Abstract
Notch receptor-mediated signaling is involved in the developmental process and functional modulation of lymphocytes, as well as in mast cell differentiation. Here, we investigated whether Notch signaling is required for antipathogen host defense regulated by mast cells. Mast cells were rarely found in the small intestine of wild-type C57BL/6 mice but accumulated abnormally in the lamina propria of the small-intestinal mucosa of the Notch2-conditional knockout mice in naive status. When transplanted into mast cell–deficient Wsh/Wsh mice, Notch2-null bone marrow-derived mast cells were rarely found within the epithelial layer but abnormally localized to the lamina propria, whereas control bone marrow-derived mast cells were mainly found within the epithelial layer. After the infection of Notch2 knockout and control mice with L3 larvae of Strongyloides venezuelensis, the abundant number of mast cells was rapidly mobilized to the epithelial layer in the control mice. In contrast, mast cells were massively accumulated in the lamina propria of the small intestinal mucosa in Notch2-conditional knockout mice, accompanied by impaired eradication of Strongyloides venezuelensis. These findings indicate that cell-autonomous Notch2 signaling in mast cells is required for proper localization of intestinal mast cells and further imply a critical role of Notch signaling in the host-pathogen interface in the small intestine.
Introduction
Mast cells are important in a wide variety of physiologic and pathologic processes, including protective immune responses to parasites and allergic disorders.1,2 In intestinal parasite infection, mast cells play a central role in the immune response.3 During the induction phase of parasite-induced inflammation, mast cells move from the submucosa to the tip of the villi, accompanying the serial changes in the protease expression pattern. Initially, they are positive for mouse mast cell protease-5 (mMCP-5) but negative for mMCP-1 and mMCP-2; eventually, they become positive for mMCP-1 and mMCP-2 but negative for mMCP-5, demonstrating convergence from connective tissue–type mast cells (CTMCs) to mature mucosal-type mast cells (MTMCs).4 The parasite-infected mice consequently experience jejunal mast cell hyperplasia,5 and the serum concentration of mMCP-1, an activation marker of small intestinal mast cells, is increased by > 1000-fold compared with that in the naive status.5
In the mammalian immune system, we and other groups have demonstrated that Notch signaling is involved in the commitment and differentiation of T cells, the development of splenic marginal zone B cells, and the differentiation and functional modulation of mature T cells, including T-helper type I (Th1)/Th2 polarization6,7 and differentiation of CD8-positive cytotoxic T cells.8 Regarding the Notch signaling in mast cells, bone marrow-derived mast cells (BMMCs) highly express Jagged19 and Notch210 among the Notch ligands and the receptors, respectively. We have previously shown that signaling through the Notch2 receptor induces mast cell development from myeloid progenitors by transcriptional up-regulation of hairy and enhancer of split homolog-1 (Hes-1) and transacting T cell–specific transcription factor GATA-3 (GATA3).11 Induction of antigen-presenting potential of mast cells by Notch signaling is also demonstrated.12 A question yet to be solved is how Notch signaling affects mast cell properties in vivo.
In this report, we examined the effect of Notch2 signaling in in vivo mast cells using Notch2-conditional knockout mice.13 We show that Notch2 signaling is specifically required for intraepithelial localization of intestinal mast cells and antiparasite immunity. In contrast, Notch2 is dispensable for either distribution or development of CTMCs.
Methods
Mice
The generation of Notch2flox/flox mice was described previously.13 Mx-Cre transgenic mice14 were crossed with Notch2flox/flox mice (N2-MxcKO mice) and the progeny were injected with polyinosinic-polycytidylic acid (pIpC; Sigma-Aldrich) 7 times every other day from 3 days after birth (25 μg/g body weight) or 3 times between 4 and 6 weeks of age (20 μg/g body weight). N2-MxcKO mice were further crossed with C57BL/6-Ly5.1 mice (a kind gift from Dr H. Nakauchi, University of Tokyo) to generate Ly5.1-N2-MxcKO mice. Notch2 deletion in bone marrow was examined by polymerase chain reaction and 3% agarose gel electrophoresis13 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Wsh/Wsh mice were purchased from The Jackson Laboratory. All experiments were done with approval from the University of Tsukuba Institutional Review Board.
Staining
Sections, fixed with Carnoid fluid, were stained with 0.5% toluidine blue (Sigma-Aldrich), pH 0.3, followed by eosin. Small intestine was embedded in optimal cutting temperature (OCT) compound (TissueTek) and cut with cryostat (Leica CM1850). The section was fixed with 4% paraformaldehyde, washed with phosphate-buffered saline (PBS), blocked in 10% horse serum and 0.1% Triton-PBS, and then stained with either 1:100 goat anti-Jagged1 antibody (C-20; Santa Cruz Biotechnology), goat anti-Delta1 antibody (Genzyme Tech), or control goat immunoglobulin G (IgG; Santa Cruz Biotechnology) overnight at 4°C. The sections were washed with PBS and stained with anti–goat Alexa 594 (Invitrogen). Sections were analyzed by fluorescence microscope (Zeiss; Axioplan2), original magnification ×200.
