Abstract
The diagnosis of primary central nervous system lymphoma (PCNSL) depends on histopathology of brain biopsies, because disease markers in the cerebrospinal fluid (CSF) with sufficient diagnostic accuracy are not available yet. MicroRNAs (miRNAs) are regulatory RNA molecules that are deregulated in many disease types, including cancer. Recently, miRNAs have shown promise as markers for cancer diagnosis. In this study, we demonstrate that miRNAs are present in the CSF of patients with PCNSL. With a candidate approach and miRNA quantification by reverse transcription polymerase chain reaction, miRNAs with significant levels in the CSF of patients with PCNSL were identified. MiR-21, miR-19, and miR-92a levels in CSF collected from patients with PCNSL and from controls with inflammatory CNS disorders and other neurologic disorders indicated a significant diagnostic value of this method. Receiver-operating characteristic analyses showed area under the curves of 0.94, 0.98, and 0.97, respectively, for miR-21, miR-19, and miR-92a CSF levels in discriminating PCNSL from controls. More importantly, combined miRNA analyses resulted in an increased diagnostic accuracy with 95.7% sensitivity and 96.7% specificity. We also demonstrated a remarkable stability of miRNAs in the CSF. In conclusion, CSF miRNAs are potentially useful tools as novel noninvasive biomarker for the diagnosis of PCNSL.
Introduction
Unraveling the cause of focal brain lesions in patients with unexplained neurologic symptoms remains a clinical challenge. Especially in patients with primary central nervous system lymphoma (PCNSL), definitive diagnosis often is not possible on the basis of radiographic features and responsiveness to corticosteroids, which both do not specifically distinguish between lymphoma and inflammatory central nervous system (CNS) disease.1,2 In most patients with suspected CNS lymphoma who present with rapidly deteriorating neurologic symptoms, stereotactic brain biopsy remains the diagnostic procedure of choice. However, CNS biopsies are associated with the risk of hemorrhage and neurologic damage, and a definitive histopathologic diagnosis cannot always be achieved.1
Because PCNSLs represent highly aggressive tumors, early diagnosis is essential for successful treatment and improvement of disease prognosis.1,3-5 Although evaluation of the cerebrospinal fluid (CSF) is less invasive than brain biopsy, cytopathologic, immunophenotypic, and genetic analyses of CSF cells are much less sensitive.6-8 Protein markers within the CSF include antithrombin,9 soluble CD27,10 and free immunoglobulin light chains.11 They have been shown to be helpful with improved diagnostic sensitivities; however, their utility in accurate diagnosis of PCNSL from the CSF has not finally been established.1
MicroRNAs (miRNAs) are small regulatory RNA molecules that bind the 3′-untranslated regions of mRNA transcripts and inhibit gene expression at a posttranscriptional level by interference with translational initiation or degradation of mRNA.12,13 In several studies, miRNAs have been shown to play key regulatory roles in a wide range of genetic pathways that control cellular differentiation, proliferation, and apoptosis in physiologic conditions as well as different human diseases. There is increasing evidence that dysfunctional expression of miRNAs is a common feature of many types of cancer, and miRNAs play a direct role in cancer because they can function as oncogenes and tumor suppressors.13 Deregulated miRNA expression is found in various malignancies, including leukemia and lymphoma.12
MiRNAs are increasingly used as markers for diagnostic and prognostic purposes.14 Expression analyses of miRNAs can be accomplished directly from tumor samples. Furthermore, circulating miRNAs stably packaged in microvesicles can be detected in human serum and plasma.14 Previously, it has been shown that high expression levels of distinctive miRNAs are detectable in sera from patients with different types of cancers and are related to disease prognosis, for example, B-cell lymphoma,15 prostate cancer,16 and non-small cell lung cancer.17 Until today, there is no report of miRNAs identified in the CSF of patients with primary diffuse large B-cell lymphomas of the CNS.
In this study, we hypothesized that miRNAs could be useful CSF-based markers for detection of PCNSL. A candidate miRNA approach assessing miRNA expression by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) in the CSF of patients with PCNSL patients and control subjects with different neurologic disorders was chosen to evaluate the potential of miRNAs as a novel class of genetic CSF marker for PCNSL.
Methods
Patient characteristics and CSF samples
Between February 2009 and August 2010, consecutive CSF samples from patients with PCNSL (n = 23) and control patients with various neurologic disorders (n = 30; patient details listed in supplemental Tables 1 and 2A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) sent to our laboratory for routine chemical and cellular diagnostics were analyzed. CSF samples were collected before chemotherapy by diagnostic lumbar puncture after written informed consent and after exclusion of large intracranial mass lesions or increased intracranial pressure or both. All patients were seronegative for HIV. The University of Bochum Ethical Committee had approved CSF sample collections.
CSF samples were centrifuged (500g, 10 minutes, room temperature) within 60 minutes of collection to remove cells and debris and were stored at −80°C until further processing. An exclusion criterion from further analyses was hemorrhagic CSF collection. Sample aliquots were used for routine CSF diagnostics, including quantification of biochemical markers, cytopathology, and immunophenotyping by flow cytometry as recently reported.6,11
RNA extraction, reverse-transcription and quantification by real-time polymerase reaction
Total RNA was extracted with the use of a mirVana RNA isolation kit (Ambion) according to the manufacturer's instructions. Briefly, CSF samples were thawed on ice, and 0.4 mL of CSF was diluted with an equal volume of mirVana PARIS 2X denaturing solution, and subsequently incubated for 5 minutes on ice. Equal volumes of acid/phenol/chloroform (Ambion) were added to each aliquot, and samples were subsequently centrifuged for 5 minutes at 10 000g. Next, glycogen was added to aqueous phases, which were subsequently mixed with 1.25 volumes of 100% ethanol. After passage through a mirVana PARIS column, several washing steps were carried out, following the manufacturer's protocol. Finally, RNA was recovered in 100 μL of elution buffer. The RNA concentration was determined by measuring a 2-μL aliquot on a NanoDrop ND-3300 Fluorospectrometer.
Sequences of all miRNAs and primers used for specific amplification are listed at: http://www.mirbase.org/ and http://www.appliedbiosystems.com/. TaqMan miRNA assays (Applied Biosystems) to quantify miRNA levels were applied as has previously been published.18 In brief, 10 μL of total RNA solution was used in reverse-transcription reactions (16°C for 30 minutes, 42°C for 30 minutes, 85°C for 5 minutes, followed by 4°C). Real-time PCR was carried out on a 7500 Real-Time PCR System according to the manufacturer's protocol (Applied Biosystems). Cycling conditions were as follows: 95°C for 10 minutes, 40 cycles of 15 seconds at 95°C, and 60 seconds at 60°C. Fluorescent data were converted into cycle threshold (Ct) measurements by the 7500 SDS system software (Version 1.2.3; Applied Biosystems). Each sample was run in duplicates. Mean Ct values and standard deviations (SDs) were calculated for all miRNAs. MiR24 was selected from several control miRNAs, because it showed uniform expression levels. The amount of target miRNA was normalized relative to the amount of miR-24 (ΔCt = ΔCtmiR − ΔCtmiR-24). Relative expression levels (RELs) were reported as 2−ΔCt (supplemental Tables 1 and 2A-B).
To determine stability of miRNAs, miR-21 was quantified in CSF samples stored at room temperature (22°C) for a maximum of 4 days. CSF samples from 5 different patients with PCNSL were thawed and divided in aliquots into separate tubes. RNA was isolated immediately after thawing or after 24, 48, and 96 hours of storage, respectively. To evaluate the resistance of CSF miRNA to cleaving enzymes, an RNase mixture (RNase Cocktail; Ambion) was added to 500 μL of CSF at a final concentration of 5 U/mL RNase A and 200 U/mL RNase T1 and then incubated at 37°C for 30 minutes. MiR-30a-5p and miR-24 in a mixture of isolated miRNA were used as positive controls. MiRNAs were purified with the use of the mirVana miRNA isolation kit (Ambion) and measured in qRT-PCR as detailed above in this section.
Statistics
All statistical analyses were performed with SPSS (Version 18; SPSS Inc) and GraphPad Prism (Version 5.0). Groupwise comparisons of distributions of clinical and biologic data were performed, applying 2-tailed Mann-Whitney U tests and Kruskal-Wallis tests with Dunn multiple comparison. Results were considered statistically significant at P values < .05. Retest reliabilities were assessed with the intraclass correlation coefficient (ICC). A 2-way mixed type consistency ICC was chosen. ICC interpretation was similar to Cohen κ for interrater reliability (ICC < 0.20, poor; 0.20-0.39, fair; 0.40-0.59, moderate; 0.60-0.79, substantial agreement; > 0.80, outstanding interrater reliability).19
Results
Patient characteristics
In this study, CSF samples from 23 patients with PCNSLs (supplemental Table 1) and 30 control patients with various neurologic disorders, including CNS inflammation (n = 20), were analyzed (supplemental Table 2A-B). The PCNSL study population consisted of 14 male and 9 female patients; age was between 42 and 77 years (mean age, 64 years). In all 23 patients with PCNSL, a histopathologic diagnosis of diffuse large B-cell type lymphoma was established by brain biopsies. At the time of CSF collection, the disease was newly diagnosed in the majority of patients with PCNSL (n = 19; 82.6%); in 4 of 23 patients (17.4%) a relapse of CNS lymphoma had been diagnosed. Leptomeningeal lymphoma dissemination was detected by concordant findings of cytopathology and flow cytometry, as recently reported,6,11 in 4 of all 23 patients with PCNSL (17.4%; supplemental Table 1). Twelve of 23 patients with PCNSL (52.2%) were treated with corticosteroids at the time of CSF sample collection.
Detection of miRNAs in the CSF of patients with PCNSL by qRT-PCR
First, we asked whether miRNAs were detectable in frozen CSF samples collected from patients with PCNSL (n = 23; supplemental Table 1). In direct measurements of total RNA with the use of a fluorospectrometer, low RNA concentrations were detected in the CSF specimens (mean ± SD, 1.8 ± 0.8 ng/mL); no obvious differences of total RNA concentrations among several CSF specimens from patients with and without neurologic disorders were found (data not shown). Considering these results, we chose a candidate approach measuring miRNAs in the CSF, exploiting specific miRNA amplification by qRT-PCR reactions.
We reasoned that a suitable disease indicator for PCNSL would be (1) expressed by lymphoma cells at moderate or high levels and (2) would be present at low or undetectable concentrations in CSF derived from control patients. A selection of potential CSF-based miRNA marker candidates for PCNSL was obtained by compiling miRNAs expressed in diffuse large B-cell lymphomas and primary CNS lymphomas, based on published miRNA expression data in lymphoma tissues.20,21 This process resulted in 6 candidate miRNAs (miR-15b, miR-19b, miR-21, miR-92a, miR-106b, miR-204) for further investigation. Four miRNAs (miR-24, RNU48, RNU6b, RNU44) were selected as controls with probable uniform expression levels and a sufficient abundance in CSF for potential normalization purposes.
We used TaqMan qRT-PCR assays for detection of miRNAs and, primarily, screened CSF samples collected from all patients with PCNSL (n = 23; supplemental Table 1) and a subset of control patients with miscellaneous neurologic disorders (n = 10; supplemental Table 2A). Results of this initial screen indicated that 3 of 6 candidate miRNAs (miR-21, miR-19b, miR-92a) showed significantly increased levels (decreased Ct values) in the CSF of patients with PCNSL compared with the CSF of control patients, respectively (Table 1). MiR-15b, miR-106b, and miR-204 were detected by qRT-PCR; however, expression levels were comparable among patients with PCNSL and control patients (Table 1). Control microRNAs RNU48, RNU6b, and RNU44 were not detectable in the CSF by qRT-PCR (mean Ct values, > 40.0; data not shown), whereas low-abundant expression of miR-24 could be measured in all samples (Table 1). Because miR-24 was not expressed in different levels in patients with PCNSL and control patients, it was chosen for normalization of miRNA expression levels in subsequent analyses of CSF miRNA expression in a larger cohort of control patients.
miRNA expression in the CSF of patients with PCNSL versus control patients
| . | Patients with PCNSL (n = 23) . | Control patients (n = 10) . | P† . | ||
|---|---|---|---|---|---|
| Ct* . | SD . | Ct* . | SD . | ||
| miR-21 | 26.29 | 1.69 | 33.25 | 1.40 | < .001 |
| miR-19b | 28.76 | 2.85 | 33.01 | 2.84 | < .001 |
| miR-92a | 27.32 | 1.17 | 30.24 | 0.74 | < .001 |
| miR-15b | 32.98 | 1.31 | 34.80 | 1.46 | .36 |
| miR-106b | 32.85 | 1.45 | 31.31 | 5.71 | .49 |
| miR-204 | 29.16 | 1.86 | 29.08 | 2.26 | .62 |
| miR-24 | 31.21 | 1.09 | 31.18 | 1.00 | .81 |
| . | Patients with PCNSL (n = 23) . | Control patients (n = 10) . | P† . | ||
|---|---|---|---|---|---|
| Ct* . | SD . | Ct* . | SD . | ||
| miR-21 | 26.29 | 1.69 | 33.25 | 1.40 | < .001 |
| miR-19b | 28.76 | 2.85 | 33.01 | 2.84 | < .001 |
| miR-92a | 27.32 | 1.17 | 30.24 | 0.74 | < .001 |
| miR-15b | 32.98 | 1.31 | 34.80 | 1.46 | .36 |
| miR-106b | 32.85 | 1.45 | 31.31 | 5.71 | .49 |
| miR-204 | 29.16 | 1.86 | 29.08 | 2.26 | .62 |
| miR-24 | 31.21 | 1.09 | 31.18 | 1.00 | .81 |
Data are means of CT values (groupwise).
Comparison of miRNA expression among patients with PCNSL and control patients with neurologic disorders and was calculated with the Mann-Whitney U test.
Diagnosis of PCNSL on the basis of miR-19b, miR-21, and miR-92a levels in the CSF
Important differential diagnoses of PCNSL include demyelinating and inflammatory CNS diseases, such as multiple sclerosis. Having demonstrated differential expression of miRNAs in the CSF of patients with PCNSL and control patients with miscellaneous neurologic disorders/symptoms, we next analyzed expression of miR-15b, miR-19b, miR-21, miR-92a, miR-106b, miR-204 in the CSF of patients with different inflammatory CNS diseases (supplemental Table 2A-B). MiRNA expression data were normalized with the use of mi-R24 levels in individual CSF specimens and reported as RELs. Comparison of CSF expression of all 6 candidate miRNAs among patients with PCNSL (n = 23) and all controls (n = 30) showed significant differences for miR-21, miR-19b, miR-92a, and miR-15b in 2-tailed Mann-Whitney tests, respectively (Figure 1A). Increased mean and median RELs of miR-21, miR-19b, and miR-92a were shown in the CSF of patients with PCNSL. For miR-15b, the mean expression level was significantly reduced in the CSF of patients with PCNSL (mean REL, 0.60) compared with control patients (mean REL, 0.91). However, the median miR-15b REL was increased in patients with PCNSL. This result indicated that the mean value for miR-15b was considerably influenced by outlying values in the control group. Accordingly, the expression profile of miR-15b needs to be further addressed in a larger cohort to clarify the meaning of outlying miR-15b expression in a subset of patients with inflammatory CNS disease. Considering only those miRNAs (miR-21, miR-19b, miR-92a) showing increased mean and median RELs in patients with PCNSL, significant differences in the REL were also found among subgroups (PCNSL vs inflammation, PCNSL vs miscellaneous) as determined by Kruskal-Wallis tests, including Dunn multiple comparison analyses (Figure 1B).
RELs of miRNAs in CSF of patients with PCNSL and control patients. (A) Scatterplots of expression levels of miR-21, miR-19b, miR-92a, miR-15b, miR-106b, and miR-204 in CSF samples from 23 patients with PCNSL compared with 30 control patients with various neurologic disorders. RELs of miRNAs (y-axis) are normalized to miR-24. The black horizontal lines represent median REL values. P values are indicated as determined in Mann-Whitney U statistical comparisons. (B) Scatterplots of expression levels of miR-21, miR-19b, and miR-92a in CSF samples from patients with PCNSL (n = 23) compared with subgroups of patients with inflammatory (n = 20) and miscellaneous (n = 10) CNS disorders. RELs of the miRNAs (y-axis) are normalized to miR-24. The black horizontal lines represent median REL values. Groupwise P values are indicated as determined in Kruskal-Wallis tests with Dunn multiple comparisons (*P < .05).
RELs of miRNAs in CSF of patients with PCNSL and control patients. (A) Scatterplots of expression levels of miR-21, miR-19b, miR-92a, miR-15b, miR-106b, and miR-204 in CSF samples from 23 patients with PCNSL compared with 30 control patients with various neurologic disorders. RELs of miRNAs (y-axis) are normalized to miR-24. The black horizontal lines represent median REL values. P values are indicated as determined in Mann-Whitney U statistical comparisons. (B) Scatterplots of expression levels of miR-21, miR-19b, and miR-92a in CSF samples from patients with PCNSL (n = 23) compared with subgroups of patients with inflammatory (n = 20) and miscellaneous (n = 10) CNS disorders. RELs of the miRNAs (y-axis) are normalized to miR-24. The black horizontal lines represent median REL values. Groupwise P values are indicated as determined in Kruskal-Wallis tests with Dunn multiple comparisons (*P < .05).
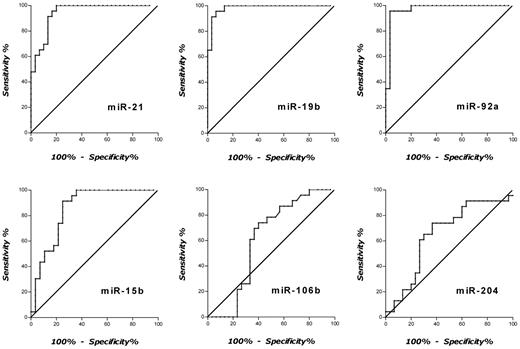
Next, RELs of all 6 candidate marker miRNAs were analyzed groupwise, and receiver-operating characteristics curves were plotted. As depicted in Figure 2, excellent separations between the groups of patients with PCNSL and control patients were observed for miR-21, miR-19b, and miR-92a, with areas under the curve (AUC) of 0.94, 0.98, and 0.97, respectively. On the contrary, AUCs for miR-15b, miR-106b, and miR-204 were calculated as 0.85, 0.60, and 0.65, respectively. On the basis of these analyses, cutoff CSF RELs with the highest accuracy were determined for miR-21, miR-19b, and miR-92a as follows: miR-21 was 8.0 REL (95.7% sensitivity; 83.3% specificity), miR-19b was 1.4 REL (95.7% sensitivity; 83.7% specificity), and miR-92a was 2.5 REL (95.7% sensitivity; 80.0% specificity).
Receiver-operating characteristics curve analyses with RELs of CSF miRNAs for discrimination of patients with PCNSL and control patients. CSF relative expression of miR-21 yielded an AUC of 0.94 (95% CI, 0.886-1.00), for miR-19b an AUC of 0.98 (95% CI, 0.955-1.01), for miR-92a an AUC of 0.97 (95% CI, 0.925-1.01), for miR-15b an AUC of 0.85 (95% CI, 0.748-0.959), for miR-106b an AUC of 0.60 (95% CI, 0.452-0.765), and for miR-204 an AUC of 0.65 (95% CI, 0.497-0.803), respectively.
Receiver-operating characteristics curve analyses with RELs of CSF miRNAs for discrimination of patients with PCNSL and control patients. CSF relative expression of miR-21 yielded an AUC of 0.94 (95% CI, 0.886-1.00), for miR-19b an AUC of 0.98 (95% CI, 0.955-1.01), for miR-92a an AUC of 0.97 (95% CI, 0.925-1.01), for miR-15b an AUC of 0.85 (95% CI, 0.748-0.959), for miR-106b an AUC of 0.60 (95% CI, 0.452-0.765), and for miR-204 an AUC of 0.65 (95% CI, 0.497-0.803), respectively.
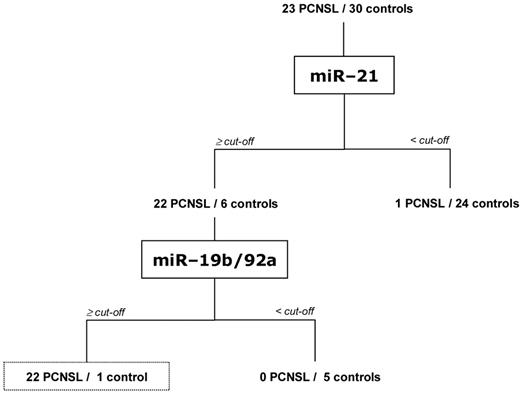
To distinguish PCNSL from other diseases on the basis of CSF miRNA expression more specifically, we combined miR-21, miR-19b, and miR-92a RELs and developed a diagnostic tree (Figure 3; Table 2). In summary, 22 of 23 patients with PCNSL (95.7%) could be correctly identified considering REL above the cutoffs for miR-21 and for one of each miR-19b or miR-92a, respectively. On the contrary, only one control patient with multiple sclerosis (patient 15; supplemental Table 2B) was falsely allocated to the PCNSL group with REL above the cutoffs for miR-21 and miR-92a. Overall, the specificity of the miRNA diagnostic tree was 96.7% in our cohort of 23 patients with PCNSL and 30 control patients (Figure 3).
CSF miRNA expression classification tree correctly diagnosing 95.7% of patients with PCNSL and 96.7% of control patients. Relative expression cutoff levels of ≥ 8.0 REL for miR-21, ≥ 1.4 REL for miR-19b, and ≥ 2.5 REL for miR-92a, respectively, were applied for diagnostic placements as depicted.
CSF miRNA expression classification tree correctly diagnosing 95.7% of patients with PCNSL and 96.7% of control patients. Relative expression cutoff levels of ≥ 8.0 REL for miR-21, ≥ 1.4 REL for miR-19b, and ≥ 2.5 REL for miR-92a, respectively, were applied for diagnostic placements as depicted.
miRNA relative expression levels (RELs) in subgroups
| . | miRNA-21 ≥ 8.0 REL . | miRNA-19b ≥ 1.4 REL . | miRNA-92a ≥ 2.5 REL . |
|---|---|---|---|
| PCNSL, n/N (%) | 22/23 (95.6) | 23/23 (100.0) | 4/23 (17.4) |
| Inflammation, n/N (%) | 6/20 (30.0) | 1/20 (5.0) | 3/20 (15.0) |
| Miscellaneous, n/N (%) | 0/10 (0.0) | 3/10 (30.0) | 3/10 (30.0) |
| . | miRNA-21 ≥ 8.0 REL . | miRNA-19b ≥ 1.4 REL . | miRNA-92a ≥ 2.5 REL . |
|---|---|---|---|
| PCNSL, n/N (%) | 22/23 (95.6) | 23/23 (100.0) | 4/23 (17.4) |
| Inflammation, n/N (%) | 6/20 (30.0) | 1/20 (5.0) | 3/20 (15.0) |
| Miscellaneous, n/N (%) | 0/10 (0.0) | 3/10 (30.0) | 3/10 (30.0) |
Reproducibility of miRNA quantification and miRNA stability in CSF
Considering the potential of miRNAs as novel marker in the CSF, we next analyzed the reproducibility of measurements in TaqMan qRT-PCR assays. Levels of miR-21, miR-19b, and miR-92a were measured twice in the same CSF samples collected from each of 6 patients with PCNSL. For miR-21, the Ct values ranged between 21.4 and 27.6, for miR-19b Ct values between 26.9 and 30.7 were measured, and for miR-92a Ct values in the range of 24.6 and 29.8 were determined by TaqMan qRT-PCR. In a second set of measurements, Ct values for each miRNA were reanalyzed in each patient, applying the same experimental conditions. Statistical analyses among independent measurements verified outstanding retest reliabilities as indicated by the after ICCs: miR-21 ICC was 0.98 (95% confidence interval [CI], 0.89-0.99; P < .001), miR-19b ICC was 0.98 (95% CI, 0.88-0.99; P < .001), and miR-92a ICC was 0.97 (95% CI, 0.78-0.99; P = .001).
An important requirement for the utility of miRNAs as markers is their stability in CSF samples ex vivo. First, we investigated the stability of miR-21, miR-19b, and miR-92a in CSF by subjecting specimens to ≤ 10 cycles of freeze-thawing. This procedure had minimal effect on miRNA levels as determined by qRT-PCR (data not shown). Prior reports have suggested that miRNAs in plasma exist in a form that is resistant to intrinsic RNase activity.16 In the CSF, low RNase activities are present.22 To address the question whether miRNAs in CSF exist in a form that is resistant to ribonucleases, we measured levels of miR-21 in CSF specimens after incubation with RNase. Although levels of extrinsic miRNAs that had been added to the CSF decreased significantly after RNase exposure, the concentrations of endogenous miRNAs (miR-21, miR-19b, and miR-92a) in the CSF were not altered by ribonucleases (data not shown). We concluded that endogenous miRNAs in the CSF exist in a form resistant to RNase cleavage, a finding that was in line with those for serum and plasma miRNAs.16
Next, we asked whether miRNAs are stable in stored CSF samples. Accordingly, CSF samples collected from 5 patients with PCNSL were kept for up to 96 hours at room temperature. Expression levels of miRNAs were measured immediately and after 24, 48, and 96 hours of storage in TaqMan qRT-PCR assays. We found miR-21 levels in stored CSF specimens to be stable over time (data not shown). Here, Ct values at 0 hour ranged between 23.4 and 25.6 (mean Ct, 24.9). At 24 and 48 hours, absolute decreases of mean CT values of 0.06 (ΔCt%, 0.23) and 0.38 (ΔCt%, 1.52) were observed, respectively. At the maximum CSF sample storage time of 96 hours at room temperature, the mean absolute CT value decreased by 1.39 (ΔCt%, 5.58). In summary, we concluded that miRNAs exist in a stable form in CSF.
Discussion
Several reports have described that deregulation of miRNAs is tightly linked to cancer.13 In addition, some miRNAs are closely associated with the clinical course of malignant tumor disease.14 Hence, expression analysis of miRNAs is of increasing interest for diagnostic and prognostic purposes. Evaluation of miRNA expression in primary tumor cells and tissues has been the basis of most studies in the field. Recently, it was discovered that tumor-derived miRNAs are present in extracellular body fluids such as serum and plasma.14-16 Most of the miRNAs circulating in peripheral blood are included in lipid or lipoprotein complexes and, therefore, are highly stable.14 Considering these findings, we raised the question whether (1) miRNAs are detectable in the extracellular liquid compartment of the CSF in patients with PCNSL and whether (2) miRNA detection in CFS may facilitate the diagnosis of PCNSL. CSF-based miRNA studies are in their early infancy. To date, only one study has reported that miRNAs can be measured in the CSF collected from healthy persons and from Alzheimer patients.23
In our study, a candidate approach was chosen, and miR-21, miR-19b, miR-92a, miR-15b, miR-106b, and miR-204 were selected as miRNAs putatively overexpressed in CSF of patients with PCNSL. We found that the levels of miR-19b, miR-21, and miR-92a, as measured in RT-PCR assays, were significantly increased in CSF samples from patients with PCNSL compared with controls with inflammatory CNS disease or other neurologic disorders. MiR-21, miR-19b, and miR-92a had a significant diagnostic value for PCNSL and yielded AUCs of 0.94, 0.98, and 0.97, respectively, in receiver-operating characteristics analyses. Inclusion of these 3 miRNAs in combined expression analyses further increased their discriminatory diagnostic value. In summary, our study of combined miR-21, miR-19b, and miR-92a analyses in patients with PCNSL showed that CSF levels of miRNA could distinguish, with considerable specificity (96.7%) and sensitivity (95.7%), patients with PCNSL in contrast to other neurologic disorders, most importantly inflammatory CNS diseases.
In our study, miR-21 was the most abundant miRNA in CSF of patients with PCNSL. Mean relative expression of miR-21 was 60.0 in CSF of PCNSL compared with 3.8 in CSF of controls. These data are consistent with Lawrie et al21 and Robertus et al20 who showed high miR-21 expression levels in samples of diffuse large B-cell lymphoma, including PCNSL. Interestingly, miR-21 has been shown to be expressed in a variety of tumors and to be associated with the down-regulation of bcl-2 and phosphatase and tensin homologue (PTEN). Both miR-19b and miR-92a are members of the poly-cistronic microRNA-17 ∼ 92 cluster located on human chromosome 13.13 In the present study, miR-19b showed 14-fold higher mean expression levels and, miR-92a was expressed 10-fold higher in the CSF of patients with PCNSL in comparison with control patients. Previously, evidence has been provided that the microRNA-17 ∼ 92 cluster is frequently overexpressed in B-cell lymphoma cell lines, the majority of diffuse large B-cell lymphomas,12 and also in PCNSL.20 Thus, the role of microRNA-17 ∼ 92 in lymphoma is further highlighted by our findings of differential expression of miR-19b and miR-92a in the CSF of patients with PCNSL.
Normalization is an important step for accurate quantification of RNA levels with qRT-PCR. For miRNA, circulating in extracellular body fluids, including those detected in the CSF, no consensus internal controls have been established yet. In our study, the yields of total RNA prepared from small volumes of CSF were below the limit of accurate quantification by fluorospectrometry. These results are confirmed in a study by Cogswell et al,23 and are similar to findings reported for plasma and serum.16 Accordingly, absolute RNA concentrations could not be used for normalization purposes in our measurements. We evaluated 4 miRNAs (RNU48, RNU6b, RNU44, miR-24) and identified miR-24 to exhibit consistent expression across patients and controls. Before our study, miR-24 has been reported as appropriate miRNA control for normalization purposes.18,23 Accordingly, miR-24 was chosen as normalizing control miRNA in our current study.
Extracellular miRNAs circulating in the peripheral blood are obviously included in cell membrane–derived particles, such as apoptotic bodies, microvesicles, and exosomes.14 There is evidence that packaging of miRNA into these particles results in protection from endogenous ribonucleases in plasma/serum.16 In agreement with these findings, miRNAs in the CSF exhibited a remarkable stability as shown here in exposure to exogenous RNase, repeated freeze-thaw cycles, and long-term storage of CSF specimens in our experiments. Possible explanations for this phenomenon include miRNA protection in exosomes or association of miRNA with other molecules, such as CSF proteins. Because the secretory mechanisms as well as the meaning of the existence of extracellular miRNAs remain largely unknown, additional studies exploring a potential biologic function of miRNAs circulating in body fluids such as peripheral blood and CSF are required. Notably, the striking stability of CSF miRNA compared with the fragility of cells within the CSF represents an important diagnostic advantage.
During the study period, 26 patients were referred to our institution for diagnosis and treatment of PCNSL. The majority of our patients (n = 24; 92.3%) had CNS lesions not accompanied by major alterations in CSF kinetics nor a hydrocephalus that prevented us from performing a lumbar puncture. However, in 2 patients (7.7%) lumbar puncture was omitted, given that space-occupying cerebellar lesions were shown in the posterior fossa. One patient had already undergone a lumbar puncture without complication in the referring hospital. In summary, in the greater group of our patients lumbar puncture was possible without a risk because of disturbed CSF kinetics or intracranial pressure. This finding was in line with those reported in the literature24 and supports the potential diagnostic value of CSF analyses such as miRNA detection in patients with PCNSL.
In conclusion, our results suggest that miRNA detected in the CSF can serve as marker for PCNSL as a model CNS disease. We anticipate that the results of our study are expanded by detection of other miRNAs in the CSF related to other neoplastic and inflammatory CNS diseases. By means of both, qRT-PCR assays and miRNA array technologies, CSF-based miRNA markers that are specific for particular CNS disorders may be discovered in the future. Although the number of patients and controls studied here is still small, our study provides the rationale for future investigations of miRNAs in the CSF for diagnostic and prognostic purposes in a variety of CNS disease, including PCNSL.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Protein Research Department (Ruhr-University) and by a grant (PURE) from the Ministry of Science, North Rhine-Westphalia, Germany.
Authorship
Contribution: A.B., A.M., and H.Z. performed the experiments; J.K., U.S., A.C., and R.G. collected clinical samples; A.B., S.A.H., J.K., U.S., A.C., R.G., A.R.S., M.D., W.S., and R.S. discussed and designed the research; A.B., S.A.H., and R.S. analyzed the results; and R.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roland Schroers, Stephan Hahn, or Wolff Schmiegel, Ruhr-University of Bochum, Knappschaftskrankenhaus Bochum-Langendreer, In der Schornau 23-25, D-44892 Bochum, Germany; e-mail: Roland@SchroersOnline.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal