Abstract
Transforming growth factor-β1 (TGF-β1) is the most important cytokine involved in the promotion of myelofibrosis. Mechanisms leading to its local activation in the bone marrow environment remain unclear. As a recent study has highlighted the role of thrombospondin-1 (TSP-1) in platelet-derived TGF-β1 activation, we investigated the role of TSP-1 in the TPOhigh murine model of myelofibrosis. Two groups of engrafted mice, WT TPOhigh and Tsp-1–null TPOhigh, were constituted. All mice developed a similar myeloproliferative syndrome and an increase in total TGF-β1 levels in the plasma and in extracellular fluids of marrow and spleen. Surprisingly, we were able to detect the active form of TGF-β1 in Tsp-1–null TPOhigh mice. Accordingly, these mice developed marrow and spleen fibrosis, with intriguingly a higher grade than in WT TPOhigh mice. Our results show that TSP-1 is not the major activator of TGF-β1 in TPO-induced myelofibrosis, suggesting the contribution of another mechanism in the megakaryocyte/platelet compartment.
Introduction
Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine involved in normal tissue repair, and its sustained production induces fibrosis in numerous organs,1 including myelofibrosis.2 Mechanisms leading to in vivo TGF-β1 activation (from inactive proforms) remain complex,3 involving interactions with integrins,4-6 thrombospondin-1 (TSP-1),7 or shear forces.8 In a murine model of myelofibrosis obtained by thrombopoietin (TPO) overexpression,9 enhanced release of TGF-β12 occurs mainly from megakaryocytes and, to a lesser extent, from monocytes.10 Because both TGF-β1 and TSP-1 are synthesized and stored in megakaryocyte α-granules,11 abnormal and concomitant release of both molecules may hypothetically lead to pathologic local TGF-β1 activation. As a recent study has highlighted the role of TSP-1 in platelet-derived TGF-β1 activation,12 we investigated the role of TSP-1 in TPO-induced myelofibrosis.
Methods
We used the TPOhigh murine model. All procedures were approved by the Institut Gustave Roussy ethics committee. Bone marrow (BM) cells from C57BL/6 wild-type (WT) or Tsp-1–null13 male littermates were infected with a retrovirus encoding murine TPO9 and engrafted into lethally irradiated WT or Tsp-1–null female hosts, respectively, leading to the following 2 engraftment combinations: WT/WT (ie, WT TPOhigh mice, n = 22) and Tsp-1–null/Tsp-1–null (ie, Tsp-1–null TPOhigh mice, n = 15). Lethally irradiated hosts were engrafted with 4 to 8 × 106 cells in 2 independent experiments. Peripheral blood was analyzed every 4 weeks during 3 months using an automated blood counter calibrated for mouse blood (MS9, Melet Schloessing). Control mice included unmanipulated WT and Tsp-1–null mice. At weeks 8 and 12 after engraftment, mice were killed for histologic analysis. Reticulin fibers revealed by silver staining were quantified using ImageJ Version 1.42q software (National Institutes of Health). Microvascular density (MVD) was determined by CD34 staining.14 TPO and TGF-β1 levels in plasma or extracellular fluids were determined with the appropriate Quantikine kits from R&D Systems.
Results and discussion
To assess the role of TSP-1 in the TGF-β1-induced BM fibrosis observed in TPOhigh mice, 2 groups of engrafted mice with BM TPO-transduced cells, WT TPOhigh and Tsp-1–null TPOhigh, were constituted. The transduction efficiency of the progenitor cells, evaluated with a colony-forming assay, was similar in the 2 mouse genotypes (77% ± 5%). Four weeks after engraftment, the TPO concentrations in plasma of WT and Tsp-1–null TPOhigh mice ranged from 1000- to 10 000-fold higher than in WT and Tsp-1–null control mice and remained elevated during the 3 months of follow-up (Figure 1A). The magnitude of this increase was comparable regardless of the TPOhigh mice groups. Chimerism, analyzed by fluorescent in situ hybridization on the Y chromosome (BM donors were male, recipients were female) on whole nucleated BM cells at weeks 8 and 12, when mice were killed for histologic analysis, showed levels more than 90% and similar in both TPOhigh mice groups (data not shown).
WT and Tsp-1–null mice display comparable TPO-induced myeloproliferative syndrome leading to similar TGF-β1 overproduction and activation. TPO level (A), platelet number (B), and leukocyte number (C) are shown in unmanipulated WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice during the 3 months of follow-up after engraftment. Results are the mean of 6 to 15 animals per experimental group. Results of statistical analysis with the Wilcoxon test are as follows: Tsp-1–null control mice versus WT control mice and Tsp-1–null TPOhigh mice versus WT TPOhigh mice: *P < .05. Total TGF-β1 (active + latent forms) levels were quantified in plasma (D) and extracellular fluids (E,G) using an enzyme-linked immunosorbent assay after acidification of samples. Active TGF-β1 levels in extracellular fluids (F,H) were determined without acidification. Total TGF-β1 plasma levels (D) are shown in WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice during the 3 months of follow-up after engraftment. No spontaneously active TGF-β1 was detected before acidification of the plasma samples. Total (E) and active TGF-β1 (F) levels in extracellular fluids of BM as well as total (G) and active TGF-β1 (H) levels in extracellular fluids of spleen are shown in WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice at times of death (8 and 12 weeks after engraftment). Note that the media supplemented with 10% fetal calf serum used to prepare extracellular fluids of BM and spleen contains less than 1.5 ng/mL TGF-β1 and no active form. Results in plasma and in extracellular fluids are presented as the mean of 6 to 15 and of 5 animals per experimental group, respectively. Results of statistical analysis with the Wilcoxon test are as follows: WT control mice versus Tsp-1–null control mice and WT TPOhigh mice versus Tsp-1–null TPOhigh mice: *P < .05.
WT and Tsp-1–null mice display comparable TPO-induced myeloproliferative syndrome leading to similar TGF-β1 overproduction and activation. TPO level (A), platelet number (B), and leukocyte number (C) are shown in unmanipulated WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice during the 3 months of follow-up after engraftment. Results are the mean of 6 to 15 animals per experimental group. Results of statistical analysis with the Wilcoxon test are as follows: Tsp-1–null control mice versus WT control mice and Tsp-1–null TPOhigh mice versus WT TPOhigh mice: *P < .05. Total TGF-β1 (active + latent forms) levels were quantified in plasma (D) and extracellular fluids (E,G) using an enzyme-linked immunosorbent assay after acidification of samples. Active TGF-β1 levels in extracellular fluids (F,H) were determined without acidification. Total TGF-β1 plasma levels (D) are shown in WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice during the 3 months of follow-up after engraftment. No spontaneously active TGF-β1 was detected before acidification of the plasma samples. Total (E) and active TGF-β1 (F) levels in extracellular fluids of BM as well as total (G) and active TGF-β1 (H) levels in extracellular fluids of spleen are shown in WT and Tsp-1–null control mice and in WT and Tsp-1–null TPOhigh mice at times of death (8 and 12 weeks after engraftment). Note that the media supplemented with 10% fetal calf serum used to prepare extracellular fluids of BM and spleen contains less than 1.5 ng/mL TGF-β1 and no active form. Results in plasma and in extracellular fluids are presented as the mean of 6 to 15 and of 5 animals per experimental group, respectively. Results of statistical analysis with the Wilcoxon test are as follows: WT control mice versus Tsp-1–null control mice and WT TPOhigh mice versus Tsp-1–null TPOhigh mice: *P < .05.
As expected from previous reports,2,9,10,15 all TPOhigh mice developed a similar myeloproliferative syndrome with thrombocytosis (Figure 1B), leukocytosis (Figure 1C), and splenomegaly associated with an increased number of blood and spleen progenitor cells (data not shown). However, Tsp-1–null TPOhigh mice showed increased thrombocytosis at week 4, which then turned out to be comparable with WT TPOhigh mice. It has been demonstrated that mice deficient in both TSP-1 and TSP-2 displayed a more rapid regeneration of thrombopoiesis after myelosuppression,16 extending previous in vitro results that indicated that TSPs negatively regulate megakaryopoiesis.17 Here, we cannot exclude that TSP-1 deficiency shortens platelet recovery under TPO stimulation. Nevertheless, we did not observe a further increase of megakaryocyte hyperplasia or thrombocytosis in Tsp-1–null TPOhigh mice compared with WT TPOhigh mice during disease progression.
Because development of fibrosis has been reported to be a direct consequence of high TGF-β1 levels in TPOhigh mice2 and TSP-1 has been demonstrated to activate TGF-β1,7 we measured total and active forms of TGF-β1 in plasma and extracellular fluids from WT and Tsp-1–null TPOhigh mice BM and spleen. Control Tsp-1–null mice exhibited a statistically significant increase in total TGF-β1 (Figure 1D) as formerly reported on the C57BL/6 background.18 As expected, the level of total TGF-β1 in the plasma of TPOhigh mice increased as early as 4 weeks after engraftment and reached a level 2- to 4-fold higher than in control mice by week 12. No active form was detected in the plasma from control or TPOhigh mice. Moreover, we observed an increased level in both total and active TGF-β1 in extracellular fluids from BM (Figure 1E-F) and spleen (Figure 1G-H) in TPOhigh mice compared with control mice. Surprisingly, we were able to detect the active form of TGF-β1 in Tsp-1–null TPOhigh mice, suggesting that alternative mechanisms are responsible for local TGF-β1 activation in this model of myelofibrosis.
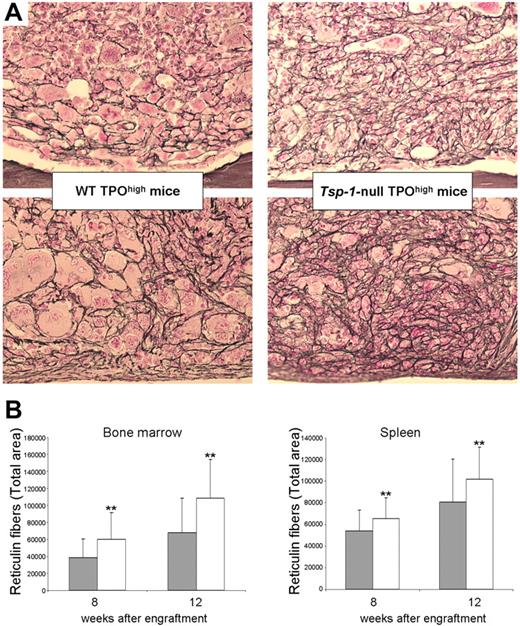
As predicted by the detection of the active form of TGF-β1, Tsp-1–null TPOhigh mice developed BM and spleen fibrosis (Figure 2A), with, intriguingly, a greater grade than in WT TPOhigh mice (Figure 2B). Our results show that local activation of TGF-β1 in the hematopoietic environment leading to BM and spleen fibrosis occurs in the absence of TSP-1, according to a previous study reporting that TSP-1 was not a major activator of TGF-β1 in platelets.18 However, Ahamed et al recently demonstrated that TSP-1 contributes to TGF-β1 activation in both serum and platelet releasates under conditions of stirring and/or shear.12 Such mechanisms are probably not involved within the BM environment. TSP-1 has been clearly demonstrated to be the major activator of TGF-β1 in lung and renal fibrosis.19,20 However, one can speculate that TGF-β1 could be regulated in a tissue-specific way, particularly in the megakaryocyte/platelet compartment. Numerous studies have shed light on the major role of integrins in TGF-β1 activation.4-6 We hypothesize that β3 integrins expressed by platelets and megakaryocytes21 may compensate for the absence of TSP-1 in this model.
Increased BM and spleen fibrosis in Tsp-1–null TPOhigh mice. (A) Representative histologic sections of femurs (top panels) and spleen (bottom panels) from WT TPOhigh mice or Tsp-1–null TPOhigh mice at week 12 after engraftment. Silver staining reveals BM and spleen fibrosis in both TPOhigh groups of mice. Images were obtained using a Leica DMRB microscope (total original magnification ×250) 25×/0.85 NA objective and acquired with a Video 3 charge-coupled device Sony Leica Power hole accumulated diode camera. (B) An increased fibrosis was observed in both BM and spleen from Tsp-1–null TPOhigh mice (□) compared with WT TPOhigh mice ( ) at weeks 8 or 12 after engraftment. Total area of reticulin fibers was quantified using ImageJ software. Results of statistical analysis with the Wilcoxon test are as follows: WT TPOhigh mice versus Tsp-1–null TPOhigh mice: **P < .001.
) at weeks 8 or 12 after engraftment. Total area of reticulin fibers was quantified using ImageJ software. Results of statistical analysis with the Wilcoxon test are as follows: WT TPOhigh mice versus Tsp-1–null TPOhigh mice: **P < .001.
Increased BM and spleen fibrosis in Tsp-1–null TPOhigh mice. (A) Representative histologic sections of femurs (top panels) and spleen (bottom panels) from WT TPOhigh mice or Tsp-1–null TPOhigh mice at week 12 after engraftment. Silver staining reveals BM and spleen fibrosis in both TPOhigh groups of mice. Images were obtained using a Leica DMRB microscope (total original magnification ×250) 25×/0.85 NA objective and acquired with a Video 3 charge-coupled device Sony Leica Power hole accumulated diode camera. (B) An increased fibrosis was observed in both BM and spleen from Tsp-1–null TPOhigh mice (□) compared with WT TPOhigh mice ( ) at weeks 8 or 12 after engraftment. Total area of reticulin fibers was quantified using ImageJ software. Results of statistical analysis with the Wilcoxon test are as follows: WT TPOhigh mice versus Tsp-1–null TPOhigh mice: **P < .001.
) at weeks 8 or 12 after engraftment. Total area of reticulin fibers was quantified using ImageJ software. Results of statistical analysis with the Wilcoxon test are as follows: WT TPOhigh mice versus Tsp-1–null TPOhigh mice: **P < .001.
Unexpectedly, the fibrosis observed in Tsp-1–null TPOhigh mice was more enhanced than the one observed in WT TPOhigh mice (Figure 2B). To explain this surprising finding, we first hypothesized that this increased fibrosis was related to an augmentation of neoangiogenesis14 mediated by the antiangiogenic function of TSP-1.16 We therefore analyzed the MVD in BM. MVD in control Tsp-1–null was higher than in control WT (10 ± 4.7 vs 3.4 ± 1.6; P < .001), as expected. However, MVD observed in Tsp-1–null TPOhigh mice (8.3 ± 4.4) did not rise above the one observed in control Tsp-1–null mice and was similar to MVD observed in WT TPOhigh mice (5.7 ± 2.9). Thus, the increase of myelofibrosis in Tsp-1–null TPOhigh mice cannot be explained by an augmentation of neoangiogenesis. We then hypothesized that the increase of BM fibrosis in Tsp-1–null TPOhigh mice could be related to an enhanced TGF-β1-mediated response by Tsp-1–null BM stromal cells. Supporting this hypothesis, it has been demonstrated that β3−/− mice exhibit a sustained TGF-β1 activation via an increase of TGF-β1 receptors I and II expression, leading to enhanced wound healing with reepithelialization.22 To this purpose, we compared the expression of several TGF-β1-signaling pathway members in Tsp-1–null and WT BM stromal cells. Unfortunately, we did not clearly demonstrate a sustained TGF-β1 activation nor an increase in TGF-β1 receptors or collagen expression in Tsp-1–null stromal cells (data not shown). Thus, mechanisms leading to the increased myelofibrosis in Tsp-1–null mice remain unclear. However, similar greater amounts of fibrosis have been recently reported in lung of Tsp-1–null mice after bleomycin treatment.23
Together, these results show that TSP-1 is not the major activator of TGF-β1 in TPO-induced BM fibrosis, suggesting the contribution of another mechanism in the megakaryocyte/platelet compartment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the animal facilities directed by Patrick Gonin, in particular Benoît Petit.
This work was supported by Inserm and La Ligue Nationale contre le Cancer (équipe labellisée 2004 and 2007). S.E. was supported by Association pour la Recherche sur le Cancer (fellowship).
Authorship
Contribution: S.E. and O.W.-B. performed animal studies, enzyme-linked immunosorbent assay, fluorescence in situ hybridization, and Western blotting; O.B. performed mRNA analysis; M.T. performed histologic analysis; P.R. performed reticulin fiber quantification; P.G. performed statistical analysis; E.Z. and J.P. performed angiogenesis analysis; A.B. provided TSP-1null mice; J.-L.V. provided MPZenTPO virus-producing GP + E86 cells; W.V. and S.G. designed studies and analyzed data; and O.W.-B. analyzed data, generated figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Orianne Wagner-Ballon, Inserm U1009, Institut Gustave Roussy, PR1, 114 rue Edouard Vaillant, 94805 Villejuif, France; e-mail: orianne.wagnerballon@hmn.aphp.fr.