BMMCs
Bone marrow cells from each mouse strain were cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 50 ng/mL stem cell factor (SCF; PeproTech), and 10 ng/mL interleukin-3 (IL-3; PeproTech) for 4 weeks. Generation of BMMCs was confirmed by staining with lineage markers, c-Kit and IgE, as previously described.11 Briefly, the cells were incubated with purified IgE (BD Biosciences) after blocking the Fcγ receptors with purified anti-CD16/32 antibody (BD Biosciences), stained with anti-IgE–fluorescein isothiocyanate (FITC; BD Biosciences), anti–Gr-1–phycoerythrin (PE), anti-Mac1–PE (eBioscience), and anti–c-Kit–allophycocyanin (APC; eBioscience), and then analyzed by FACSCalibur (BD Biosciences).
Peritoneal mast cells
Five milliliters ice-cold PBS was injected into the peritoneal cavity, and then 3 mL PBS was recovered. c-Kit and IgE receptor (FcϵRI) expression was used to define the cells as peritoneal mast cells. Ly5.1 and Notch2 were stained with anti-Ly5.1–PE (BD Biosciences) or biotinylated anti-Notch2 antibody (clone HMN2-35)8 followed by streptavidin PE (eBioscience), respectively.
Bone marrow transplantation
C57BL/6 mice and Wsh/Wsh mice were lethally irradiated with a total dose of 9.5 Gy and then transplanted with 1 × 107 whole bone marrow cells from either N2-MxcKO-Ly5.1 mice or Notch2flox/flox-Ly5.1 mice from the tail vein. Tissues of transplanted mice were assessed at 3 to 4 months after transplantation. Donor-cell engraftment was assessed by fluorescence-activated cell sorting (FACS) analysis of peripheral blood, which was stained by anti-Ly5.2–FITC (BD Biosciences) and anti-Ly5.1–PE.
S venezuelensis infection
Mice were infected by subcutaneous injection of third-stage infective larvae of Strongyloides venezuelensis. The degree of infection was monitored by counting the number of eggs per gram of feces. Mast cells were counted and presented as the number per 10 villus crypt units. BMMCs were washed with PBS twice and then cultured with 10 ng/mL IL-4 and 10 ng/mL IL-10 for 3 days. These Th2-conditioned BMMCs were injected at day 3 and day 6 of experiments.15 In contrast to the bone marrow transplantation, mice were not irradiated before BMMC injection.
Statistical analysis
The data for the number of mast cells and the S venezuelensis infection data were analyzed by the t test. P values < .05 were considered significant.
Results
Notch signaling affects the number and localization of mast cells in the small intestine
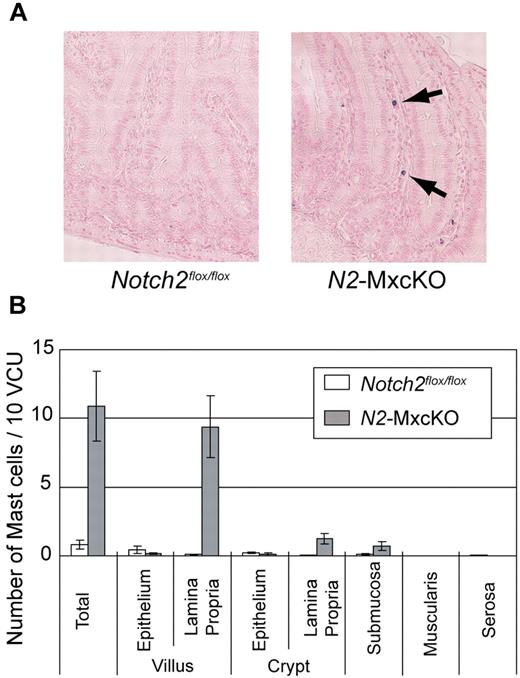
We have previously reported that Notch2 regulates mast cell differentiation in vitro.11 To examine whether Notch2 controls the differentiation or development of MTMCs in vivo, we examined intestinal mast cells by toluidine blue staining in C57BL/6 mice carrying the Notch2flox/flox allele with or without the Mx1-Cre transgene (N2-MxcKO mice or Notch2flox/flox mice, respectively) after pIpC treatment.13 Mast cells were only sparsely detected in the small intestine of Notch2flox/flox mice, mainly within the epithelium. However, the total number of mast cells in the small intestine of N2-MxcKO mice was unanticipatedly greater than that of Notch2flox/flox mice. Furthermore, those mast cells were mainly localized to the lamina propria, and very few mast cells were found within the epithelium (Figure 1A-B).
Mature mast cells were abnormally accumulated in the lamina propria of the small intestine of Notch2-deficient mice. (A) Sections of the small intestine of N2-MxcKO or littermate control Notch2flox/flox mice. Toluidine blue staining, followed by eosin. Original magnification ×200. (B) The numbers of mast cells per 10 villus crypt units (vcus) distributing to various layers of the small intestine. Data are presented as means ± SEM; Notch2flox/flox (n = 10) versus N2-MxcKO (n = 8); P = .000461 (total), P = .000261 (villus, lamina propria), P = .001918 (crypt, lamina propria), P = .046874 (submucosa).
Mature mast cells were abnormally accumulated in the lamina propria of the small intestine of Notch2-deficient mice. (A) Sections of the small intestine of N2-MxcKO or littermate control Notch2flox/flox mice. Toluidine blue staining, followed by eosin. Original magnification ×200. (B) The numbers of mast cells per 10 villus crypt units (vcus) distributing to various layers of the small intestine. Data are presented as means ± SEM; Notch2flox/flox (n = 10) versus N2-MxcKO (n = 8); P = .000461 (total), P = .000261 (villus, lamina propria), P = .001918 (crypt, lamina propria), P = .046874 (submucosa).
Localization of MTMCs is abnormal in wild-type mice transplanted with N2-MxcKO bone marrow cells, reminiscent of that in N2-MxcKO mice
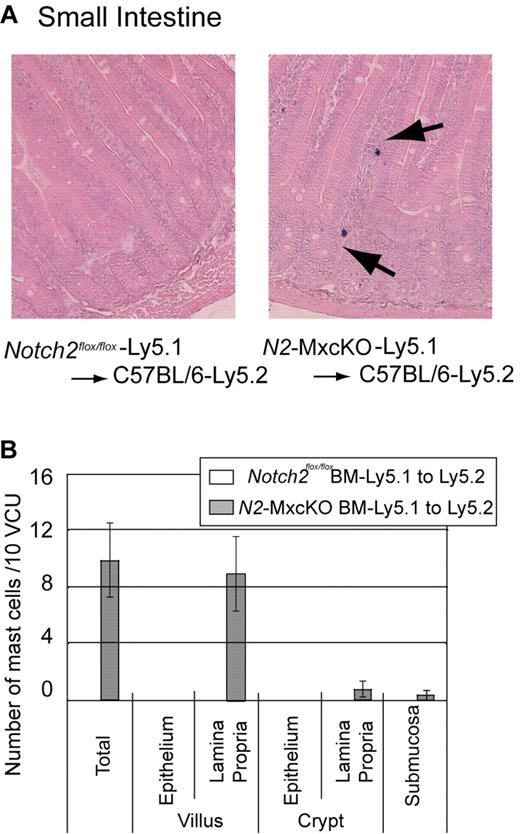
Because the Mx-Cre–based conditional knockout system deletes target genes not only in the bone marrow cells but also, albeit partially, in the intestinal cells,14 there was a possibility that Notch2 deletion in the intestinal cells was responsible for the distinct distribution pattern or increased number of mast cells in N2-MxcKO mice compared with control mice. To exclude this possibility, we transplanted Notch2-null bone marrow cells carrying the Ly5.1 marker to irradiated wild-type C57BL/6-Ly5.2 mice. A chimerism of donor-derived Ly5.1-positive fraction accounted for more than 70% in the peripheral blood (data not shown). The recipients of bone marrow cells from Notch2flox/flox mice showed that the intestinal mast cell distribution was virtually the same as that in wild-type mice, whereas the recipients of Notch2-null bone marrow cells showed an increase in mast cells mainly in the lamina propria in an indistinguishable manner from the N2-MxcKO mice (Figure 2A-B). This result indicates that deletion of Notch2 in bone marrow-derived cells alters the distribution pattern and increases the number of mast cells in the small intestine.
Localization of intestinal mast cells is abnormal in wild-type mice transplanted with N2-MxcKO-Ly5.1 bone marrow cells, reminiscent of that in N2-MxcKO mice. (A) Bone marrow cells from either N2-MxcKO- Ly5.1 mice or littermate Notch2flox/flox- Ly5.1 mice were transplanted into lethally irradiated (9.5 Gy) C57BL/6-Ly5.2 mice. Toluidine blue staining, followed by eosin. Original magnification ×200. (B) The numbers of mast cells per 10 vcus distributing to various layers of the small intestine. Data are presented as means ± SEM; Mast cells in C57BL/6-Ly5.2 mice transplanted with Notch2flox/flox-Ly5.1 (n = 3) versus N2-MxcKO-Ly5.1 (n = 3). P = .020594 (total) and P = .030123 (villus, lamina propria).
Localization of intestinal mast cells is abnormal in wild-type mice transplanted with N2-MxcKO-Ly5.1 bone marrow cells, reminiscent of that in N2-MxcKO mice. (A) Bone marrow cells from either N2-MxcKO- Ly5.1 mice or littermate Notch2flox/flox- Ly5.1 mice were transplanted into lethally irradiated (9.5 Gy) C57BL/6-Ly5.2 mice. Toluidine blue staining, followed by eosin. Original magnification ×200. (B) The numbers of mast cells per 10 vcus distributing to various layers of the small intestine. Data are presented as means ± SEM; Mast cells in C57BL/6-Ly5.2 mice transplanted with Notch2flox/flox-Ly5.1 (n = 3) versus N2-MxcKO-Ly5.1 (n = 3). P = .020594 (total) and P = .030123 (villus, lamina propria).
Notch-ligand expression in the small intestine
Notch signaling is known to be activated through Notch ligand-receptor binding.16 We examined the expression pattern of Notch ligands in the small intestine with antibodies against Notch ligands Jagged1 and Delta1 and found that the epithelial layer was clearly stained with anti-Jagged1 but not with anti-Delta1 antibody (Figure 3). The staining with the anti-Jagged1 antibody was confined to the surface of epithelial cells, especially at their basal side rather than the apical side (Figure 3). The Jagged1 expression pattern suggests a possibility that Jagged1-Notch2 interaction between the basal side of the epithelial cells and mast cells has an important role for mast cell migration from the lamina propria across the basement membrane toward the epithelium (Figure 3). Furthermore, the ligand-receptor binding itself might contribute to mast cell–epithelial cell adhesion to some extent, based on our observation that Notch2-expressing BMMCs attached to the Jagged1-expressing Chinese hamster ovary (CHO) cells, while Notch2-null BMMCs did not (supplemental Figure 2).
Jagged1 is strongly expressed on the surface of the epithelial cells, especially at their basal side. A section of small intestine prepared using cryostat was stained with goat anti-Jagged1 and goat anti-Delta1 antibodies followed by anti–goat Alexa594. Original magnification ×200.
Jagged1 is strongly expressed on the surface of the epithelial cells, especially at their basal side. A section of small intestine prepared using cryostat was stained with goat anti-Jagged1 and goat anti-Delta1 antibodies followed by anti–goat Alexa594. Original magnification ×200.
Notch2 is dispensable for the CTMC development and distribution
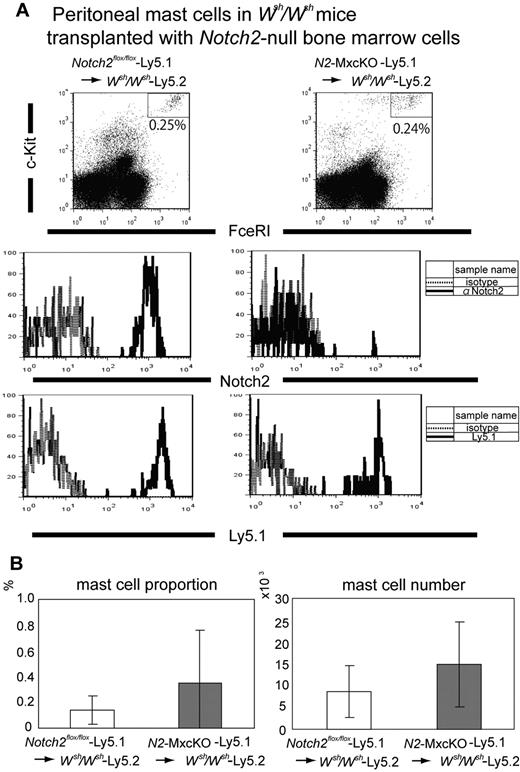
We next investigated the roles of Notch2 in the development of CTMCs. The localization and the number of CTMCs in the skin and peritoneal cavity were not significantly different between N2-MxcKO and littermate Notch2flox/flox mice more than 4 weeks after the treatment with pIpC (data not shown). This observation might simply indicate that the Mx-Cre system was inefficient in the tissue-resident mast cells, as a great majority of peritoneal mast cells of pIpC-treated N2-MxcKO mice still expressed Notch2 (data not shown). Therefore, to clarify the requirement of Notch2 in the CTMC development, we examined peritoneal mast cells in mast cell-deficient Wsh/Wsh mice after transplantion of Notch2-null bone marrow cells carrying the Ly5.1 marker. In this system, mast cells exclusively develop from transplanted bone marrow progenitors, in which the Cre recombinase under the Mx-promoter is quite effective 14 (supplemental Figure 1). In this experiment, we found that the proportion and absolute number of peritoneal mast cells was not significantly different between those developed from the N2-MxcKO-Ly5.1 bone marrow cells and those developed from littermate Notch2flox/flox-Ly5.1 bone marrow cells (Figure 4A-B). Notch2 was not expressed in the peritoneal mast cells derived from N2-MxcKO-Ly5.1 bone marrow cells but was expressed in those derived from littermate Notch2flox/flox-Ly5.1 bone marrow cells (Figure 4A middle), indicating that Notch2 was deleted efficiently. These results suggest that Notch2 is dispensable for the development and distribution of CTMCs.
Notch2 is not required for peritoneal mast cell development. (A) Bone marrow cells from N2-MxcKO-Ly5.1 mice or control Notch2flox/flox-Ly5.1 mice were transplanted into lethally irradiated Wsh/Wsh mice. Peritoneal mast cells were stained with anti–c-Kit–APC, IgE, and biotinylated anti-Notch2 antibody (HMN2-35), followed by anti-IgE–FITC and streptavidin-PE, or they were stained with anti–c-Kit–APC, IgE, and anti-Ly 5.1–PE, followed by anti-IgE–FITC; they were then analyzed by FACSCalibur (BD Biosciences). (B) The proportion (left) and the absolute number (right) of peritoneal mast cells were not significantly different between Wsh/Wsh mice transplanted with Notch2-WT bone marrow cells and those transplanted with Notch2-null bone marrow cells. P = .210642 (mast cell proportion) and P = .196045 (mast cell number).
Notch2 is not required for peritoneal mast cell development. (A) Bone marrow cells from N2-MxcKO-Ly5.1 mice or control Notch2flox/flox-Ly5.1 mice were transplanted into lethally irradiated Wsh/Wsh mice. Peritoneal mast cells were stained with anti–c-Kit–APC, IgE, and biotinylated anti-Notch2 antibody (HMN2-35), followed by anti-IgE–FITC and streptavidin-PE, or they were stained with anti–c-Kit–APC, IgE, and anti-Ly 5.1–PE, followed by anti-IgE–FITC; they were then analyzed by FACSCalibur (BD Biosciences). (B) The proportion (left) and the absolute number (right) of peritoneal mast cells were not significantly different between Wsh/Wsh mice transplanted with Notch2-WT bone marrow cells and those transplanted with Notch2-null bone marrow cells. P = .210642 (mast cell proportion) and P = .196045 (mast cell number).
Cell-autonomous Notch2 signaling in mast cells is important for mast cell migration across the basement membrane in the small intestine
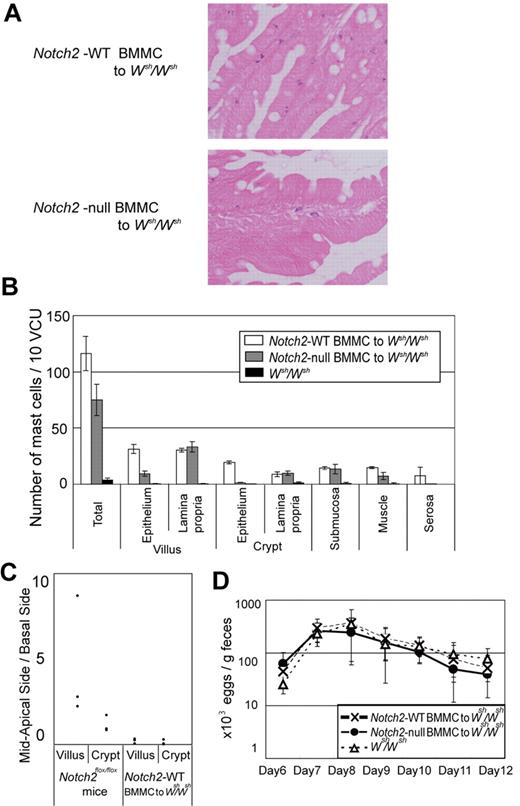
We then asked a question whether aberrant mast cell migration in the small intestine in N2-MxcKO mice is dependent on Notch2 signaling in mast cells per se. We intravenously infused Notch2-null or control BMMCs into nonirradiated Wsh/Wsh mice after S venezuelensis infection, because it is reported that BMMCs could only transiently reconstitute intestinal mast cells in mast-cell deficient mice if these recipient mice are in naive status.17 In tissue sections, we found that the distribution of mast cells in the small intestine was different between control BMMCs-reconstituted mice and Notch2-null BMMCs-reconstituted mice; control BMMCs were mainly migrated into the epithelial layer, while a majority of Notch2-null BMMCs remained in the lamina propria. This observation indicates that mast cell-autonomous Notch2 expression contributes to mast cell migration across the basement membrane from lamina propria into the epithelial layer (Figure 5A-B). Even in the control BMMC-infused mice, however, a substantial proportion of mast cells still remained in the lamina propria, submucosa, and smooth muscle layers, and the distribution of mast cells within the epithelium was confined to the basement membrane side of the epithelial layer (Figure 5B-C). This mast cell localization pattern was different from that in the Notch2flox/flox mice with S venezuelensis infection, in which mast cells were present mainly at the mid to apical side of the epithelial layer (Figure 5C). The numbers of S venezuelensis eggs in the stool were virtually the same in the S venezuelensis–infected Wsh/Wsh mice infused with Notch2-null and control BMMCs and in the S venezuelensis–infected Wsh/Wsh mice without any BMMC infusion throughout the period after infection (Figure 5D).
Mast cell–autonomous Notch2 expression is required for mast cell migration toward the epithelium. Wsh/Wsh mice infected with S venezuelensis were intravenously infused with Th2-conditioned Notch2-null or control BMMCs on days 3 and 6 of infection. (A) Notch2-null BMMCs poorly migrated toward the epithelium compared with control BMMCs. Toluidine blue staining followed by eosin staining. Original magnification ×200. (Top) Control BMMCs; (Bottom) Notch2-null BMMCs. (B) The number of mast cells per 10 vcus in the small intestine on day 12 after S venezuelensis infection in Wsh/Wsh mice, without BMMC infusion, with control BMMC infusion, and with Notch2-null BMMC infusion. Data are presented as means ± SEM; n = 3 (control BMMC infusion) and n = 4 (Notch2-null BMMC infusion), P = .004080 (villus, epithelium) and P = .000020 (crypt, epithelium). Note that mast cells in Wsh/Wsh mice infused with Notch2-null BMMCs abnormally resided in the lamina propria, whereas most of those in Wsh/Wsh mice infused with control BMMCs had intraepithelially migrated. (C) Mast cell number in mid to apical side of the epithelial layer was divided with that in the basal side of the epithelial layer. (D) Time course of S venezuelensis egg numbers in the stool. The number of excreted eggs was not significantly different between Wsh/Wsh mice infused with Notch2-null and control BMMCs. Data are presented as means ± SEM.
Mast cell–autonomous Notch2 expression is required for mast cell migration toward the epithelium. Wsh/Wsh mice infected with S venezuelensis were intravenously infused with Th2-conditioned Notch2-null or control BMMCs on days 3 and 6 of infection. (A) Notch2-null BMMCs poorly migrated toward the epithelium compared with control BMMCs. Toluidine blue staining followed by eosin staining. Original magnification ×200. (Top) Control BMMCs; (Bottom) Notch2-null BMMCs. (B) The number of mast cells per 10 vcus in the small intestine on day 12 after S venezuelensis infection in Wsh/Wsh mice, without BMMC infusion, with control BMMC infusion, and with Notch2-null BMMC infusion. Data are presented as means ± SEM; n = 3 (control BMMC infusion) and n = 4 (Notch2-null BMMC infusion), P = .004080 (villus, epithelium) and P = .000020 (crypt, epithelium). Note that mast cells in Wsh/Wsh mice infused with Notch2-null BMMCs abnormally resided in the lamina propria, whereas most of those in Wsh/Wsh mice infused with control BMMCs had intraepithelially migrated. (C) Mast cell number in mid to apical side of the epithelial layer was divided with that in the basal side of the epithelial layer. (D) Time course of S venezuelensis egg numbers in the stool. The number of excreted eggs was not significantly different between Wsh/Wsh mice infused with Notch2-null and control BMMCs. Data are presented as means ± SEM.
Taken together, the BMMC-Wsh/Wsh transplantation model demonstrated that Notch2 in the mast cells indeed determines their intraepithelial migration from lamina propria; nevertheless, this model was not adequate to examine the physiologic mast cell distribution pattern and subsequent parasite expulsion that depends on mast cells.
Notch2 signaling regulates antiparasite immunity of mast cells in the intestine
The BMMC-Wsh/Wsh reconstitution model could not completely reflect physiologic mast cell distribution pattern in the small intestine. Therefore, to further assess the effect of Notch2 signaling on the mucosal immune response of intestinal mast cells under a pathologic condition, N2-MxcKO or control Notch2flox/flox mice were infected with S venezuelensis. Total mast cell number was increased in Notch2flox/flox mice much more than in N2-MxcKO mice, especially in the epithelium in both crypts and villi 8 days after infection (Figure 6A-B). Thirteen days after infection, mast cells in the epithelium in Notch2flox/flox mice were still more abundant than those in N2-MxcKO mice (Figure 6C-D), while mast cell accumulation in the lamina propria in N2-MxcKO mice was more prominent in both villi and crypt than that in the earlier stage of infection (Figure 6A,C). In particular, dense aggregation of mast cells was prominent in the lamina propria of N2-MxcKO mice at the tip of the villi (Figure 6D). As a consequence, the total number of mast cells in the intestine of N2-MxcKO mice became equivalent to those of Notch2flox/flox mice 13 days after infection (Figure 6C,E). The number of S venezuelensis eggs in the stool was gradually decreased during day 8 to 10 in control Notch2flox/flox mice but not in N2-MxcKO mice (Figure 6F). Furthermore, the worms were still observed in N2-MxcKO mice but not in Notch2flox/flox mice 12 days after infection (Figure 6G). These data suggest that Notch2 deficiency alters the distinct distribution pattern of mast cells in the small intestine, which is responsible for the defective eradication of S venezuelensis.
Notch2 is essential for antiparasite immunity of mast cells in the intestine. N2-MxcKO or control Notch2flox/flox mice were subcutaneously injected with third-stage infective larvae of S venezuelensis. (A) The number of mast cells per 10 vcus in the small intestine on day 8 after S venezuelensis infection. Data are presented as means ± SEM. The number of mast cells was much less in N2-MxcKO mice; n = 3, P = .008592 (total), P = .005695 (villus, epithelium), P = .000715 (villus, lamina propria), P = .005245 (crypt, epithelium), and P = .045466 (crypt, lamina propria). Note that mast cells in N2-MxcKO mice were abnormally clustered in the lamina propria, whereas most of those in the control Notch2flox/flox mice were intraepithelially migrated. (B) Toluidine blue staining followed by eosin staining of the small intestine on day 8; original magnification ×200. (C) The number of mast cells per 10 vcus in the small intestine on day 13 after S venezuelensis infection. Data are presented as means ± SEM; n = 3, P = .026076 (villus, epithelium), P = .00194 (villus, lamina propria), P = .021177 (crypt, epithelium), and P = .019324 (crypt, lamina propria), P = .047445 (submucosa). (D) Toluidine blue staining followed by eosin staining of the small intestine on day 13. Original magnification ×200. (E) The total number of mast cells per 10 vcus on day 0, day 8, and day 13 of infection. The total number of mast cells was significantly lower in N2-MxcKO mice at the early phase (day 8) and almost equal at the later phase (day 13) to that of control mice. Data are presented as means ± SEM; n = 10 and 8 (day 0, Notch2flox/flox and N2-MxcKO); n = 3 and 3 (day 8, Notch2flox/flox and N2-MxcKO); n = 4 and 4 (day 13, Notch2flox/flox and N2-MxcKO). (F) Time course of egg number in the stool. The number of excreted eggs was significantly greater in N2-MxcKO mice compared with those in Notch2flox/flox mice. Data are represented as means ± SEM; n = 4; P = .0291 (day 8) and P = .0219 (day 9). (G) Hematoxylin-eosin staining of the small intestine on day 12. Original magnification ×200. Arrows indicate worms. Worms were still observed in the villi in the jejunum of N2-MxcKO, but not of Notch2flox/flox mice.
Notch2 is essential for antiparasite immunity of mast cells in the intestine. N2-MxcKO or control Notch2flox/flox mice were subcutaneously injected with third-stage infective larvae of S venezuelensis. (A) The number of mast cells per 10 vcus in the small intestine on day 8 after S venezuelensis infection. Data are presented as means ± SEM. The number of mast cells was much less in N2-MxcKO mice; n = 3, P = .008592 (total), P = .005695 (villus, epithelium), P = .000715 (villus, lamina propria), P = .005245 (crypt, epithelium), and P = .045466 (crypt, lamina propria). Note that mast cells in N2-MxcKO mice were abnormally clustered in the lamina propria, whereas most of those in the control Notch2flox/flox mice were intraepithelially migrated. (B) Toluidine blue staining followed by eosin staining of the small intestine on day 8; original magnification ×200. (C) The number of mast cells per 10 vcus in the small intestine on day 13 after S venezuelensis infection. Data are presented as means ± SEM; n = 3, P = .026076 (villus, epithelium), P = .00194 (villus, lamina propria), P = .021177 (crypt, epithelium), and P = .019324 (crypt, lamina propria), P = .047445 (submucosa). (D) Toluidine blue staining followed by eosin staining of the small intestine on day 13. Original magnification ×200. (E) The total number of mast cells per 10 vcus on day 0, day 8, and day 13 of infection. The total number of mast cells was significantly lower in N2-MxcKO mice at the early phase (day 8) and almost equal at the later phase (day 13) to that of control mice. Data are presented as means ± SEM; n = 10 and 8 (day 0, Notch2flox/flox and N2-MxcKO); n = 3 and 3 (day 8, Notch2flox/flox and N2-MxcKO); n = 4 and 4 (day 13, Notch2flox/flox and N2-MxcKO). (F) Time course of egg number in the stool. The number of excreted eggs was significantly greater in N2-MxcKO mice compared with those in Notch2flox/flox mice. Data are represented as means ± SEM; n = 4; P = .0291 (day 8) and P = .0219 (day 9). (G) Hematoxylin-eosin staining of the small intestine on day 12. Original magnification ×200. Arrows indicate worms. Worms were still observed in the villi in the jejunum of N2-MxcKO, but not of Notch2flox/flox mice.
Discussion
There is a growing body of evidence that Notch signaling modulates cellular migration and adhesion in endothelial, neural, and lymphoid lineage cells, as well as cancer cells.18 We have shown that Notch2 signaling induces the development of mast cells.11 However, it has remained unclear whether Notch2 signaling is involved in the distribution of mast cells in the intestinal mucosa or connective tissues or in controlling the functions of mast cells against microorganisms. Here, we investigated the role of Notch2 signaling in mast cells in terms of their distribution and functions using cell-specific Notch2-deficient mice. We found that in N2-MxcKO mice, mast cells were abnormally accumulated in the lamina propria of the small intestine, suggesting that Notch2-null mast cells have some defect in the migration toward the epithelium. Furthermore, N2-MxcKO mice failed to eradicate S venezuelensis and exhibited a distinct mast cell migration pattern in the intestine compared with control mice, suggesting that mast cells regulate the host-microbial interface in the intestine through Notch2 signaling.
Mast cell number was rather increased in the intestinal mucosa of N2-MxcKO mice compared with control mice in naive status. Mast cell progenitors were supposed to reside in the submucosa and gradually move toward the villi, accompanied by their differentiation into mature mast cells. Based on our observation in an S venezuelensis–infection model, mast cells increase in number in the epithelium in control Notch2flox/flox mice, while they abnormally aggregate in lamina propria in N2-MxcKO mice, especially in the later stage of infection. This suggests that mast cell migration from lamina propria toward the epithelium across the basement membrane is impaired in N2-MxcKO mice. Consequently, mast cell turnover might be prolonged in N2-MxcKO mice. Given that the mechanism of mast cell migration from lamina propria toward the epithelium is common in naive status and infection status, such migration defect may also explain the mast cell increase in N2-MxcKO mice in naive status that we observed.
The defect of mast cell migration toward intraepithelium of the small intestine in N2-MxcKO mice is very similar to that in integrin β6-deficient mice,19 in which activation of transforming growth factor (TGF)–β signaling is impaired.20 A crosstalk between Notch signaling and TGF-β signaling might occur in intestinal mast cells as well as the cases of other cell types.21 Alternatively, Notch signaling might directly regulate a downstream target of TGF-β1 in intestinal mast cell migration (eg, the induction of integrin αE expression).19,22 Integrin αE, forming an integrin αEβ7 complex on mast cells, binds to E-cadherin on epithelial cells and is involved in mast cell localization in the epithelium.22 The expression level of integrin αEβ7, measured by flow cytometric analysis, however, was not affected by Notch-ligand stimulation in BMMCs (unpublished data).
In the previous paper we showed that Notch signaling facilitates mast cell lineage development at the expense of granulocyte/macrophage development from both common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs) in vitro.11 Mast cells, however, were not depleted in N2-MxcKO mice in naive status in vivo, but rather slightly increased in the small intestine of N2-MxcKO mice. This clearly indicates that Notch2 signaling is dispensable for steady-state mast cell generation in vivo. However, the dynamic increase of mast cells during the early phase of intestinal parasite infection was markedly impaired in N2-MxcKO mice. The mechanisms underlying the Notch2 signaling requirement only in parasite-infected mice remain to be clarified. Nevertheless, rapidly increasing intestinal mast cells have to be supplied by mast cell progenitors. The pathways and mechanisms responsible for mast cell progenitor recruitment and trafficking are likely to be dynamic and susceptible to modification during inflammation.1 Such a modulation of the mast cell generation pathway during intestinal infection might underlie the requirement of Notch2 only during parasite infection. This is similar to IL-3–deficient mice. IL-3 is essential for mast cell differentiation in vitro; however, IL-3–deficient mice have the normal number of mast cells at the steady state, whereas mast cell hyperplasia is impaired upon intestinal parasite infection.23
Our data showed that parasite expulsion was impaired in N2-MxcKO mice. We could not exclude the possibility that the Notch2 deletion in immune cells other than mast cells modulate the response against the nematode infection. If we could show that Th2-conditioned wild-type BMMCs successfully eradicate S venezuelensis in Wsh/Wsh mice and that Notch2-null BMMCs do not, it would be clearer that Notch2 signaling in mast cells per se but not in other immune cells should be critically important for defense against S venezuelensis infection. The failure of rescue experiments may be caused by the abnormal mast cell distribution pattern of wild-type BMMCs in Wsh/Wsh mice. Nevertheless, the result of this experiment supported the previous finding that the proper epithelial migration of mast cells is required for efficient expulsion of S venezuelensis24 and thus provides an insight that the impaired S venezuelensis expulsion in N2-MxcKO mice is attributed to the mast cell-autonomous deletion of Notch2.
In conclusion, our data clearly indicate that Notch2 receptor signaling is specifically required for proper intestinal mast cell distribution in a cell-autonomous manner. Furthermore, involvement of Notch2 signaling in mucosal immunity was proven, particularly for eradication of infected parasites, although whether this is due to the Notch2 signaling in mast cells is yet to be elucidated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Shigeo Koyasu for kind advice on the parasite infection experiment and Dr Cliff Takemoto, Dr Eichi Morii, and Dr Keisuke Oboki for kind advice on the histochemistry. We also thank Dr Hiromitsu Nakauchi for the Ly5.1 mice.
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI #21790905 to M.S.-Y. and #18659276 to S.C.) and by grants from Mitsubishi Pharma Research Foundation and Takeda Science Foundation.
Authorship
Contribution: M.S.-Y. designed and performed the research, analyzed the data, and wrote the paper; T.S., Y. Miyake, and Y. Morishita performed the research; T.I.S., H.M., and H.Y. contributed new reagents; E.N.-Y., K.K., M.F., S.O., and M.K. provided vital discussion; K.Y. designed the research; and S.C. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shigeru Chiba, Department of Clinical and Experimental Hematology, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8575, Japan; e-mail: schiba-tky@umin.net.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal