Abstract
The protein tyrosine phosphatase CD45 is an important regulator of Src-family kinase activity. We found that in the absence of CD45, natural killer (NK) cells are defective in protecting the host from mouse cytomegalovirus infection. We show that although CD45 is necessary for all immunoreceptor tyrosine–based activation motif (ITAM)–specific NK-cell functions and processes such as degranulation, cytokine production, and expansion during viral infection, the impact of CD45 deficiency on ITAM signaling differs depending on the downstream function. CD45-deficient NK cells are normal in their response to inflammatory cytokines when administered ex vivo and in the context of viral infection. Syk and ζ chain–associated protein kinase 70 (Zap70) are thought to play redundant roles in transmitting ITAM signals in NK cells. We show that Syk, but not Zap70, controls the remaining CD45-independent, ITAM-specific NK-cell functions, demonstrating a functional difference between these 2 Syk-kinase family members in primary NK cells.
Introduction
Natural killer (NK) cells confer immunity against tumors and pathogens by producing cytokines such as interferon γ (IFNγ), tumor necrosis factor α, RANTES (regulated on activation normal T-cell expressed and secreted), macrophage inflammatory protein 1α, and macrophage inflammatory protein 1β, and by killing transformed or infected cells through the release of lytic granules.1 These effector functions are triggered by the binding of activating NK-cell receptors (NKRs) to their ligands on target cells, which include immunoglobulin G (IgG), viral antigens, adhesion molecules, and stress-induced glycoproteins that are induced after viral infection or cellular transformation.2 These activating signals are modulated by inhibitory NKRs that recognize self–major histocompatibility complex class I molecules and prevent damage of healthy tissues.
Many of the human and mouse activating NKRs associate with signaling adapters containing immunoreceptor tyrosine–based activation motifs (ITAMs) via charged amino acids within the transmembrane domains. These NKRs, which include CD16, Ly49H, NKp46, and an isoform of NKG2D, associate with the ITAM-containing adapters CD3ζ, FcϵRIγ, and/or DAP12.3 Ligation and cross-linking of these ITAM-bearing receptors initiate a signaling cascade whereby the tyrosines within the ITAM are phosphorylated by the Src-family kinases. The 2 members of the Syk-kinase family, Syk and ζ chain–associated protein kinase 70 (Zap70), both of which are expressed in NK cells,4,5 bind the doubly phosphorylated tyrosine residues in the ITAMs via tandem SH2 domains. Src-family kinases then phosphorylate and activate ITAM-bound Syk and Zap70, which in turn propagate the activating signal through trans-autophosphorylation and phosphorylation of molecules such as LAT and SLP-76.6 A variety of downstream molecules, such as the Vav-family members and the mitogen-activated protein kinases (MAPKa), JNK, ERK, and p38, are activated, resulting in NK cell–mediated cytotoxicity and cytokine production.3
NK cells are important in the protection of both humans and mice from herpesvirus infection, including human and mouse cytomegalovirus (MCMV), respectively7-9 MCMV-infected cells express a viral glycoprotein, m157, on the cell surface, which is a ligand for the mouse ITAM-associated NKR Ly49H that is expressed on a subset of NK cells in C57BL/6 mice.10 Antibody blockade or ablation of Ly49H results in susceptibility of C57BL/6 mice to MCMV.11,12 In addition to target cell killing and cytokine production, ITAM-dependent NKR activation also results in the expansion of NK cells, as observed during infection of mice with MCMV. By day 7 after infection, a significant expansion of Ly49H+ NK cells occurs, which requires the presence of m157 on the infected cells, as well as the presence and function of Ly49H on the NK cells.13-15 NK cells also expand and/or produce cytokines such as IFNγ independently of ITAM-based NKR signaling through stimulation with type I IFNs and cytokines such as IL-2, IL-15, IL-12, and IL-18 that are produced during viral infections.9
A large number of Src-family kinases are expressed in NK cells, including Fyn, Lck, Lyn, Hck, c-Fgr, Src, and, c-Yes.16-18 Although Src-kinase activity is required for ITAM-receptor functions in NK cells, no prior study has reported defective ITAM-based NKR functions in mice lacking specific Src kinases.19,20 This is likely because of functional redundancy between Src family members. Src-family kinases are controlled in part by the tyrosine phosphatase CD45, which dephosphorylates the negative regulatory tyrosine of Src-family kinases, allowing these kinases to be further activated.21 CD45 is expressed on all hematopoietic cells and is necessary for T- and B-cell development and function. We and others have previously shown that CD45 is necessary in NK cells for ITAM-dependent cytokine production, but is not required for ITAM-dependent cell-mediated cytotoxicity in vitro.20,22-25 MAPK signaling downstream of ITAM-dependent receptors is severely defective in CD45-deficient NK cells.22,23 We found it striking that the stimulation of a single ITAM-signaling receptor would result in such a dichotomy of resultant effector functions. In this study, we have addressed the cellular functions that are dependent on CD45 during viral infection and identified genetic requirements for the CD45-independent ITAM-dependent receptor functions.
Methods
Mice and infections
Inbred wild-type (WT) and Rag2−/− × IL2rγ−/− C57BL/6 mice were purchased from Charles River Laboratories or the National Cancer Institute. Ptprc (CD45)−/− mice (exon 6 disruption), Zap70−/−, Sykb (Syk)+/−, and Tyrobp (DAP12)−/− mice were backcrossed at least 8 generations, 7 generations, 11 generations, and 12 generations, respectively, onto the C57BL/6 genetic background.5,26-29 All Syk-deficient mice (Sykb+/− mice and Ptprc−/− × Sykb+/−) mice were maintained as heterozygotes because Syk-deficiency results in embryonic lethality. Sykb+/− or Ptprc−/− × Syk+/− mice were bred to generate Sykb−/− or Ptprc−/− × Sykb−/− embryos. Fetal livers were harvested from 15.5- to 16.5-day-old embryos. Sykb−/− or Ptprc−/− × Sykb−/− embryos were visually identified and confirmed by polymerase chain reaction. WT and Syk-deficient fetal liver cells were used to reconstitute lethally irradiated WT CD45.1 adult C57BL/6 recipients via intravenous injection. WT, Ptprc−/−, and Ptprc−/− × Sykb−/− fetal liver cells (approximately 25% of a fetal liver per recipient) were used to reconstitute irradiated Rag2−/− × IL2rγ−/− adult recipients via intravenous injection. Mixed bone marrow chimeras were generated by injecting mixed bone marrow (1:1 ratio) from WT (CD45.2 or CD45.1 × CD45.2) and Ptprc−/− C57BL/6 mice via intravenous injection along with 200 μg of anti-CD4 and anti-asialo GM1 per mouse via intraperitoneal injection into irradiated WT adult recipients (CD45.1). Recipients were irradiated with 1000 rads before reconstitution. Individual adult mice and mixed bone marrow chimeras were infected with a salivary gland stock of MCMV (Smith strain) at 5 × 104 PFUs (plaque-forming units) per mouse via intraperitoneal injection. Newborn (approximately 2 days old) DAP12-deficient mice were infected with 2 × 103 PFU one day after receiving 1 × 105 enriched Ly49H+ NK cells (∼ 50% NK-cell purity). Mice were housed in a specific pathogen–free facility and experiments were performed according to University of California-San Francisco Institutional Animal Care and Use Committee guidelines.
Flow cytometry
Fc receptors on freshly isolated splenic cells and IL-2–cultured NK cells were blocked with anti-CD16+CD32 monoclonal antibody (mAb) 2.4G2 before staining with the indicated antibodies. Antibodies were obtained from BD Biosciences, eBioscience, and BioLegend. Flow cytometry was performed using a FACScan or LSRII (BD Biosciences) and data were analyzed using FlowJo software Version 9.2 (TreeStar).
Preparation of NK cells
For isolation of fresh NK cells for neonatal experiments and ex vivo stimulations, splenocytes were stained with anti–TER-119 mAb (to remove red blood cells), anti-CD4, anti-CD8, anti-CD5, anti-CD19, and anti-GR-1 mAbs, and then incubated with goat anti–rat IgG-coated magnetic beads (QIAGEN), resulting in an enriched NK-cell population. For the generation of IL-2–expanded NK cells, splenocytes were isolated and red blood cells were lysed with ACK buffer (BioWhittaker). Splenocytes were stained with anti-CD4, anti-CD8, and anti-CD19 mAbs, and then incubated with goat anti–rat IgG-coated magnetic beads (QIAGEN) to deplete T cells and B cells. Anti-CD45.1 or anti-CD45 mAbs were used in NK-cell enrichments from chimeric mice, including Syk-deficient or CD45-deficient (including both CD45 and Syk-deficient) mice, to remove any contaminating host cells. NK cells were positively selected using a DX5 mAb-conjugated magnetic bead system (Miltenyi Biotec). The enriched NK cells were cultured with 4000 U/mL of human IL-2 (National Institutes of Health Biological Resources Branch Preclinical Repository), and harvested for functional analysis after 6-8 days.
51Cr-release assays
Mouse Ba/F3 pro-B cells, Ba/F3 cells transduced with Rae-1ϵ, Ba/F3 transduced with MCMV m157, and the RMA (H-2b–positive) T-cell lymphoma cell line (provided by Dr J. Ryan, University of California-San Francisco) were cultured in RPMI 1640 medium containing 10% fetal calf serum, 2mM glutamine, 50 U/mL of penicillin, and 50 μg/mL of streptomycin. IL-2–activated NK cells were used as effector cells in a standard 4-6-hour 51Cr cytotoxicity assay at 37°C.22 For antibody-dependent cell-mediated cytotoxicity (ADCC),22 RMA target cells were incubated in the presence or absence of 4 μg/mL of anti-CD90 (provided by Drs Salim Dhanji and Hung-sai Teh, University of Vancouver, Vancouver, BC), and mixed with the effector NK cells. NK cells were incubated in the presence or absence of 40 μg/mL of anti-CD16 + CD32 mAb (2.4G2) (to block Fc receptors) for 30 minutes, and then mixed with RMA or anti-CD90 mAb–coated RMA target cells at various effector:target ratios. The 2.4G2 mAb remained in the medium during the assay.
