Abstract
In Hodgkin lymphoma (HL), the malignant cells are surrounded by a large number of reactive infiltrating inflammatory cells, including OX40-expressing T cells and interleukin 10 (IL-10)–producing regulatory T (T-reg) cells. These T-reg cells can suppress the immune response and thus contribute to the maintenance of immune tolerance and to insufficient antitumor response. The engagement of OX40L with the OX40 receptor is essential for the generation of antigen-specific memory T cells and for the induction of host antitumor immunity. In the present study, we investigated whether histone deacetylase inhibitors (HDACis) may induce a favorable antitumor immune response by regulating the expression of OX40L in HL. We found that HDACis up-regulated OX40L surface expression in HL cell lines in a dose-dependent manner. Small interfering RNAs (siRNAs) that selectively inhibited HDAC11 expression, significantly up-regulated OX40L and induced apoptosis in HL cell lines, and silencing HDAC11 transcripts increased the production of tumor necrosis-α (TNF-α) and IL-17 in the supernatants of HL cells. Furthermore, HDACI-induced OX40L inhibited the generation of IL-10–producing type 1 T-reg cells. These results demonstrate for the first time that HDAC11 plays an essential role in regulating OX40L expression. Pharmacologic inhibition of HDAC11 may produce a favorable antitumor immune response in patients with HL.
Introduction
Classic Hodgkin lymphoma (cHL) is a B-cell lymphoid malignancy that is characterized by a relatively small number of malignant Hodgkin and Reed-Sternberg (HRS) cells surrounded by an overwhelming number of inflammatory cells, including a large number of OX40-expressing T cells, interleukin 10 (IL-10)–producing T-regulatory (T-reg) cells (Tr1 cells) and CD4+CD25+Foxp3+ T-reg cells that are known to play a role in the maintenance of peripheral immune tolerance.1-3 This unique pathology is generated by a variety of cytokines, chemokines, and growth factors that are secreted by HRS cells, including thymus- and activation-regulated chemokine (TARC)/CCL17 and transforming growth factor-β, which attract CD4+ T-helper-2 (Th2) and T-reg cells, in addition to contributing to the known ineffective cellular immune response.3-5 Strategies to reverse the immune-suppression status in the HL microenvironment and to restore an effective anti-HRS immunity in vivo are currently being explored as novel treatments for patients with cHL.4,6,7
OX40 ligand (OX40L), a member of the tumor necrosis factor (TNF) superfamily, is expressed predominantly by professional antigen-presenting cells such as dendritic cells, activated B cells, and macrophages, in addition to T cells and endothelial cells.8,9 OX40 receptor (CD134) is transiently expressed as a costimulatory protein by activated T cells, natural killer T cells, and T-reg cells.10 The engagement of OX40L with the OX40 receptor is essential for the generation of antigen-specific memory T cells and for the induction of host antitumor immunity.11 Consequently, strategies to activate the OX40-OX40L pathway are being explored to break immune tolerance for the treatment of cancer.12-15
We recently reported that histone deacetylase inhibitors (HDACis) may induce a favorable antitumor immune response in HL by down-regulating the expression and secretion of thymus- and activation-regulated chemokine (TARC) in HRS and dendritic cells in vitro and by altering the balance of inflammatory cytokines to favor a Th1-type response.16 This in vitro effect was reproduced in vivo, because HDACI therapy decreased serum TARC levels in patients with relapsed HL.17 These observations suggested that the favorable clinical activity of HDACis in patients with HL may be related to combined antiproliferative and immunomodulatory effects.17-19 In the work reported here, we extended our previous studies by examining how HDACis may modulate the immune response. Recently, we and others reported that OX40 triggering can inhibit the suppressive function of IL-10–producing Tr1 cells and CD4+CD25+Foxp3+ T-reg cells, and can also inhibit the transforming growth factor-β–induced conversion of antigen-specific CD4+ naive T cells into CD4+CD25+Foxp3+ T-reg cells.20-22 Therefore, we investigated whether HDACis enhance the immune response by regulating the expression of OX40L in cHL.
Methods
Cell lines, culture conditions, and reagents
The human HRS–derived cell lines HD-LM2, L428, and KM-H2 were obtained from the German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures (Braunschweig, Germany). All cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), 1% L-glutamine, and penicillin/streptomycin in a humid environment of 5% CO2 at 37°C. Antibodies to caspase 8 and caspase 9 were from Cell Signaling Technology, antibody to β-actin was from Sigma-Aldrich, and antibodies to CD40 and OX40L were from BD Biosciences. Anti-OX40L antibody for the blocking experiment was from R&D Systems. The HDACI suberoylanilide hydroxamic acid (vorinostat) was purchased from BioVision. MGCD0103 was kindly provided by MethylGene and SNDX-275 was kindly provided by Syndax.
Flow cytometry
Cell surface expression was determined by fluorescence-activated cell sorting (FACS) as described previously. Apoptosis was determined by annexin-V–FLUOS and propidium iodide double staining (Roche Molecular Biochemicals) according to the manufacturer's instructions and as described previously.23 Data were collected on a FACSCalibur flow cytometer (BD Biosciences) as described previously.23 Results were obtained by analyzing data with FlowJo Version 7.6.1 software (TreeStar). Results represent the mean value of 3 independent experiments.
Western blot analysis
Total cellular proteins were extracted by incubating the cells in lysis buffer (Cell Signaling Technology) for 30 minutes on ice and then centrifuging to remove cellular debris. The protein in the resulting supernatant was quantified by the bicinchoninic acid assay (Pierce) according to the manufacturer's instructions. Protein was then diluted 1:2 in protein sodium dodecyl sulfate loading buffer (Cell Signaling Technology) and heated to 95°C for 5 minutes. A total of 30 μg of protein was loaded onto 12% Tris-HCl sodium dodecyl sulfate-polyacrylamide electrophoresis Ready Gels (Bio-Rad), transferred to a nitrocellulose transfer membrane (Bio-Rad), and detected using SuperSignal West Dura Extended Duration Substrate (Pierce), as described previously.23,24
Real-time RT-PCR
Total RNA from control (untreated) and HDACI-treated cell lines was extracted using the TRIzol method (Invitrogen), purified using RNeasy chromatography (QIAGEN), and reverse transcribed using the SuperScript method (Invitrogen). Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed by standard procedures using the SYBR Green/ROX PCR Master Mix (SA Biosciences) and OX40L quantitative PCR (qPCR) primers (Hs00182411; Applied Biosystems) in a 7500 Fast Real-Time PCR System (Applied Biosystems).
Selective inhibition of HDAC expression by siRNA
Three sets (for each HDAC) of double-stranded small interfering RNA (siRNA) for HDAC1, -2, -3, -8, and -11, and of the silencer-negative siRNA as a control, were purchased from Invitrogen. The sequences of the siRNAs are available on request. The HL cell lines HD-LM2, L428, and KM-H2 were plated at 1 × 106 cells/mL in 12-well plates. Double-stranded siRNAs (2μM) were transfected using the Nucleofector kit (Amaxa). Cells were harvested after 24 hours and subjected to FACS and Western blot analysis. This protocol gave a transfection efficiency of 70%-80%.
Generation of IL-10–producing Tr1 cells
Total CD4+ T cells (purity > 90%) were isolated from peripheral blood mononuclear cells using a human CD4+ T-cell enrichment cocktail kit (StemCell Technologies). A total of 20 × 104 freshly purified CD4+ T cells were cultured with IL-2 (50 units/mL; R&D Systems), anti-CD28 monoclonal antibody (mAb; 0.2 μg/mL), dexamethasone (5 × 10−8 M; Life Technologies), and 1α,25-dihydroxyvitamin D3 (1 × 10−7 M; Life Technologies) on 8 × 104 irradiated CD32/ICOSL-L cells that had been precoated with anti-CD3 mAb (OKT3, 0.2 μg/mL, in a 48-well plate). In some experiments, CD4+ T cells were stimulated to differentiate into Tr1, in the presence of 6 × 104 Mino or L428 cells treated with dimethylsulf-oxide (DMSO) or SNDX-275 (for 48 hours). In addition, L428 cells were treated with 1.5 μg/mL of anti-OX40L–neutralizing antibody or IgG1 control mAb to verify if the effect of SNDX-275 on IL-10–producing Tr1 cells was truly mediated by OX40L. We used RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM l-glutamine, penicillin G, and streptomycin for our T-cell cultures. T cells were harvested after 8 days of culture and used for IL-10 intracellular staining.
Enzyme-linked immunosorbent assay
HL cell lines were incubated with 1μM MGCD0103 or DMSO for 24 hours before supernatants were collected and examined by Luminex (R&D Systems) according to the manufacturer's instructions. Each experiment was performed in triplicate, and the results represent the mean value from the 3 independent experiments.
Statistical analysis
Procedures to determine the effects of certain conditions on cell proliferation, apoptosis, and cytokine production were performed in 3 independent experiments. The 2-tailed Student t test was used to estimate the statistical significance of the differences in results from the 3 experiments. Significance was set at P < .05.
Results
OX40L is not expressed on unstimulated cultured HL-derived cells
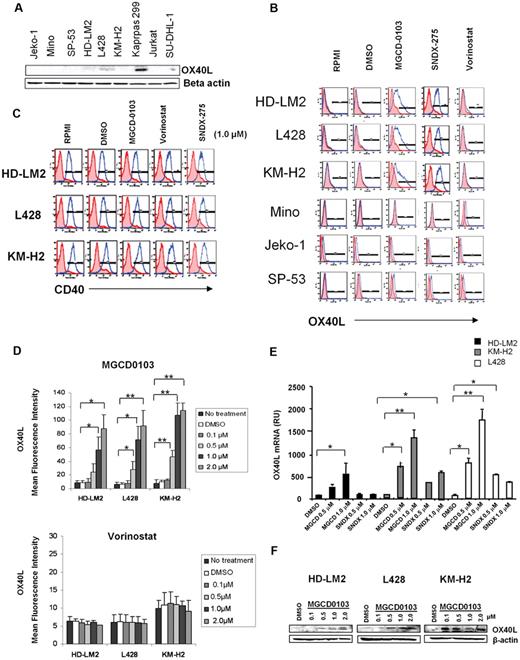
We examined the expression of OX40L in 3 well-characterized HL-derived cell lines and compared the results with those in mantle-cell lymphoma (MCL) cell lines and anaplastic large-cell lymphoma cell lines. Using Western blot analysis, we found that the HL and MCL cells did not express OX40L (Figure 1A). In contrast, Karpas 299, an anaplastic large-cell lymphoma line that has a CD4+ T-reg phenotype, did express OX40L.25 We further confirmed the lack of OX40L expression in the HL and MCL cells using FACS analysis (Figure 1B).
HDACis up-regulate the expression of OX40L in HL. (A) Baseline expression of OX40L in lymphoid cell lines by Western blot analysis. (B) OX40L expression in HL and MCL cells. Cells were incubated with DMSO, MGCD0103, vorinostat, and SNDX-275 (1μM) for 24 hours before OX40L levels were analyzed by FACS. MGCD0103 and SNDX-275 increased OX40L expression in HL cells but not in MCL cells. (C) CD40 expression in HL cells. (D) OX40L expression in HL cells. Cells were incubated with DMSO, MGCD0103, and vorinostat (0.1-2.0μM) for 48 hours, collected, and their OX40L expression was analyzed by FACS. MGCD0103 increased OX40L expression in a dose-dependent manner. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (E) Effect of HDACis on OX40L mRNA levels in HL cells. Results of qRT-PCR after 48 hours of incubation in the absence or presence of MGCD0103 (0.5-1.0μM) or SNDX-275 (0.5-1.0μM). Data are presented as mRNA relative units (RU) ± SEM; n = 3 for each condition and kinetic point. *P < .05; **P < .005. (F) Effect of MGCD0103 on OX40L expression. HL cells were incubated with MGCD0103 (0.1-2.0μM) for 48 hours. Western blot analysis was performed on whole-cell lysates. The effect was more evident with higher doses of MGCD0103 in all HL cell lines.
HDACis up-regulate the expression of OX40L in HL. (A) Baseline expression of OX40L in lymphoid cell lines by Western blot analysis. (B) OX40L expression in HL and MCL cells. Cells were incubated with DMSO, MGCD0103, vorinostat, and SNDX-275 (1μM) for 24 hours before OX40L levels were analyzed by FACS. MGCD0103 and SNDX-275 increased OX40L expression in HL cells but not in MCL cells. (C) CD40 expression in HL cells. (D) OX40L expression in HL cells. Cells were incubated with DMSO, MGCD0103, and vorinostat (0.1-2.0μM) for 48 hours, collected, and their OX40L expression was analyzed by FACS. MGCD0103 increased OX40L expression in a dose-dependent manner. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (E) Effect of HDACis on OX40L mRNA levels in HL cells. Results of qRT-PCR after 48 hours of incubation in the absence or presence of MGCD0103 (0.5-1.0μM) or SNDX-275 (0.5-1.0μM). Data are presented as mRNA relative units (RU) ± SEM; n = 3 for each condition and kinetic point. *P < .05; **P < .005. (F) Effect of MGCD0103 on OX40L expression. HL cells were incubated with MGCD0103 (0.1-2.0μM) for 48 hours. Western blot analysis was performed on whole-cell lysates. The effect was more evident with higher doses of MGCD0103 in all HL cell lines.
Regulation of OX40L expression in HL-derived cell lines by HDACis
Because HDACis are known to modulate the expression of a variety of TNF superfamily ligands and receptors,26-28 we investigated whether HDACis up-regulate OX40L expression in HL cells. We incubated HL and MCL cells with 3 different HDACis known to inhibit both class I and class IV (SNDX-275 and MGCD0103) and class I and II (vorinostat) HDAC enzymes, and examined OX40L surface expression by FACS analysis (Figure 1B).29-31 Both MGCD0103 and SNDX-275 up-regulated OX40L surface expression in HL cell lines, but had no effect on MCL cell lines. In contrast, the pan-HDACI vorinostat failed to up-regulate OX40L in any of the cell lines (Figure 1B). In comparison, none of the HDACis modulated CD40 expression in HL cells (Figure 1C). The induction of OX40L surface expression by MGCD0103 was dose dependent, as determined by FACS (Figure 1D), qRT-PCR (Figure 1E), and Western blot (Figure 1F) analyses. In contrast, vorinostat had no significant effect on OX40L (Figure 1D bottom).
Essential role for HDAC11 in regulating OX40L expression
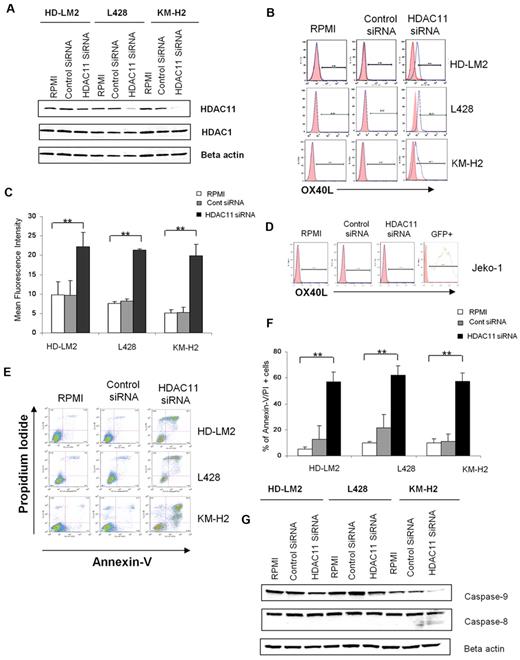
MGCD0103 is known to preferentially inhibit HDAC1, -2, -3, -8, and -11 in purified cell-free enzyme assays.32 We hypothesized that the ability of this HDACI to up-regulate OX40L in HL cells is likely to be related to inhibiting the function of one of these HDACs. We used siRNAs to selectively inhibit the expression of these HDAC enzymes in HL cell lines and determined the functional consequences of such inhibition. siRNA knock-down of HDAC11 expression significantly up-regulated OX40L in the HL cell lines (Figure 2A-C). Consistent with the lack of effect of MGCD0103 or SNDX-275 on OX40L expression in MCL cell lines, siRNA knock-down of HDAC11 expression in the MCL cell line Jeko-1 had no effect on OX40L expression (Figure 2D). Because MCL and HL cells express comparable levels of class I and IV HDACs,33 these results suggest that OX40L expression is differentially regulated in different cell types.
Silencing of HDAC11 gene expression by siRNA up-regulates OX40L in HL cell lines. (A) HL cell lines were transfected with HDAC11 or control siRNA (3 μg), and after 48 hours the cellular level of HDAC11 was determined by Western blot. Results represent 3 independent experiments showing the efficacy of HDAC11 siRNA in down-regulating HDAC11 expression. (B) HL cell lines were analyzed by FACS after 48 hours of HDAC11 siRNA transfection. Blocking HDAC11 increased OX40L expression. (C) Mean fluorescence intensity of 3 independent experiments (± SEM). *P < .05; **P < .005. (D) The Jeko-1 cell line was analyzed by FACS after 24 hours of HDAC11 siRNA transfection. Down-regulating HDAC11 did not have any effect on OX40L expression. (E) HDAC11 siRNA induced apoptosis in HL cell lines. (F) Representative experiment demonstrating the effect of down-regulating HDAC11 on induction of apoptosis in 3 HL cell lines as determined by propidium iodide and annexin V staining and FACS analysis. Results are shown after 48 hours of incubation. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (G) Effect of HDAC11 siRNA on the caspase pathway. Down-regulation of HDAC11 decreased expression of caspase 9 and caspase 8 in HL cells, as determined after 24 hours of incubation.
Silencing of HDAC11 gene expression by siRNA up-regulates OX40L in HL cell lines. (A) HL cell lines were transfected with HDAC11 or control siRNA (3 μg), and after 48 hours the cellular level of HDAC11 was determined by Western blot. Results represent 3 independent experiments showing the efficacy of HDAC11 siRNA in down-regulating HDAC11 expression. (B) HL cell lines were analyzed by FACS after 48 hours of HDAC11 siRNA transfection. Blocking HDAC11 increased OX40L expression. (C) Mean fluorescence intensity of 3 independent experiments (± SEM). *P < .05; **P < .005. (D) The Jeko-1 cell line was analyzed by FACS after 24 hours of HDAC11 siRNA transfection. Down-regulating HDAC11 did not have any effect on OX40L expression. (E) HDAC11 siRNA induced apoptosis in HL cell lines. (F) Representative experiment demonstrating the effect of down-regulating HDAC11 on induction of apoptosis in 3 HL cell lines as determined by propidium iodide and annexin V staining and FACS analysis. Results are shown after 48 hours of incubation. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (G) Effect of HDAC11 siRNA on the caspase pathway. Down-regulation of HDAC11 decreased expression of caspase 9 and caspase 8 in HL cells, as determined after 24 hours of incubation.
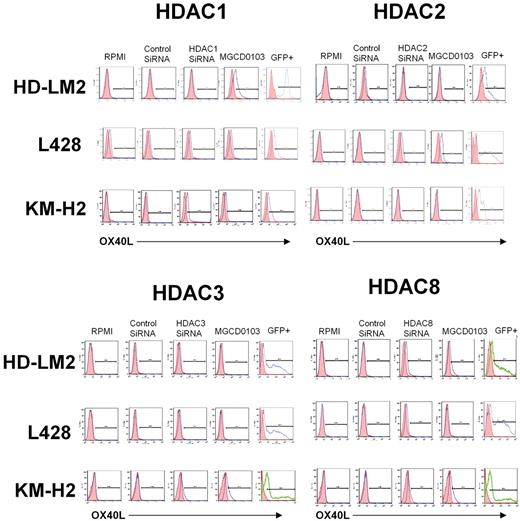
To better understand the functional role of HDAC11 in HL, we examined the effect of disrupting HDAC11 expression by siRNA on HL cell survival. We found that silencing HDAC11 induced apoptosis in all 3 HL cell lines (Figure 2E-F), but had no significant effect on MCL Jeko-1 cells (data not shown). This effect was associated with a cleavage of caspase 9, indicating activation of the intrinsic caspase pathway (Figure 2G). In contrast, inhibition of the expression of HDAC1, -2, -3, or -8 had little or no effect on OX40L expression in the HL cell lines (Figure 3).
Effect of HDAC1, -2, -3, and -8 siRNA transfection on OX40L expression. HL cell lines were transfected with HDAC1, -2, -3, and -8 siRNA and cells were analyzed for OX40L expression after 48 hours of incubation. Inhibition of expression of HDAC1, -2, -3, or -8 had little or no effect on OX40L expression in HL cells.
Effect of HDAC1, -2, -3, and -8 siRNA transfection on OX40L expression. HL cell lines were transfected with HDAC1, -2, -3, and -8 siRNA and cells were analyzed for OX40L expression after 48 hours of incubation. Inhibition of expression of HDAC1, -2, -3, or -8 had little or no effect on OX40L expression in HL cells.
Silencing of HDAC11 expression increases TNF-α production by HL cells
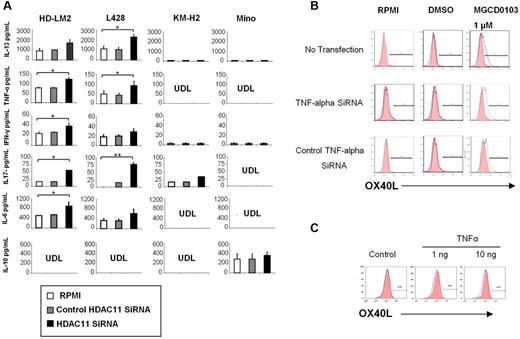
We have previously reported that HDACis can alter the balance of cytokines and chemokines in HL cells to favor Th1-type cytokines, some of which have been reported to either induce OX40L expression in a variety of cell types or to enhance OX40L-mediated blocking of T-reg survival.16,34 Furthermore, we have previously reported that OX40L converts IL-10–producing T-reg cells into a TNF-α-producing inflammatory Th1-cell response.20,35 Silencing HDAC11 expression by siRNA resulted in an increase in the secretion of TNF-α, interferon-γ, and IL-6 into the supernatants of cultured HL cell lines but not the Mino MCL cells. In contrast to findings reported for professional antigen-presenting cells, silencing HDAC11 transcription in HL cells had no effect on IL-10 production (Figure 4A).36 Similarly, silencing HDAC11 did not have a significant effect on IL-4 or IL-12 production in HL cells (data not shown). Interestingly, silencing HDAC11 resulted in an increase in IL-13 production in HL cells, perhaps reflecting a negative feedback loop, as we previously reported with HDACis.16 Finally, silencing HDAC11 also increased IL-17 production in the HL cell lines, but not in the Mino MCL cells (Figure 4A). These data demonstrate that silencing HDAC11 transcripts preferentially alters cytokine secretion in HL cells to favor a Th1- and Th17-type response.
HDAC11 regulates the production of cytokines and chemokines in HL. (A) We analyzed 4 cell lines: HD-LM2, L428, KM-H2, and Mino. Cell culture supernatants were collected after 24 hours of HDAC11 siRNA transfection. HDAC11 siRNA increased the production of IL-13, TNF-α, interferon-γ, IL-6, and IL-17. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (B) Expression of OX40L after TNF-α siRNA transfection. The L428 cell line was analyzed by FACS after TNF-α siRNA transfection and after 48 hours of treatment with MGCD0103 (1.0μM). MGCD0103 increased OX40L expression. (C) OX40L expression in HD-LM2 cells. HD-LM2 cells were incubated with recombinant TNF-α (1-10 ng) for 48 hours and then analyzed by FACS. Recombinant TNF-α did not have a significant effect on OX40L expression in these cells.
HDAC11 regulates the production of cytokines and chemokines in HL. (A) We analyzed 4 cell lines: HD-LM2, L428, KM-H2, and Mino. Cell culture supernatants were collected after 24 hours of HDAC11 siRNA transfection. HDAC11 siRNA increased the production of IL-13, TNF-α, interferon-γ, IL-6, and IL-17. Each value is the mean of 3 independent experiments (± SEM). *P < .05; **P < .005. (B) Expression of OX40L after TNF-α siRNA transfection. The L428 cell line was analyzed by FACS after TNF-α siRNA transfection and after 48 hours of treatment with MGCD0103 (1.0μM). MGCD0103 increased OX40L expression. (C) OX40L expression in HD-LM2 cells. HD-LM2 cells were incubated with recombinant TNF-α (1-10 ng) for 48 hours and then analyzed by FACS. Recombinant TNF-α did not have a significant effect on OX40L expression in these cells.
HDAC11-induced up-regulation of OX40L in HL is independent of TNF-α
Several cytokines have been reported to induce OX40L expression in a cell-type–dependent manner. For example, in professional antigen-presenting cells, OX40L can be induced by activating CD40, Toll-like receptors, thymic stromal lymphopoietin, and IL-18.37 In other cell types, such as natural killer, endothelial, mast, and smooth muscle cells, OX40L has been reported to be induced by several inflammatory cytokines, including TNF-α.37 We have previously reported that MGCD1013 can induce TNF-α production in HL cells.38 Because HDAC11 siRNA also induced TNF-α secretion in HL cell lines, we examined whether OX40L up-regulation is mediated by TNF-α in HL cells. We induced OX40L expression in HD-LM2 cells by incubating them with 1μM MGCD0103 for 24 hours (Figure 4B). Silencing TNF-α expression by siRNA, however, did not decrease MGCD0103-induced OX40L expression, suggesting that TNF-α may not be involved in MGCD0103-induced OX40L expression. Furthermore, incubating HD-LM2 cells with recombinant TNF-α did not have a significant effect on OX40L expression in these cells (Figure 4C). These data indicate that TNF-α does not play a significant role in inducing OX40L expression in HL cells.
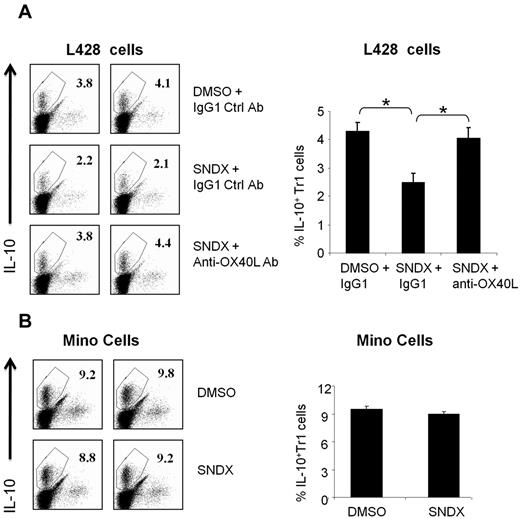
HDACI-induced OX40L inhibited the generation of IL-10–producing Tr1 cells
We previously reported that OX40L plays an important role in reversing peripheral tolerance by inhibiting the generation of IL-10–producing Tr1 cells.20 We examined whether HDACI-induced OX40L has a similar immunomodulatory function. We generated IL-10–producing Tr1 cells from CD4+ T cells as described previously.20 Tr1 cells were then cocultured with L428 HL cells or Mino MCL cells that were stimulated with either SNDX-275 or DMSO. The percentage of IL-10–producing Tr1 cells was reduced by 40%–50% in the presence of SNDX-275–treated L428 cells (Figure 5). This reduction of TR1 cells was specifically mediated by OX40L, because it was reversed with anti-OX40L blocking antibodies but not with nonspecific isotype control antibodies (Figure 5). A similar effect was seen with MGCD0103, although the effect was less prominent, because MGCD0103 was more lethal to the HL cells (data not shown). Finally, when Mino MCL cells were used instead of HL cells, there was no effect on the generation of IL-10–producing Tr1 cells regardless of whether they were stimulated with HDACI (Figure 5). Because HDACI did not up-regulate OX40L in MCL cells (Figure 1), these data further confirm that HDACI-induced OX40L can inhibit the immunosuppressive function of Tr1 cells.
HDACI-induced OX40L expression in HL blocks the generation of IL-10–producing Tr1 cells. CD4+ T cells were stimulated to differentiate into Tr1, as described in “Generation of IL-10–producing Tr1 cells,” in the presence of 6 × 104 Mino or L428 cells. (A) L428 cells were treated with DMSO or SNDX-275 (for 48 hours) in the presence of 1.5 μg/mL of anti-OX40L–neutralizing antibody or IgG1 control mAb. For the T-cell culture, cells were incubated with RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM L-glutamine, penicillin G, and streptomycin. T cells were harvested after 8 days of culture before the intracellular level of IL-10 was measured by FACS analysis. Results from 2 representative experiments are shown in the left panel; a summary graph of 4 independent experiments is shown in the right panel. Each value is the mean (± SEM). *P < .05. (B) Results for Mino cells are shown for comparison. Results from 2 representative experiments are shown in the left panel; a summary graph of 4 independent experiments is shown in the right panel. Each value is the mean (± SEM).
HDACI-induced OX40L expression in HL blocks the generation of IL-10–producing Tr1 cells. CD4+ T cells were stimulated to differentiate into Tr1, as described in “Generation of IL-10–producing Tr1 cells,” in the presence of 6 × 104 Mino or L428 cells. (A) L428 cells were treated with DMSO or SNDX-275 (for 48 hours) in the presence of 1.5 μg/mL of anti-OX40L–neutralizing antibody or IgG1 control mAb. For the T-cell culture, cells were incubated with RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM L-glutamine, penicillin G, and streptomycin. T cells were harvested after 8 days of culture before the intracellular level of IL-10 was measured by FACS analysis. Results from 2 representative experiments are shown in the left panel; a summary graph of 4 independent experiments is shown in the right panel. Each value is the mean (± SEM). *P < .05. (B) Results for Mino cells are shown for comparison. Results from 2 representative experiments are shown in the left panel; a summary graph of 4 independent experiments is shown in the right panel. Each value is the mean (± SEM).
Discussion
This study demonstrates for the first time that HDACis can up-regulate OX40L in HL cells by inhibiting HDAC11, and that HDACI-induced OX40L reverses the immunosuppressive function of Tr1 cells. These data provide an additional mechanistic rationale for the development of HDACI as a treatment strategy for HL. Our data also add to the complexity of HDACI mechanisms of action in cancer in general and in HL specifically. Previous studies focused on the direct antiproliferative effect of a variety of HDACis in a variety of cancer types.39-42 We recently reported that vorinostat and MGCD0103 may also work in HL by modulating key cytokines and chemokines in the microenvironment to create a favorable antitumor environment.16 The current study provides additional clues as to how HDACis may induce a favorably antitumor immune response. Furthermore, our data add to the complexity of the functional consequences of OX40 signaling, which may vary in different cell types and under different cytokine milieu.43-45 These data suggest that pharmacologic inhibition of HDACs may produce favorable clinical results in patients with HL by a direct antitumor effect (mainly by inhibiting HDAC1 and -2) and by an indirect immunomodulatory effect by reversing the immunosuppressive properties of T-reg cells (mainly by inhibiting HDAC11). Moreover, we have recently reported that HDACis may down-regulate the immunosuppressant PD-1 expression on peripheral blood T lymphomocytes in vivo,19 but the critical HDAC that mediates this effect remains unknown.
HDAC11, the sole member of HDAC class IV, was identified in 2002 as a new member of the zinc-dependent HDAC family and was found to contain conserved residues in the catalytic core regions that are shared with both class I and class II HDACs.46 Its transcripts are highly expressed in kidney, heart, brain, skeletal muscle, and testis. The function of HDAC11 remains poorly understood, although recent experiments indicated that it is expressed by antigen-presenting cells and is involved in regulating the immune response.36 Furthermore, the role of HDAC11 in regulating cell growth and survival is currently unknown, because there are no published data on the phenotype of HDAC11-knockout mice. Our data provide insight into the functional role of HDAC11 in HL. In addition to up-regulating OX40L, HDAC11 also played a role in regulating tumor cell survival, because silencing its expression resulted in apoptotic cell death. However, from a mechanistic point of view, it will be important in future studies to further dissect the pathway linking HDAC11 to OX40L and apoptosis.
From a clinical point of view, it will be important to validate our observations in the clinical setting in patients receiving treatment with HDACis by performing serial biopsies to examine the effect of HDACis on OX40L in vivo. Furthermore, our data raise the question of whether HDACis may also up-regulate OX40L expression in professional antigen-presenting cells. If so, then HDAC11-targeted therapy, either as a single modality or in conjunction with tumor vaccines, can be explored in the future to enhance the immune response against other types of cancers. Moreover, these data suggest that HDACis can be explored in 2 complimentary treatment strategies: in combination with chemotherapy and other anticancer agents to improve the remission rate and in an adjuvant setting after achieving clinical remission to prevent disease relapse by enhancing an effective antitumor immune response. Our results support examining new strategies in clinical trials using HDACis that inhibit HDAC11 function to restore antitumor immunity in patients with HL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Karen Muller for editorial assistance.
This work was supported in part by National Cancer Institute grant 1R21CA117070-01 (A.Y.); by Lymphoma Specialized Programs of Research Excellence (SPORE) grant 1P50CA136411-01A1 to (A.Y.); by the Clay Chiles Lymphoma Fund (A.Y.); by the Jack L. Stotsky Memorial Fund (A.Y); and by National Institutes of Health grants R01 AI065796-05 (H.M.-V.) and R01 AI061645-01 and U19 AI071130-01 (Y.-J.L.).
National Institutes of Health
Authorship
Contribution: D.B. and N.M.K. conceived of the study, performed the experiments, analyzed data, prepared the figures, and wrote the paper; K.S.V. performed experiments and prepared Figure 4; H.M.-V. performed experiments and helped prepare Figure 1; Y.-J.L. helped design experiments, analyzed data, and approved the final manuscript; and A.Y. conceived of the study, helped design the experiments, evaluated the results, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anas Younes, MD, Department of Lymphoma/Myeloma, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ayounes@mdanderson.org.
References
Author notes
D.B. and N.M.K contributed equally to the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal