Abstract
Complete response (CR) was an uncommon event in elderly myeloma patients until novel agents were combined with standard oral melphalan-prednisone. This analysis assesses the impact of treatment response on progression-free survival (PFS) and overall survival (OS). We retrospectively analyzed 1175 newly diagnosed myeloma patients, enrolled in 3 multicenter trials, treated with melphalan-prednisone alone (n = 332), melphalan-prednisone-thalidomide (n = 332), melphalan-prednisone-bortezomib (n = 257), or melphalan-prednisone-bortezomib-thalidomide (n = 254). After a median follow-up of 29 months, the 3-year PFS and OS were 67% and 27% (hazard ratio = 0.16; P < .001), and 91% and 70% (hazard ratio = 0.15; P < .001) in patients who obtained CR and in those who achieved very good partial response, respectively. Similar results were observed in patients older than 75 years. Multivariate analysis confirmed that the achievement of CR was an independent predictor of longer PFS and OS, regardless of age, International Staging System stage, and treatment. These findings highlight a significant association between the achievement of CR and long-term outcome, and support the use of novel agents to achieve maximal response in elderly patients, including those more than 75 years. This trial was registered at www.clinicaltrials.gov as #NCT00232934, #ISRCTN 90692740, and #NCT01063179.
Introduction
Multiple myeloma (MM) is an incurable plasma-cell neoplasm. The main goal of treatment is to improve progression-free survival (PFS) and overall survival (OS). The International Staging System (ISS) classification and the cytogenetic status are the most relevant prognostic factors.1-4 In patients eligible for high-dose therapy and autologous transplantation, the achievement of complete response (CR)5,6 or at least very good partial response (VGPR),7 is associated with prolonged PFS and OS. In patients not eligible for autologous transplantation, CR was quite rare until the novel agents thalidomide or bortezomib were added to conventional chemotherapies. Five trials reported an improvement in PFS with the combination melphalan-prednisone-thalidomide (MPT) compared with melphalan-prednisone (MP), but in only 2 of them this translated into an increase in OS.8-13 A randomized trial that compared melphalan-prednisone-bortezomib (VMP) with MP showed an improvement in both PFS and OS with the 3-drug combination.14,15 The 4-drug combination melphalan-prednisone-thalidomide-bortezomib followed by bortezomib-thalidomide maintenance (VMPT-VT) was superior to the novel combination VMP.16 The CR rates were in the range of 3% to 4% with MP,8-15 6% to 16% with MPT,8-13 24% to 33% with VMP,14-16 and raised to 38% with VMPT-VT.16
In this study, we compared PFS and OS of newly diagnosed patients who achieved CR after MP, MPT, VMP, or VMPT-VT with those whose best response was VGPR or partial response (PR) only.
Methods
Study design
Patients with newly diagnosed MM, not eligible for high-dose therapy and autologous transplantation because of age (≥ 65 years) or coexisting comorbidities, enrolled in the GISMM-2001 MP vs MPT, the Dutch-Belgian Cooperative Trial Group for Hematology Oncology (HOVON) MP vs MPT, and the GIMEMA MM0305 VMP vs VMPT-VT phase 3 trials were retrospectively analyzed. Details on treatment regimens and results of these studies have previously been reported.8,12,13,16 Briefly, 331 patients were randomly assigned to receive 6 courses of MP or MPT followed by maintenance with thalidomide until progression,8 344 patients with 8 courses of MP or MPT followed by maintenance with thalidomide until progression,12 and 511 patients with 9 cycles of VMP or VMPT followed by continuous VT as maintenance.16 Trials were approved by the Independent Ethics Committees/Institutional Review Boards at all participating centers. Patients provided written informed consent before entering the studies, which were performed in accordance with the Declaration of Helsinki. Patients who received at least one dose of the study drug and for whom best response to treatment was available were included in this analysis.
Assessment
In the GISMM-2001 MP vs MPT and in the HOVON MP vs MPT studies, responses were initially determined by investigator assessment using the European Group for Blood and Marrow Transplantation criteria.17 All responses were confirmed at least in 2 consecutive assessments 6 weeks apart. In the GIMEMA MM0305 VMP vs VMPT-VT, responses were initially determined by investigator assessment using the International Myeloma Working Group criteria.18,19 All responses were confirmed at least in 2 consecutive assessments made at any time. In this retrospective analysis, responses of patients enrolled in the GISMM-2001 MP vs MPT and the HOVON MP vs MPT were reevaluated using the International Myeloma Working Group criteria.18,19 Briefly, a PR was defined as a 50% or higher decrease in the serum monoclonal protein (M-protein) levels from baseline and a reduction 90% or greater in 24-hour urine M-protein excretion or less than 200 mg/24 hours; for patients with soft tissue plasmacytomas, a 50% or higher reduction was required. A VGPR required a 90% or greater reduction in serum M-protein and urinary M-protein less than 100 mg/24 hours or M-protein detectable by immunofixation but not by electrophoresis. A CR was defined as negative serum and urine immunofixation, disappearance of any soft tissue plasmacytoma, and less than 5% plasma cells on bone marrow examination. Disease that did not satisfy the criteria for PR, VGPR, CR, or progressive disease was classified as stable disease. Disease progression required any of the following: 25% or greater increase from lowest response value in serum M-protein (absolute ≥ 0.5 g/dL) or urine M-protein (absolute ≥ 200 mg/24 hours).
PFS was calculated from the time of diagnosis until the date of progression, relapse, death from any cause, or the date the patient was last known to be in remission. OS was calculated from the time of diagnosis until the date of death or the date the patient was last known to be alive. Duration of CR was calculated from the time of CR achievement until the date of progression, relapse, death from any cause, or the date the patient was last known to be in remission.
Statistical analysis
Data cut-off was May 1, 2010. For this retrospective non-preplanned analysis, patients treated with MP, MPT, VMP or VMPT-VT were pooled together and stratified according to best response achieved. Patient characteristics were compared using the Pearson χ2 test for discrete variables or the Mann-Whitney test for continuous variables. PFS, OS, and duration of CR were estimated according to the Kaplan-Meier method and analyzed by univariate and multivariate Cox proportional hazards models, comparing the 2 arms by the Wald test and calculating 95% confidence intervals (CIs). A landmark analysis with landmark point at 6 months was performed. The following variables were assessed for potential association with PFS and OS: age at diagnosis ( > 75 vs ≤ 75 years), gender, Durie-Salmon and ISS stages, baseline creatinine ( > 1.2 vs ≤ 1.2 mg/dL), treatment regimen (MP/MPT/VMP/VMPT-VT), and best response achieved (CR/VGPR/PR/stable disease/progressive disease). Best response was always treated as a time-dependent variable. All reported P values were 2-sided, at the conventional 5% significance level.
Results
Patients
A total of 1175 patients were retrospectively analyzed: 332 received MP, 332 MPT, 257 VMP, and 254 VMPT-VT. Best response to treatment was available in 1136: CR was reported in 195 (17%), VGPR in 212 (19%), PR in 397 (35%); the remaining patients achieved less than PR. Baseline demographics and disease characteristics were similar in patients who obtained CR, VGPR, PR, or in the entire study population. Patients older than 75 years were 29% in the PR group, 21% in the VGPR group, and 21% in the CR group. Patients with ISS stage I, II, and III were equally distributed in the CR, VGPR, or PR groups. Response rates varied according to treatment regimens, and accordingly the proportion of patients treated with MP, MPT, VMP, or VMPT-VT was different in the CR, VGPR, and PR groups. In the CR group, 49% of patients received VMPT-VT, 31% VMP, 15% MPT, and only 5% MP; in the VGPR group, 25% received VMPT-VT, 31% VMP, 32% MPT, and 13% MP; and in the PR group, 19% received VMPT-VT, 20% VMP, 32% MPT, and 29% MP (Table 1).
Patient demographics and baseline characteristics
| Variable . | All patients (n = 1175) . | CR (n = 195) . | VGPR (n = 212) . | PR (n = 397) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 613 (52) | 89 (46) | 105 (50) | 217 (55) |
| Age, y | ||||
| Median | 72 | 71 | 71 | 72 |
| Range | 54-89 | 54-86 | 61-85 | 56-87 |
| > 75 | 314 (27) | 40 (21) | 44 (21) | 117 (29) |
| ISS stage* | ||||
| I | 223 (25) | 43 (28) | 39 (24) | 90 (29) |
| II | 430 (48) | 74 (47) | 79 (49) | 149 (47) |
| III | 241 (27) | 39 (25) | 43 (27) | 75 (24) |
| Missing | 281 | — | — | — |
| Durie and Salmon Staging System stage* | ||||
| II | 298 (32) | 34 (30) | 41 (26) | 113 (35) |
| III | 622 (68) | 78 (70) | 117 (74) | 206 (65) |
| Missing | 255 | — | — | — |
| Creatinine* | ||||
| > 1.2 mg/dL | 353 (30) | 48 (25) | 71 (33) | 110 (28) |
| Missing | 3 | — | — | — |
| Therapy | ||||
| MP | 332 (28) | 9 (5) | 27 (13) | 117 (29) |
| MPT | 332 (28) | 30 (15) | 67 (32) | 127 (32) |
| VMP | 257 (22) | 61 (31) | 65 (31) | 79 (20) |
| VMPT-VT | 254 (22) | 95 (49) | 53 (25) | 74 (19) |
| Variable . | All patients (n = 1175) . | CR (n = 195) . | VGPR (n = 212) . | PR (n = 397) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 613 (52) | 89 (46) | 105 (50) | 217 (55) |
| Age, y | ||||
| Median | 72 | 71 | 71 | 72 |
| Range | 54-89 | 54-86 | 61-85 | 56-87 |
| > 75 | 314 (27) | 40 (21) | 44 (21) | 117 (29) |
| ISS stage* | ||||
| I | 223 (25) | 43 (28) | 39 (24) | 90 (29) |
| II | 430 (48) | 74 (47) | 79 (49) | 149 (47) |
| III | 241 (27) | 39 (25) | 43 (27) | 75 (24) |
| Missing | 281 | — | — | — |
| Durie and Salmon Staging System stage* | ||||
| II | 298 (32) | 34 (30) | 41 (26) | 113 (35) |
| III | 622 (68) | 78 (70) | 117 (74) | 206 (65) |
| Missing | 255 | — | — | — |
| Creatinine* | ||||
| > 1.2 mg/dL | 353 (30) | 48 (25) | 71 (33) | 110 (28) |
| Missing | 3 | — | — | — |
| Therapy | ||||
| MP | 332 (28) | 9 (5) | 27 (13) | 117 (29) |
| MPT | 332 (28) | 30 (15) | 67 (32) | 127 (32) |
| VMP | 257 (22) | 61 (31) | 65 (31) | 79 (20) |
| VMPT-VT | 254 (22) | 95 (49) | 53 (25) | 74 (19) |
Data are number and (%).
— indicates not applicable.
Percentage was calculated based on the number of patients whose data were available.
Impact of CR on outcome
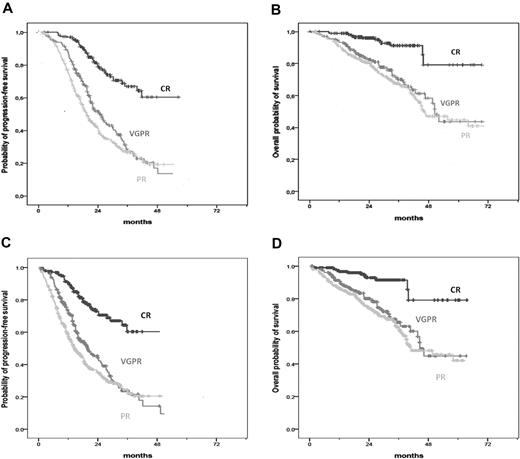
After a median follow-up of 29 months (range, 1-81 months), 3-year PFS and OS for the entire study population were 29% and 65%, respectively. The 3-year PFS was 67% in patients who achieved CR, 27% in patients with VGPR (hazard ratio [HR] = 0.16, 95% CI, 0.10-0.24, P < .001), and 27% in those with PR only (HR = 0.07, 95% CI, 0.04-0.13, P < .001; Figure 1A). Similarly, the 3-year OS was 91% in patients who obtained CR, 70% in patients with VGPR (HR = 0.15, 95% CI, 0.08-0.28, P < .001), and 67% in those with PR only (HR = 0.08, 0.04-0.16, P < .001; Figure 1B). A landmark analysis for PFS and OS, with landmark point at 6 months, was performed: patients who achieved CR had prolonged PFS and OS compared with patients who achieved VGPR and PR only (Figure 1C-D).
Survival curves according to response in all patients. (A) PFS in patients achieving CR, VGPR, and PR. (B) OS in patients achieving CR, VGPR, and PR. (C) Landmark analysis of PFS (landmark point at 6 months) in patients achieving CR, VGPR, and PR. (D) Landmark analysis of OS (landmark point at 6 months) in patients achieving CR, VGPR, and PR.
Survival curves according to response in all patients. (A) PFS in patients achieving CR, VGPR, and PR. (B) OS in patients achieving CR, VGPR, and PR. (C) Landmark analysis of PFS (landmark point at 6 months) in patients achieving CR, VGPR, and PR. (D) Landmark analysis of OS (landmark point at 6 months) in patients achieving CR, VGPR, and PR.
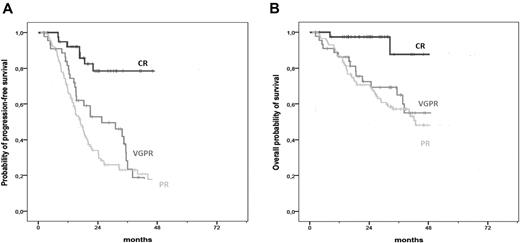
The 3-year PFS was 26% in patients older than 75 years and 29% in those 75 years or younger (HR = 1.23, 95% CI, 1.04-1.45, P = .014), whereas the 3-year OS was 54% and 65% (HR = 1.59, 95% CI, 1.29-1.96, P < .001). However, the impact of CR on both PFS and OS was similar in patients older or younger than 75 years. In the analysis restricted to patients older than 75 years, the 3-year PFS was 79% in patients who achieved CR and 24% in those who obtained VGPR (HR = 0.26, 95% CI, 0.12-0.58, P = .001) and 23% in those who attained PR (HR = 0.20, 95% CI, 0.10-0.41, P < .001; Figure 2A). The 3-year OS was 88% in patients who achieved CR, 65% in those who reached VGPR (HR = 0.13, 95% CI, 0.03-0.58, P = .007), and 57% in those who obtained PR (HR = 0.12, 95% CI, 0.03-0.51, P = .004; Figure 2B).
Survival curves according to response in patients older than 75 years. (A) PFS in patients older than 75 years achieving CR, VGPR, and PR. (B) OS in patients older than 75 years achieving CR, VGPR, and PR.
Survival curves according to response in patients older than 75 years. (A) PFS in patients older than 75 years achieving CR, VGPR, and PR. (B) OS in patients older than 75 years achieving CR, VGPR, and PR.
Subgroup analysis of OS in CR patients according to treatment regimen (bortezomib vs nonbortezomib regimens) was performed. There were no significant differences in PFS between the 2 groups (HR = 0.67, 95% CI, 0.34-1.32, P = .248), whereas there was a trend toward a better OS for bortezomib patients compared with nonbortezomib patients (HR = 0.31, 95% CI, 0.08-1.29, P = .107).
Achievement of CR and longer OS with the use of highly efficacious regimens may lead to greater toxicity. We therefore analyzed the toxicities in patients who achieved CR, VGPR, and PR. There were no significant differences in rate of grade 3 or 4 adverse events in patients who obtained CR, VGPR, or PR. In particular, rate of grade 3 or 4 peripheral neuropathy was 10% in patients who achieved CR, 13% in patients who obtained VGPR, and 10% in those who attained PR.
Impact of time to CR on outcome
Overall, most CRs were reached during the first 6 months of therapy: 34% at 4 months, 62% at 6 months, and 85% at 9 months of therapy. There were no significant differences in either PFS (HR = 1.06, 95% CI, 0.49-2.27, P = .878) or OS (P = .676) or duration of CR (HR = 0.66, 95% CI, 0.30-1.45, P = .305) between patients who achieved CR during the first 6 months of therapy or later.
Multivariate analysis
Multivariate analysis was performed on the 681 patients for whom complete baseline assessment and data on best response were available. The achievement of CR was the dominant factor associated with significantly longer PFS compared with VGPR (HR = 0.22; 95% CI, 0.13-0.38; P < .001) and PR (HR = 0.13; 95% CI, 0.07-0.26; P < .001), regardless of baseline patient characteristics, staging and treatment administered. Age more than 75 years was not associated with shorter PFS (HR = 1.14; 95% CI, 0.93-1.40; P = .220), whereas ISS stage II and III were. The addition of bortezomib or thalidomide to MP correlated with a significant improvement in PFS (Table 2). Similarly, the achievement of CR was the variable most strongly associated with significantly prolonged OS compared with VGPR (HR = 0.25, 95% CI, 0.11-0.55, P = .001) and PR (HR = 0.16, 95% CI, 0.06-0.39, P < .001). There was a trend for shorter OS for age more than 75 years (HR = 1.30, 95% CI, 1.00-1.70, P = .053). The addition of bortezomib or bortezomib-thalidomide to MP was associated with longer OS, whereas the addition of thalidomide only was not (Table 3).
Univariate and multivariate analysis (Cox model) of progression-free survival
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Response* | < .001 | < .001 | ||||
| CR vs VGPR | 0.16 | 0.10-0.24 | < .001 | 0.22 | 0.13-0.38 | < .001 |
| CR vs PR | 0.07 | 0.04-0.13 | < .001 | 0.13 | 0.07-0.26 | < .001 |
| CR vs SD | 0.03 | 0.01-0.05 | < .001 | 0.06 | 0.03-0.12 | < .001 |
| CR vs PD | 0.007 | 0.003-0.01 | < .001 | 0.02 | 0.007-0.04 | < .001 |
| Age, y | ||||||
| > 75 vs ≤ 75 | 1.23 | 1.04-1.45 | .014 | 1.14 | 0.93-1.40 | .220 |
| ISS | < .001 | .016 | ||||
| II vs I | 1.45 | 1.17-1.81 | .001 | 1.36 | 1.06-1.74 | .015 |
| III vs I | 1.59 | 1.24-2.03 | < .001 | 1.47 | 1.12-1.95 | .006 |
| Durie and Salmon stage | ||||||
| III vs II | 1.31 | 1.10-1.756 | .003 | 1.33 | 1.08-1.63 | .007 |
| Therapy | < .001 | < .001 | ||||
| MPT vs MP | 0.70 | 0.59-0.84 | < .001 | 0.78 | 0.63-0.98 | .029 |
| VMP vs MP | 0.42 | 0.34-0.53 | < .001 | 0.52 | 0.35-0.76 | .001 |
| VMPT-VT vs MP | 0.27 | 0.21-0.36 | < .001 | 0.51 | 0.35-0.75 | .001 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Response* | < .001 | < .001 | ||||
| CR vs VGPR | 0.16 | 0.10-0.24 | < .001 | 0.22 | 0.13-0.38 | < .001 |
| CR vs PR | 0.07 | 0.04-0.13 | < .001 | 0.13 | 0.07-0.26 | < .001 |
| CR vs SD | 0.03 | 0.01-0.05 | < .001 | 0.06 | 0.03-0.12 | < .001 |
| CR vs PD | 0.007 | 0.003-0.01 | < .001 | 0.02 | 0.007-0.04 | < .001 |
| Age, y | ||||||
| > 75 vs ≤ 75 | 1.23 | 1.04-1.45 | .014 | 1.14 | 0.93-1.40 | .220 |
| ISS | < .001 | .016 | ||||
| II vs I | 1.45 | 1.17-1.81 | .001 | 1.36 | 1.06-1.74 | .015 |
| III vs I | 1.59 | 1.24-2.03 | < .001 | 1.47 | 1.12-1.95 | .006 |
| Durie and Salmon stage | ||||||
| III vs II | 1.31 | 1.10-1.756 | .003 | 1.33 | 1.08-1.63 | .007 |
| Therapy | < .001 | < .001 | ||||
| MPT vs MP | 0.70 | 0.59-0.84 | < .001 | 0.78 | 0.63-0.98 | .029 |
| VMP vs MP | 0.42 | 0.34-0.53 | < .001 | 0.52 | 0.35-0.76 | .001 |
| VMPT-VT vs MP | 0.27 | 0.21-0.36 | < .001 | 0.51 | 0.35-0.75 | .001 |
SD indicates stable disease; and PD, progressive disease.
Treated as a time-dependent variable.
Univariate and multivariate analysis (Cox model) of overall survival
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Response* | < .001 | < .001 | ||||
| CR vs VGPR | 0.15 | 0.08-0.28 | < .001 | 0.25 | 0.11-0.55 | .001 |
| CR vs PR | 0.08 | 0.04-0.16 | < .001 | 0.16 | 0.06-0.39 | < .001 |
| CR vs SD | 0.03 | 0.01-0.06 | < .001 | 0.07 | 0.02-0.20 | < .001 |
| CR vs PD | 0.01 | 0.005-0.03 | < .001 | 0.03 | 0.009-0.11 | < .001 |
| Age, y | ||||||
| > 75 vs ≤ 75 | 1.59 | 1.29-1.96 | < .001 | 1.30 | 1.00-1.70 | .053 |
| ISS | < .001 | < .001 | ||||
| II vs I | 1.73 | 1.24-2.43 | .001 | 1.41 | 0.99-2.01 | .056 |
| III vs I | 2.84 | 2.00-4.04 | < .001 | 2.17 | 1.49-3.15 | < .001 |
| Durie and Salmon stage | ||||||
| III vs II | 1.38 | 1.10-1.73 | .006 | 1.38 | 1.04-1.82 | .024 |
| Therapy | < .001 | .003 | ||||
| MPT vs MP | 0.88 | 0.71-1.10 | .263 | 1.00 | 0.76-1.31 | .991 |
| VMP vs MP | 0.25 | 0.17-0.38 | < .001 | 0.30 | 0.14-0.64 | .002 |
| VMPT-VT vs MP | 0.23 | 0.15-0.36 | < .001 | 0.50 | 0.26-0.95 | .035 |
| . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Response* | < .001 | < .001 | ||||
| CR vs VGPR | 0.15 | 0.08-0.28 | < .001 | 0.25 | 0.11-0.55 | .001 |
| CR vs PR | 0.08 | 0.04-0.16 | < .001 | 0.16 | 0.06-0.39 | < .001 |
| CR vs SD | 0.03 | 0.01-0.06 | < .001 | 0.07 | 0.02-0.20 | < .001 |
| CR vs PD | 0.01 | 0.005-0.03 | < .001 | 0.03 | 0.009-0.11 | < .001 |
| Age, y | ||||||
| > 75 vs ≤ 75 | 1.59 | 1.29-1.96 | < .001 | 1.30 | 1.00-1.70 | .053 |
| ISS | < .001 | < .001 | ||||
| II vs I | 1.73 | 1.24-2.43 | .001 | 1.41 | 0.99-2.01 | .056 |
| III vs I | 2.84 | 2.00-4.04 | < .001 | 2.17 | 1.49-3.15 | < .001 |
| Durie and Salmon stage | ||||||
| III vs II | 1.38 | 1.10-1.73 | .006 | 1.38 | 1.04-1.82 | .024 |
| Therapy | < .001 | .003 | ||||
| MPT vs MP | 0.88 | 0.71-1.10 | .263 | 1.00 | 0.76-1.31 | .991 |
| VMP vs MP | 0.25 | 0.17-0.38 | < .001 | 0.30 | 0.14-0.64 | .002 |
| VMPT-VT vs MP | 0.23 | 0.15-0.36 | < .001 | 0.50 | 0.26-0.95 | .035 |
SD indicates stable disease; and PD, progressive disease.
Treated as a time-dependent variable.
Discussion
The addition of bortezomib or thalidomide to standard oral MP has dramatically increased CR rates and extended PFS and OS.8-16 We performed a retrospective analysis on pooled data of 1175 elderly patients with newly diagnosed MM, treated with MP and novel agents. The median survival of the entire population was 50 months, significantly longer than 29 months previously reported in a large meta-analysis of 6633 patients (58% of them younger than 65 years of age) who received MP or conventional chemotherapy.20 The achievement of CR was associated with improved PFS and OS: the 3-year PFS was 67% in patients who achieved CR and 27% in those in VGPR or PR, whereas the 3-year OS rates were 91% in patients who obtained CR and 67% to 70% in those in VGPR or PR. A similar impact of CR was observed in patients more than 75 years.
In younger patients treated with conventional chemotherapy without novel agents, the achievement of CR significantly prolonged OS compared with the achievement of PR only.21 In elderly patients treated with VMP in the VISTA phase 3 trial, CR was associated with a significantly improved time to progression compared with VGPR and PR. However, no significant differences in OS were reported, probably because of the small sample size of the study.22 In our analysis, the achievement of CR predicted long-term outcome. This finding is consistent with a large meta-analysis of 4990 younger patients who underwent autologous transplantation,23 and with the post-hoc analysis of elderly patients treated with VMP in the VISTA trial.22 Of notice, it may be difficult to accurately assess a serum M-protein decrease higher than 90% in patients with small M-protein size at diagnosis. Therefore, in some cases, response could be underestimated. This should be considered while comparing outcome of patients who achieved VGPR or PR. When VGPR and CR were pooled together, their achievement significantly improved outcome if compared with PR only. In clinical practice, the achievement of CR rather than VGPR correlates with improved outcome.
In our study, the impact of CR on long-term outcome has been confirmed regardless of baseline patient characteristics, including age. In the era of novel agents, CR has become an achievable goal not only in young but also in elderly patients. The increased life expectancy of the general population and the better performance status of many aged patients should change the treatment paradigm, with a significant shift from a more palliative therapy to a more effective intensive approach. In previous studies, an increased response rate did not translate into an increased OS, particularly in patients older than 70 to 75 years, where treatment-related toxicities greatly impaired efficacy.9,24-26 Highly efficacious regimens may be associated with higher toxicity, and this should be carefully considered. The toxicity profile related to the treatment regimens evaluated in this analysis have been published elsewhere.8,12,13,16 We did not find any significant difference in rate of grade 3 or 4 adverse events in patients who obtained CR, VGPR, or PR. In the present analysis, the higher CR rate has been reported with the bortezomib combinations. In the VMPT-VT vs VMP GIMEMA study, the protocol was amended and bortezomib schedule was reduced from twice-weekly to once-weekly infusions to decrease toxicity. Extrahematologic grade 3 or 4 adverse events were reported in 35% of once-weekly patients and 51% of twice-weekly patients (P = .003) and the incidence of grade 3 or 4 peripheral neuropathy was 8% in the once-weekly and 28% in the twice-weekly group (P < .001). A post-hoc analysis assessed the impact of the schedule change on efficacy and safety. The treatment schedule change did not adversely impact on efficacy because long-term outcomes and CR rates were similar between once-weekly and twice-weekly patients.27 Our retrospective analyses suggest that efficacy (high CR rate) and feasibility (weekly administration of bortezomib,16,27 low-dose thalidomide8,10,12,13,16 ) are both essential to improve outcome in frail and very elderly patients. The achievement of CR correlates with improved outcome regardless of patient age, whereas individual dose adjustments should be adopted to minimize excessive toxicities that in turn jeopardize efficacy.
There is a correlation between rate of CR and treatment. Both factors impact on outcome. Subgroup analysis of outcome in CR patients who received bortezomib versus patients who did not show differences in PFS, but a trend toward a better OS in bortezomib-treated patients, was seen. Unfortunately, this analysis has some limitations: patients who received bortezomib regimens accounted for 80% of the CRs, whereas nonbortezomib regimens accounted for 20% of the CRs; median follow-up for bortezomib patients was 24 months, whereas for nonbortezomib patients it was 39 months; and a limited number of events occurred.
By multivariate analysis, the association of the achievement of CR with better outcome was independent of ISS stage: patients with either stage II/III or stage I disease benefited from profound cytoreduction. Moreover, the addition of bortezomib, but not of thalidomide only, was associated with longer OS. As previously reported, combinations including MP plus bortezomib were correlated with a significant improvement in OS,14-16 whereas combinations including MP plus thalidomide failed to show such advantage.8,11-13 Comparing the clinical outcomes of the treatment regimens used in the 3 trials shows, however, some limitations given the lack of a direct randomized comparison, differences in treatment schedules, and length of follow-up. Unfortunately, complete baseline data were not available for all patients, and the multivariate analysis was consequently performed on 681 of 1175 patients. Moreover, data on chromosomal abnormalities were not available for the majority of patients, and these variables were not included in the multivariate analysis.
In our study, 34% of CRs were reported at 4 months of treatment, 62% at 6 months, and 85% at 9 months, suggesting that 9 months could be considered a reasonable length of induction therapies. The long-term advantages linked with the achievement of CR were similar in patients who obtained it before or after the first 6 months of therapy. These results, consistent with previous findings,22,28 support the need to identify an optimal length of treatment and to adjust its intensity to prevent toxicities and early discontinuation in elderly patients.
Free-light chain assay, multiparameter flow cytometry, and polymerase chain reaction have recently been used to define more stringent response criteria.29,30 The greater depth of response, detectable with these more sensitive techniques compared with standard immunofixation, could further help clinicians to set the optimal level of response and individualize treatment intensity and duration.
Consolidation and maintenance therapy can improve outcome. Consolidation after autologous transplantation with bortezomib-thalidomide-dexamethasone improved the CR rate.29 Maintenance treatment with lenalidomide improved PFS in younger and elderly patients.31,32 Ideally, treatment strategies should include induction regimens associated with the highest CR rate followed by maintenance treatment.
In conclusion, the achievement of CR was an independent predictor of long-term outcome regardless of age and ISS stage. These findings support the use of novel agents to achieve maximal response, even in elderly patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who agreed to participate in this study, the data managing staff (Giulia Lupparelli, Tiziana Marangon), the nursing staff (Castorina Giacomo, Piccin Nadia Nives), editorial assistant Giorgio Schirripa, and the Dutch-Belgian Cooperative Trial Group for Hematology Oncology (HOVON).
This work was supported by Associazione Italiana Ricerca Cancro, Milan, Italy; Associazione Italiana Leucemie, Rome, Italy; Compagnia di S. Paolo, Turin, Italy; Fondazione Neoplasie Sangue Onlus, Turin, Italy; Ministero Università Ricerca Scientifica e Tecnologica, Rome, Italy; and Consiglio Nazionale delle Ricerche, Rome, Italy.
Authorship
Contribution: F.G., A.L., M.B., and A.P. designed and performed the research and collected data; F.G., B.B., R.P., and A.P. analyzed and interpreted data; F.G., A.L., and R.P. performed statistical analysis; F.G., M.B., A.P., and P.S. gave the final approval to the manuscript; and all authors recruited patients, provided clinical data, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: F.G. has received honoraria from Celgene, Janssen-Cilag, and Roche. F.C. has received honoraria from Celgene and Janssen-Cilag. M.T.P. has received honoraria from Janssen-Cilag and Celgene. F.P. has received honoraria from Roche, Janssen-Cilag, and Celgene and Advisory Board from Shering Plough. M.O. has received honoraria from Janssen-Cilag and Celgene. P.M. has received research founds and honoraria from Celgene. M.B. has received consultancy, advisory committees, and research funding from Celgene, Janssen-Cilag, and Pharmion. P.S. has received advisory boards from Johnson & Johnson and Celgene. A.P. has received advisory committee from Celgene and Janssen-Cilag and honoraria from Celgene, Janssen-Cilag, Merck, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Antonio Palumbo, Myeloma Unit, Division of Hematology, University of Torino, AOU San Giovanni Battista, Torino, Italy; e-mail: appalumbo@yahoo.com.