Stimulation of NK cells
Ninety-six-well tissue-culture plates were coated with anti-Ly49H, anti-NKG2D, anti-NKp46, or control mAbs, as described previously.22 Approximately 2 × 105 NK cells per well were plated, gently centrifuged, and incubated for 4-5 hours at 37°C. For the detection of intracellular IFNγ, cells were incubated before and during stimulation in the presence of GolgiPlug (BD Pharmingen), and then stained for surface markers and intracellular IFNγ using an intracellular cytokine staining kit (BD Pharmingen). Degranulation was measured by incubating the NK cells in the presence of anti-CD107a (LAMP1) mAb (BD Pharmingen) during stimulation and then staining for other surface markers after the stimulation. No differences were detected when stimulations were performed in the absence and presence of anti-CD16 + CD32 mAb (2.4G2) (to block Fc receptors). Thus, the stimulations were specific for the receptor tested.
Results
CD45 is necessary for NK cell–mediated protection from MCMV
We examined whether CD45 controls the ability of NK cells to protect the host against MCMV infection. We used a neonatal protection assay whereby transferred NK cells protect the young mice from viral infection. Newborn mice lack mature NK cells and, consequently, the transferred cells are the only NK cells giving potential protection against the infection.30 CD45 is expressed on all nucleated hematopoietic cells.21 We used the neonatal model because it allowed us to test the role of CD45 specifically on NK cells and avoid any indirect effects of the lack of CD45 on other cell types. We adoptively transferred equal numbers of Ly49H+ NK cells enriched from WT or CD45-deficient spleens isolated from adult mice into newborn DAP12-deficient mice. To completely exclude any role of endogenous NK cells in the neonates, we used DAP12-deficient mice as recipients because DAP12-deficient NK cells are defective in their response to MCMV.15,31 CD45-deficient NK cells were defective in their ability to protect the neonates from MCMV infection compared with WT NK cells (Figure 1), indicating that CD45 plays a critical role in NK cell–mediated protection against MCMV. We have previously demonstrated that the protection of neonates by the adoptive transfer of adult NK cells is ablated by treatment with a blocking anti-Ly49H antibody, demonstrating that NK cells are responsible for protection.15
CD45 is required for protection from MCMV infection. Survival of DAP12-deficient neonatal mice given 1 × 105 Ly49H+ WT NK cells, CD45-deficient NK cells, or PBS and infected with 2 × 103 PFU of MCMV. P values were determined using the Mantel-Cox test. Data were combined from 2 separate experiments with each experiment giving similar results.
CD45 is required for protection from MCMV infection. Survival of DAP12-deficient neonatal mice given 1 × 105 Ly49H+ WT NK cells, CD45-deficient NK cells, or PBS and infected with 2 × 103 PFU of MCMV. P values were determined using the Mantel-Cox test. Data were combined from 2 separate experiments with each experiment giving similar results.
IFNγ production by CD45-deficient NK cells during early MCMV infection
Having shown that CD45 is important for the NK cell–mediated protection against MCMV infection, we addressed why CD45-deficient NK cells fail to respond optimally to MCMV challenge. During early infection and independent of ITAM signaling, NK cells are activated and produce IFNγ, presumably through stimulation by pro-inflammatory cytokines such as IL-12 that are produced by dendritic cells.13,14,32 We compared the ability of CD45-deficient and WT NK cells to respond to MCMV within the same mouse by generating mixed bone marrow chimeras with bone marrow from CD45-null mice and WT mice. When stained for intracellular IFNγ 1.5 days after infection with MCMV, CD45-deficient NK cells produced IFNγ at levels equivalent to WT NK cells (Figure 2A). We further demonstrated that ex vivo WT and CD45-deficient NK cells made equal amounts of IFNγ when stimulated with the cytokines IL-12 and IL-18 (Figure 2B). Thus, NK cells have the capacity to make IFNγ independently of their CD45 expression in the context of viral infection or in the presence of pro-inflammatory cytokines.
Normal ITAM-independent IFNγ production by CD45-deficient NK cells. (A) Mixed bone marrow chimeric mice (1:1 mixture of WT and CD45-deficient bone marrow) were infected with 5 × 104 PFU of MCMV. Intracellular staining of NK cells for IFNγ at day 1.5 after infection (solid line) compared with NK cells from uninfected control mice (gray fill). WT splenocytes (left panel) gated on CD45+NK1.1+CD3− and CD45-deficient splenocytes (right panel) gated on CD45−NK1.1+CD3−. Three independent sets of mixed bone marrow chimeras were made with approximately 10 mice per set. Similar results were seen in all groups of chimeric mice. (B) Enriched WT or CD45-deficient NK cells were incubated with IL-12 (20 ng/mL), IL-18 (10 ng/mL), and brefeldin A, followed by intracellular staining for IFNγ. A representative dataset is shown on top, and the average ± SEM of results from 5 independent experiments is shown below. NS indicates no significant differences between genotypes found using a 2-tailed unpaired t test.
Normal ITAM-independent IFNγ production by CD45-deficient NK cells. (A) Mixed bone marrow chimeric mice (1:1 mixture of WT and CD45-deficient bone marrow) were infected with 5 × 104 PFU of MCMV. Intracellular staining of NK cells for IFNγ at day 1.5 after infection (solid line) compared with NK cells from uninfected control mice (gray fill). WT splenocytes (left panel) gated on CD45+NK1.1+CD3− and CD45-deficient splenocytes (right panel) gated on CD45−NK1.1+CD3−. Three independent sets of mixed bone marrow chimeras were made with approximately 10 mice per set. Similar results were seen in all groups of chimeric mice. (B) Enriched WT or CD45-deficient NK cells were incubated with IL-12 (20 ng/mL), IL-18 (10 ng/mL), and brefeldin A, followed by intracellular staining for IFNγ. A representative dataset is shown on top, and the average ± SEM of results from 5 independent experiments is shown below. NS indicates no significant differences between genotypes found using a 2-tailed unpaired t test.
CD45-deficient NK cells are defective in Ly49H-specific expansion in MCMV infection
Because the Ly49H receptor has been shown to be essential for NK-cell responses to MCMV, we tested the ability of the Ly49H+ NK-cell population to expand during infection in our mutant mice. We infected adult WT and CD45-deficient mice with MCMV and uncovered a mild yet consistent defect in the increase in the percentages of Ly49H+ NK cells at day 7 after MCMV infection in CD45-deficient mice compared with WT mice (Figure 3A). We next compared the ability of CD45-deficient and WT NK cells to expand in response to MCMV within the same mouse by generating mixed bone marrow chimeras. After 8-10 weeks, we noticed that in a majority of the chimeric mice, the repopulated cells were biased in favor of CD45-deficient NK cells (Figure 3B top panel), similar to results shown previously.23,25 Thus, CD45-deficient NK cells are not intrinsically deficient in proliferation at steady state. In uninfected mice at 36 hours after infection there was no change in the ratio between CD45-deficient and WT Ly49H+ NK cells (Figure 3B). However, by day 7 after infection, expansion of CD45-deficient Ly49H+ NK cells was significantly less compared with that of WT cells, as can be seen by the shift from CD45-deficient Ly49H+ NK cells to WT Ly49H+ NK cells (Figure 3B-C). Thus, CD45 plays a role in driving Ly49H-dependent NK-cell expansion during MCMV infection.
Defective expansion of CD45-deficient NK cells during MCMV infection. (A) WT and CD45-deficient mice were infected with 5 × 104 PFU of MCMV. Splenocytes gated on NK1.1+CD3− NK cells. Data are the average ± SD of results from 3-9 individual experiments. Percentages above each bar graph are the percentage increase of Ly49H+ NK cells in infected mice compared with uninfected mice. (B-C) Mixed bone marrow chimeric mice (1:1 mixture of WT and CD45-deficient bone marrow) were infected with 5 × 104 PFU of MCMV. (B) Histogram of representative staining for CD45 in NK1.1+CD3−Ly49H+ cells. (C) Change of percentage of CD45+ (left panel) or CD45− (right panel) cells within the Ly49H+NK1.1+CD3− splenic population in individual mice from 1 set of mixed bone marrow chimeric mice. Three independent sets of mixed bone marrow chimeras were made, with approximately 10 mice per set. Similar results were seen in all chimeric mice.
Defective expansion of CD45-deficient NK cells during MCMV infection. (A) WT and CD45-deficient mice were infected with 5 × 104 PFU of MCMV. Splenocytes gated on NK1.1+CD3− NK cells. Data are the average ± SD of results from 3-9 individual experiments. Percentages above each bar graph are the percentage increase of Ly49H+ NK cells in infected mice compared with uninfected mice. (B-C) Mixed bone marrow chimeric mice (1:1 mixture of WT and CD45-deficient bone marrow) were infected with 5 × 104 PFU of MCMV. (B) Histogram of representative staining for CD45 in NK1.1+CD3−Ly49H+ cells. (C) Change of percentage of CD45+ (left panel) or CD45− (right panel) cells within the Ly49H+NK1.1+CD3− splenic population in individual mice from 1 set of mixed bone marrow chimeric mice. Three independent sets of mixed bone marrow chimeras were made, with approximately 10 mice per set. Similar results were seen in all chimeric mice.
CD45-deficient NK cells are defective in degranulation after receptor engagement
Prior studies have demonstrated that both target cell killing and IFNγ are needed for protection against MCMV.33,34 We tested the ability of CD45-deficient NK cells to make IFNγ ex vivo, and were unable to detect intracellular IFNγ after Ly49H cross-linking compared with WT NK cells (Figure 4A). However, IFNγ production by CD45-deficient NK cells was equivalent to WT NK cells when receptor-mediated signals were bypassed with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Figure 4A). We have shown previously that CD45-deficient NK cells kill target cells at equivalent levels to WT NK cells.20,22-25 To further delineate the role of CD45 in NK cell–mediated target killing, we examined the ability of CD45-deficient NK cells to degranulate in response to antibody cross-linking of NK cell–activating receptors. Cytotoxic granules express CD107a (Lamp-1), which is detectable on the cell surface after degranulation. We measured a greater than 2-fold decrease in the frequency of CD45-deficient NK cells degranulating compared with WT cells after Ly49H, NKp46, or NKG2D cross-linking as measured by surface expression of CD107a (Figure 4B and data not shown). We observed a small difference in the percentage of CD45-deficient NK cells expressing Ly49H (42% ± 1.7%) compared with WT NK cells (52.3% ± 2.3%). This difference in cells expressing Ly49H was not great enough to account for the degranulation defect. The defect in degranulation remains at least 2-fold even after accounting for this difference in Ly49H expression. Moreover, equal frequencies of CD45-deficient NK cells degranulated as WT NK cells when receptor-mediated signals were bypassed with PMA and ionomycin (Figure 4C). We considered the possibility that CD45-deficient mice have a higher proportion of immature NK cells with a reduced ability to perform effector functions compared with mature NK cells. We examined CD45-deficient NK cells for the expression of CD11b and CD27, markers for mature NK cells,35-37 and found approximately equal NK-cell maturation levels in WT and CD45-deficient mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), suggesting that a difference in the maturation state of the NK cells in the CD45-deficient mice was not responsible for their reduced level of degranulation.
Defective degranulation downstream of ITAM-based receptors in CD45-deficient NK cells. Enriched WT or CD45-deficient NK cells were incubated on plates coated with the indicated mAbs or with PMA (25 ng/mL) and ionomycin (1 μg/mL). Gated on NK1.1+CD3− cells. (A) Intracellular staining for IFNγ after incubation with brefeldin A. (B-C) Incubation was performed in the presence of anti-CD107a mAb whereby only those cells that degranulate stained positively for CD107a. Graphs show the average ± SEM of 2-4 experiments using 4-9 mice per genotype. The P values for significant differences between genotypes are located above each condition in each graph. NS indicates no significant differences between genotypes were found using a 2-tailed unpaired t test.
Defective degranulation downstream of ITAM-based receptors in CD45-deficient NK cells. Enriched WT or CD45-deficient NK cells were incubated on plates coated with the indicated mAbs or with PMA (25 ng/mL) and ionomycin (1 μg/mL). Gated on NK1.1+CD3− cells. (A) Intracellular staining for IFNγ after incubation with brefeldin A. (B-C) Incubation was performed in the presence of anti-CD107a mAb whereby only those cells that degranulate stained positively for CD107a. Graphs show the average ± SEM of 2-4 experiments using 4-9 mice per genotype. The P values for significant differences between genotypes are located above each condition in each graph. NS indicates no significant differences between genotypes were found using a 2-tailed unpaired t test.
NK cell–mediated cytotoxicity is intact in Syk-deficient and Zap70-deficient NK cells
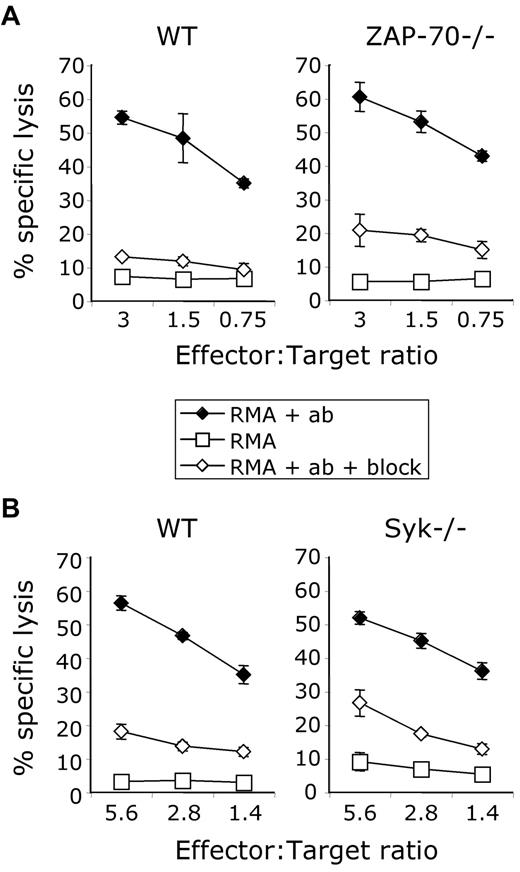
NK-cell receptor signaling must still occur in CD45-deficient NK cells because they can kill target cells. We therefore sought to understand what signaling molecules are responsible for this CD45-independent killing activity. We focused our attention on the Syk-family kinases, consisting of Syk and Zap70, which are both expressed in NK cells and whose ITAM-binding abilities and kinase activities are regulated by Src-family kinases and CD45. Downstream effector functions (both killing and cytokine production) have been shown to still be intact in the absence of Syk or Zap70 individually.4,5 The absence of both Syk-family kinases, Syk and Zap70, renders ITAM-dependent NKRs nonfunctional.38 These data demonstrate redundancy between these 2 molecules. Recent evidence from thymocytes, in which Syk and Zap70 are briefly expressed concurrently in early development, suggests that Zap70 and Syk are not completely redundant in that each plays distinct roles in T-cell receptor (TCR) signaling.39 Therefore, we hypothesized that in the context of CD45 deficiency, Zap70 and Syk are regulated differently and play separate roles in NK-cell ITAM-dependent signaling. First, we tested the function of splenic NK cells from Zap70−/− mice, Sykb−/− chimeric mice, or the appropriate control mice in an ADCC assay. Because Sykb−/− mice die in utero, lethally irradiated WT mice (CD45.1+) were reconstituted with fetal livers from Sykb−/− embryos as a source of hematopoietic precursors.27,29 Both Syk-deficient and Zap70-deficient NK cells killed antibody-coated target cells via CD16 at levels equivalent to their WT counterparts (Figure 5A-B). We found that Zap70−/− and Sykb−/− NK cells also killed normally via Ly49H and NKG2D compared with WT NK cells (data not shown). Thus, Syk and Zap70 individually are not required for target cell killing and are redundant for the function of NK-cell ITAM-linked receptors.4,5
Normal antibody-dependent cell-mediated cytotoxicity in Zap70-deficient and Syk-deficient NK cells. (A-B) ADCC induced by coating a chromium-labeled RMA cell line with anti-CD90 in the absence (♦) or presence (◇) of CD16-blocking antibody 2.4G2; cells alone served as an additional control (○). (A) Comparison between WT and Zap70−/− NK cells. (B) Comparison between WT and Sykb−/− NK cells from chimeric mice. Data are representative of 2-3 independent experiments.
Normal antibody-dependent cell-mediated cytotoxicity in Zap70-deficient and Syk-deficient NK cells. (A-B) ADCC induced by coating a chromium-labeled RMA cell line with anti-CD90 in the absence (♦) or presence (◇) of CD16-blocking antibody 2.4G2; cells alone served as an additional control (○). (A) Comparison between WT and Zap70−/− NK cells. (B) Comparison between WT and Sykb−/− NK cells from chimeric mice. Data are representative of 2-3 independent experiments.
Syk, but not Zap70, is required for cell-mediated cytotoxicity in the absence of CD45
We addressed whether Syk or Zap70 is responsible for the CD45-independent target cell cytotoxicity that we have described. Syk and Zap70 are both expressed at WT levels in CD45-deficient NK cells.23 We generated chimeric mice deficient for both CD45 and Syk by reconstituting lethally irradiated mice with fetal livers from Ptprc−/− × Sykb−/− embryos. We generated CD45-deficient chimeric mice to use as appropriate controls. We generated mice deficient for both CD45 and Zap70 by breeding because these mice appear healthy in our colony and can survive through breeding age as homozygotes. We tested the ability of NK cells from Ptprc−/− × Zap70−/− mice to kill target cells through ADCC via the ITAM-associated receptor CD16 and found no difference compared with CD45-deficient NK cells (Figure 6A). We found, however, that Ptprc−/− × Sykb−/− NK cells were incapable of killing target cells compared with CD45-deficient NK cells (Figure 6B). We confirmed that these NK cells were capable of secreting cytokine in response to PMA and ionomycin (data not shown). Thus, Syk is necessary for CD45-independent target cell killing.
Syk is required for CD45-independent target cell killing. (A-B) ADCC induced by coating a chromium-labeled RMA cell line with anti-CD90 in the absence (♦) or presence of CD16-blocking antibody 2.4G2 (◇); cells alone served as an additional control (□). (A) Comparison between NK cells that are CD45 deficient and that are both CD45- and Zap70-deficient. (B) Comparison between NK cells that are CD45-deficient and that are both CD45- and Syk-deficient from chimeric mice. Data are representative of 3 independent experiments.
Syk is required for CD45-independent target cell killing. (A-B) ADCC induced by coating a chromium-labeled RMA cell line with anti-CD90 in the absence (♦) or presence of CD16-blocking antibody 2.4G2 (◇); cells alone served as an additional control (□). (A) Comparison between NK cells that are CD45 deficient and that are both CD45- and Zap70-deficient. (B) Comparison between NK cells that are CD45-deficient and that are both CD45- and Syk-deficient from chimeric mice. Data are representative of 3 independent experiments.
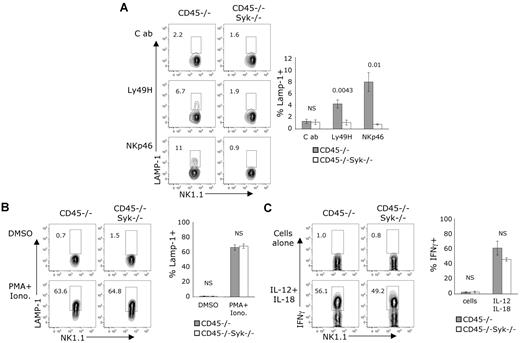
Syk is required for CD45-independent ITAM-mediated function
The inability of NK cells deficient in both CD45 and Syk to kill via CD16 (ADCC) suggested that other ITAM-coupled receptors might also be nonfunctional in these NK cells. We examined the ability of Ptprc−/− × Sykb−/− NK cells to respond ex vivo to stimulation by cross-linking of NKp46 and Ly49H. These ITAM-linked receptors were incapable of inducing degranulation, as measured by surface CD107a staining, compared with CD45-deficient NK cells (Figure 7A). A slightly lower percentage of Ptprc−/− × Sykb−/− NK cells (36.6 ± 4.8) expressed Ly49H compared with Ptprc−/− NK cells (45.3 ± 5.8), but this does not account for the undetectable degranulation seen in Ptprc−/− × Sykb−/− NK cells. We confirmed that the NK cells deficient in both CD45 and Syk were capable of degranulating compared with CD45-deficient NK cells when receptor-mediated signals were bypassed through PMA and ionomycin stimulation (Figure 7B). We demonstrated that the Ptprc−/− × Sykb−/− NK cells were also capable of producing IFNγ compared with Ptprc−/−NK cells when exposed to the inflammatory cytokines IL-12 and IL-18 (Figure 7C). Thus, Syk is responsible for transmitting the ITAM-dependent activity in CD45-deficient NK cells.
Syk is required for CD45-independent, ITAM-dependent degranulation. (A-C) Enriched NK cells were incubated on plates coated with the indicated mAbs or with the indicated stimuli. Cells shown were gated on NK1.1+CD3− cells. (A-B) Incubation was done in the presence of anti-CD107a whereby only cells that degranulate stained positively for CD107a. (A) Data are representative of 3-5 independent experiments. (B) Stimulation with PMA (25 ng/mL) and ionomycin (1 μg/mL). Data are representative of 4 independent experiments. (C) Cells were incubated with IL-12 (20 ng/mL), IL-18 (10 ng/mL), and brefeldin A, followed by intracellular staining for IFNγ. Data are representative of 3 independent experiments. (A-C) Graphs to the right show the average ± SEM of results of 3 independent experiments. The P values for significant differences between genotypes are located above each condition in each graph. NS indicates no significant differences between genotypes were found using a 2-tailed unpaired t test.
Syk is required for CD45-independent, ITAM-dependent degranulation. (A-C) Enriched NK cells were incubated on plates coated with the indicated mAbs or with the indicated stimuli. Cells shown were gated on NK1.1+CD3− cells. (A-B) Incubation was done in the presence of anti-CD107a whereby only cells that degranulate stained positively for CD107a. (A) Data are representative of 3-5 independent experiments. (B) Stimulation with PMA (25 ng/mL) and ionomycin (1 μg/mL). Data are representative of 4 independent experiments. (C) Cells were incubated with IL-12 (20 ng/mL), IL-18 (10 ng/mL), and brefeldin A, followed by intracellular staining for IFNγ. Data are representative of 3 independent experiments. (A-C) Graphs to the right show the average ± SEM of results of 3 independent experiments. The P values for significant differences between genotypes are located above each condition in each graph. NS indicates no significant differences between genotypes were found using a 2-tailed unpaired t test.
Discussion
In the present study, we have addressed the role of CD45 in NK-cell responses necessary to control MCMV infection and have determined the effects of CD45 deficiency on the Syk- and Zap70-mediated activation of ITAM-coupled NKRs involved in host defense. We and others have previously shown that NK-cell target killing is minimally affected by the absence of CD45.20,22,23 In this study, we further dissected the mechanism of this killing phenotype (or lack thereof) and found defective, yet detectable, degranulation downstream of ITAM-coupled receptors expressed on CD45-deficient NK cells. We and others have shown that cytokine production and downstream signaling events are abrogated after activation of individual specific ITAM-linked receptors.20,22,23 We used MCMV infection to induce Ly49H-specific expansion driven predominantly through the ITAM adaptor DAP12.13-15,31 We have shown previously that CD45-deficient NK cells are defective in this expansion, yet have an advantage over WT NK cells undergoing homeostatic expansion when repopulating irradiated recipients.23,25 Thus, our findings demonstrate that upon stimulation of a specific ITAM-associated receptor, CD45 regulates all downstream NK-cell biologic processes, including degranulation, cytokine production, and expansion, but is not required for responsiveness to inflammatory cytokines during viral infection.
We demonstrate that CD45 contributes significantly to NK cell–specific protection from MCMV infection. Perforin-dependent killing and IFNγ production are both important in protection against MCMV.33,34 Direct cell-mediated killing of MCMV-infected target cells in C57BL/6 mice requires the ITAM-coupled Ly49H receptor. In contrast, IFNγ production can be induced either by the Ly49H receptor or by stimulation of IL-12 and IL-18 receptors on NK cells during viral infection.13,32 In our present studies, we found that ITAM-induced cytokine production is undetectable in CD45-deficient NK cells, but that these cells make abundant IFNγ at 36 hours after MCMV infection, when inflammatory cytokines have been shown to contribute the most to IFNγ production independently of Ly49H.13,15 Furthermore, we show that CD45-deficient NK cells produce IFNγ equal to WT NK cells in response to stimulation ex vivo by IL-12 and IL-18, indicating that these non–ITAM-dependent response pathways function normally in the absence of CD45.
During MCMV infection, Ly49H+ NK-cell expansion is driven predominantly by the ITAM-bearing adapter DAP12.13,15,31 Although the proliferation of Ly49H+ NK cells is not required for the early control of MCMV, this expansion of MCMV-specific NK cells may be important in the control of MCMV at later times by increasing the number of NK cells that can respond specifically to infected host cells. Thus, the defects in Ly49H-induced degranulation, cytokine production, and expansion found in CD45-deficient NK cells likely all contribute to their inability to properly protect the host against MCMV.
Our findings that CD45 deficiency does not completely abrogate certain effector functions downstream of ITAM-dependent signaling led us to determine what is responsible for this ITAM-specific, CD45-independent activity. We focused on cell-mediated cytotoxicity and NK-cell degranulation, because it is the least perturbed in CD45-deficient NK cells and provides a robust assay system. We hypothesized that the 2 members of the Syk-kinase family, Syk and Zap70, might be responsible for this CD45-independent target cell cytotoxicity. Src-kinases, controlled by CD45, phosphorylate the tyrosine residues of the ITAMs to which the Syk-family kinases bind. The Syk kinases then propagate this signal, allowing for the activation of effector functions in T cells, B cells, myeloid cells, and NK cells. Although most mature hematopoietic cells express either Syk or Zap70, NK cells are the exception and express abundant amounts of both kinases.38 NK cells deficient in either molecule kill target cells normally, as we have shown, but deficiency in both kinases renders NK cells unable to respond via ITAM-associated receptors.4,5,38 This evidence indicates that there is redundancy between the 2 kinases for ITAM-dependent functions in NK cells. We demonstrate for the first time in primary cells that in the absence of CD45, these 2 molecules are not redundant and that Syk, but not Zap70, controls NK-cell ITAM signaling and is responsible for the NK-cell degranulation and target cell killing downstream of a variety of NKRs.
The level of Src activity downstream of CD45 most likely controls the requirement for Syk and Zap70 in NK-cell ITAM-dependent functions. In transformed cell lines, Syk does not require the presence of Src kinases to activate TCR signaling.40-42 In vitro and in cell lines, Syk alone can phosphorylate ITAMs, whereas ITAM phosphorylation is defective in Syk-deficient macrophages, which do not express Zap70.42-44 Conversely, in transformed lines, Zap70 is unable to phosphorylate ITAM-tyrosines in vitro and can only transmit TCR-mediated signaling in the presence of Src kinases.40-42,44 Thus, we propose that effector functions downstream of ITAM-coupled receptors are normal in NK cells deficient for Syk, because the Src family kinases can still transmit ITAM signals via Zap70. ITAM-associated NKRs can function in the absence of Zap70 because of activity from both Syk and the Src kinases. In the absence of CD45, Src kinase activity is defective, and therefore the stringent requirement of Zap70 for Src activity renders Zap70 nonfunctional in CD45-deficient NK cells. The ability of Syk to signal independently of Src kinases allows partial NK-cell effector function to occur in the absence of CD45 and in the presence of defective Src activity. Consistent with this, we have found that ITAM-dependent NK-cell degranulation is less sensitive to Src kinase inhibition in the absence of CD45 (supplemental Figure 2). However, when both Syk and CD45 are missing, ITAM-associated NKRs are rendered completely nonfunctional, because Zap70 (the only Syk-family kinase present) cannot be activated due to defective Src activity.
In summary, we have demonstrated a role for CD45 in NK cells in the control of MCMV and in the regulation of effector functions downstream of ITAM-associated NKRs. In addition, we have provided mechanistic insight into the control of ITAM activity occurring in the absence of CD45 in NK cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Cliff Lowell, Natalie Bezman, Mark Orr, Marine Champsaur, Michelle Hermiston, Susan Levin, and Byron Au-Yeung for discussions, technical assistance, and/or supplying mice, as well as the entire Lanier laboratory and Weiss laboratory for support.
The work is supported by National Institutes of Health grants (AI06812 to L.L.L. and AI35297 to A.W.) and by the Howard Hughes Medical Research Institute (to A.W.). D.G.T.H is a Special Fellow of the Leukemia & Lymphoma Society, and L.L.L. is an American Cancer Society Professor.
National Institutes of Health
Authorship
Contribution: D.G.T.H., J.C.S., J.N.B., and S.R.W. performed experiments; E.H.P. and A.W. contributed ideas and reagents; and D.G.T.H. and L.L.L. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of J.C.S. is Memorial Sloan-Kettering Cancer Center, New York, NY. The current affiliation of J.N.B. is Novo Nordisk, Seattle, WA. The current affiliation of S.R.W. is Xoma, Berkeley, CA. The current affiliation of E.H.P. is Celera Corporation, Alameda, CA.
Correspondence: Lewis L. Lanier, University of California, Department of Microbiology and Immunology, 513 Parnassus Ave, HSE 1001G, Box 0414, San Francisco, CA 94143-0414; e-mail: lewis.lanier@ucsf.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal