic mice on the NOD-SCID background; however, engraftment of NSG mice with human HSCs generates substantially higher percentages of human CD45+ cells in host bone marrow than with similarly treated NOD-SCID mice.3,4 Therefore, we crossed NOD-SCID-SGM3 mice to the NSG background. Overall engraftment was similarly robust in NSG and NSG-SGM3 mice, and as reported previously, we observed increases in myeloid cells and reduction of hematopoietic progenitor cell frequencies in the bone marrow.20 However, the most striking phenotype was the selective increase in CD4+FoxP3+ regulatory T-cell numbers. These data suggest that the selective expression of human cytokines in humanized mice might affect the complex process of hematopoiesis in an unexpected way and should therefore be considered carefully. In NSG-SGM3 mice, a constitutively active cytomegalovirus promoter drives transgene expression; however, to accurately reproduce the complex spatiotemporal expression pattern of cytokines in vivo, it will be important to include bacterial artificial chromosome transgenic and knock-in approaches in future strain development. Furthermore, other factors may influence the ability of exogenous cytokines to function properly besides their timing and site of expression, such as other human-murine mismatches in humanized mice, and these will need to be understood and solved.
Regulatory T cells play a pivotal role in controlling immune responses and maintaining immune system homeostasis.24 They have been implicated in several pathologic processes, including cancers, infectious diseases, and autoimmune diseases. Treg cells can be divided into 2 major subsets: natural Treg cells and induced Treg cells.32 Whereas natural Treg cells develop in the thymus, induced Treg cells are generated from effector T-cell precursors in the periphery during an immune response. Regulatory T-cell lineage development has been studied intensely, but some aspects remain opaque. Genetic studies in both mice and humans have identified Scurfin or FoxP3, a forkhead transcription factor, as a determinant of Treg cell development.25,26,33-35 Mice carrying spontaneous mutations in FoxP3 that result in the Scurfin phenotype or with targeted disruptions in the FoxP3 gene25,26 lack functional Treg cells and exhibit lymphoproliferative diseases and autoimmune phenotypes. Similarly, humans with mutations in the human FoxP3 gene lack functional Treg cells and consequently suffer from severe autoimmune symptoms in multiple organs.35 However, regulatory T-cell populations, specifically those that are induced in the periphery, are very heterogeneous in phenotype and function, and not all of them express FoxP3. The mechanisms of peripheral regulatory T-cell induction are complex and may include a specific mode of antigen presentation or exposure to anti-inflammatory cytokines such as IL-10 or TGF-β.36 Recent studies indicate that DCs may play a major role in this process.37
Human regulatory T-cell development in humanized mice has not been studied in detail thus far. It was reported previously that approximately 1%-4% of human CD4+ T cells present in thymus, spleen, lymph nodes, and blood of humanized BRG (BALB/c Rag2−/− IL2Rγnull) mice display both Treg phenotype (CD25+ FoxP3+) and Treg function.38 In NSG recipients, human CD4+ T cells preferentially develop into Th1 cells, although Th2 and Th17 cells are also generated.11 Here, we confirm these observations in both HIS and HIS-SGM3 mice and additionally provide evidence that human CD4+FoxP3+ regulatory T cells also develop in both strains, albeit at significantly increased frequencies in HIS-SGM3 recipients. Treg cells in HIS-SGM3 mice express high levels of CD25 and CTLA-4 and are fully functional, because they can suppress T-cell proliferation ex vivo.
The mechanisms by which regulatory T cells are expanded in HIS-SGM3 mice remain to be determined. Our data demonstrate that human mature T lymphocytes in humanized mice do not express the receptors for SCF, GM-CSF, and IL-3, and thus, it is not likely that they respond directly to these cytokines. Although c-KIT, CD116, and CD123 may be expressed on developing thymocytes, these receptors usually are not expressed in cells committed toward the lymphoid lineage.39 We found equal numbers of CD3+ thymocytes and comparable frequencies of CD4+FoxP3+ cells in the thymus of HIS and HIS-SGM3 mice. Although HIS-SGM3 mice showed higher levels of lineage-committed single-positive thymocytes at week 12 after HSC transplantation, we did not observe a skewing toward the CD4+ T-cell lineage in the thymus. Thus, Treg cell expansion in transgenic mice is more likely mediated via an indirect mechanism in the periphery rather than via enhanced thymic development. Future studies should address the question of whether the large increase in human Treg cells in HIS-SGM3 mice is because of peripheral proliferation of thymically derived natural Treg cells or because of the conversion of effector T cells into induced regulatory T cells. Studying the mechanisms underlying this expansion might yield important insights into human Treg cell biology and tolerance maintenance.
DCs play a pivotal role in maintaining peripheral tolerance. Studies in mice and humans demonstrated that several subsets of DCs are able to regulate Treg cell expansion.40-43 For example, a DC subset in the gut characterized by expression of CD103+ is specialized to induced FoxP3+ Treg cells in the absence of any exogenous factors and participates in maintenance of oral tolerance.44,45 Similarly, in mice, it was shown that splenic CD8+ DEC-205+ DCs have the ability to induce antigen-specific FoxP3+ Treg cells.43,46 The human equivalent of mouse CD8+ DCs was identified recently, characterized by a CD1− CD141+ Clerc9A+ Necl2+ XCR1+ surface phenotype.47-50 DCs in the skin, called Langerhans cells, may also play a role in maintaining tolerance by controlling FoxP3+ Treg cell frequencies.43 It was further demonstrated that a subset of more mature DCs in the human thymus, after stimulation with thymic stromal lymphopoietin, can lead to expansion of FoxP3+ Treg cells.51
Previous studies13,52 and the present data suggest that all major subsets of human DCs develop in humanized mice. In addition, we found increased levels of BDCA-1 (CD1c)+ myeloid DCs in the bone marrow of HIS-SGM3 recipients. Thus, human DC subsets capable of inducing Treg cells might be one possible mechanism for the increased number of human CD4+FoxP3+ Treg cells in HIS-SGM3 mice.
In conclusion, the humanized NSG-SGM3 mouse model might be a useful in vivo system to study human Treg cell development and the possible mechanism underlying their peripheral generation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank James Mulloy for sharing NOD/SCID-SGM3 mice with us.
This work was supported in part by Clinical and Translational Science Award pilot grant CCL3001018 (to A.P.) and Clinical and Translational Science Award grant UL1 RR024143 (to Rockefeller University) from the National Center for Research Resources, a component of the National Institutes of Health; a U19 AI057266 subcontract with Emory University (to C.M.R. and A.P.); the National Institutes of Health through the National Institutes of Health Roadmap for Medical Research, grant 1 R01 DK085713-01 (information on this Roadmap Transformative R01 Program can be found at http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-08-029.html); and the Greenberg Medical Institute. E.B. and M.D. are recipients of a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft.
National Institutes of Health
Authorship
Contribution: E.B., W.T.B., K.M., and M.D. performed research; and E.B., C.M.R, and A.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Ploss, Laboratory of Virology and Infectious Disease, The Rockefeller University, 1230 York Ave, New York, NY 10065; e-mail: aploss@rockefeller.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal